Abstract
Autoimmune phenomena including vasculitis are known to be associated with malignancy, especially those that are haematological in origin. Vasculitis syndromes associated with malignant disease include cutaneous leucocytoclastic vasculitis, polyarteritis nodosa, Churg-Strauss syndrome, microscopic polyangiitis, Wegener's granulomatosis and Henoch-Schönlein purpura. We describe a patient whose initial presentation with vasculitis led to the diagnosis of hairy cell leukaemia.
Background
Autoimmune phenomena including vasculitides are known to be associated with malignancies. Statistically, vasculitides have been shown to be associated with malignancy in 5% cases, especially those that are haematologic in origin.1 2 Vasculitis syndromes associated with malignant disease include cutaneous leukocytoclastic vasculitis (LCV), polyarteritis nodosa, Churg-Strauss syndrome, microscopic polyangiitis, Wegener's granulomatosis and Henoch-Schönlein purpura.2–7 Of these, cutaneous LCV is the most common.8 We describe a patient whose initial presentation with cutaneous LCV ultimately led to the diagnosis of a haematologic malignancy.
Case presentation
A 61-year-old male patient, previously known to be in good general health was admitted to our hospital with a 3-month history of a profound weight loss (30 pounds over a 3-month period), anorexia, night sweats, malaise and a new skin rash. Previously he had been admitted and treated at another institution for mild fever and rash, where he was diagnosed with biopsy-proven LCV.
Investigations
Laboratory evaluation at that time had revealed leucopaenia, white cell count of 1250/μl (nl 4800–10800/μl), neutropenia with an absolute neutrophil count of 600/μl (nl>1500/μl) and macrocytic anaemia with a haemoglobin of 9.6 g/dl (nl 14–17.5 g/dl) with an mean corpuscular volume of 105 fl (nl 80–99 fl). Platelet count was normal. Systemic oral steroids had been started in-hospital and were continued at home with instructions to follow-up with a rheumatologist for further management. However, persistent fever and malaise led to his presentation to our institution. Examination at this time revealed an ill appearing man with a temperature of 39.5°C. A skin rash, best described as palpable purpura on the ventral surface of the upper and lower extremities was readily apparent (figure 1). He also had purpuric lesions on the face, especially around the eyes and lips. Abdominal exam revealed hepatosplenomegaly. Laboratory data included a complete blood count with a white cell count of 700/μl, with an absolute neutrophil count of 343/μl, haemoglobin of 6.9 g/dl, haematocrit 20.1% (nl 39–53%), mean corpuscular volume 101.2 fl. Platelet count was 110/μl (nl 130–400/μl). Erythrocyte sedimentation rate was elevated at >140mm/h (nl 0–15 mm/h) and the C reactive protein was 23.58 mg/dl (nl 0–0.7 mg/dl). Rheumatological work-up, including an antineutrophil antibody and antineutrophilcyoplasmic antibody were negative with normal complement levels. Chemistries including serum creatinine were normal. Hepatitis A, B and C panels and HIV testing were negative. CT scan of the chest and abdomen demonstrated consolidation in the right lower lung with enlarged intrathoracic lymph nodes and splenomegaly. The patient underwent bronchoscopic lavage and biopsy which did not demonstrate infection or malignancy.
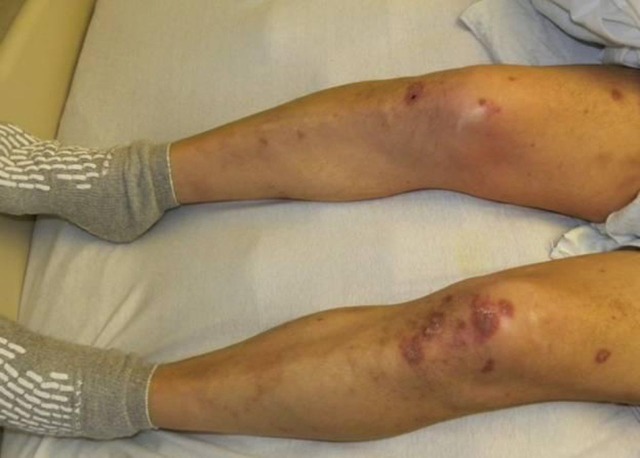
Figure 1.
Vasculitic lesions involving bilateral lower extremities. Depicted are palpable purpuras involving the lower extremities. Similar lesions were present on the upper extremities and face.
Differential diagnosis
Systemic symptoms especially weight loss in the setting of a rash with an evidence of pancytopenia were atypical for an uncomplicated vasculitic process. Haematological malignancies, especially since these have been associated with vasculitic phenomena, and lymphoproliferative or malignant processes affecting the bone marrow were included in the differential diagnosis.
Treatment
Antibiotics were empirically started for hospital-acquired pneumonia and steroids were continued for vasculitis.
Outcome and follow-up
Peripheral blood flow cytometry revealed a small population of immunophenotypically abnormal B cells which coexpressed CD10, CD103, CD11c and CD25, suggesting a diagnosis of hairy cell leukaemia (HCL). A subsequent bone marrow biopsy revealed subtotal replacement of the bone marrow with atypical lymphocytes with hairy cell morphology (figure 2). The atypical lymphocytes strongly expressed CD20 (figure 3) as well as CD10, CD103, CD11c and CD25, confirming the diagnosis of HCL. The patient's condition deteriorated after a right basal ganglion infarct, likely secondary to cerebral vasculitis. The patient died during the hospitalisation before chemotherapy could be initiated.
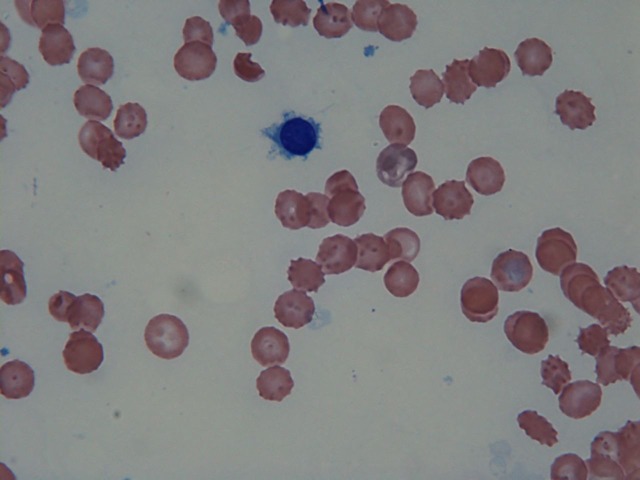
Figure 2.
Bone marrow aspirate smear. The haemodilute aspirate specimen demonstrated atypical lymphocytes with cytoplasmic projections.
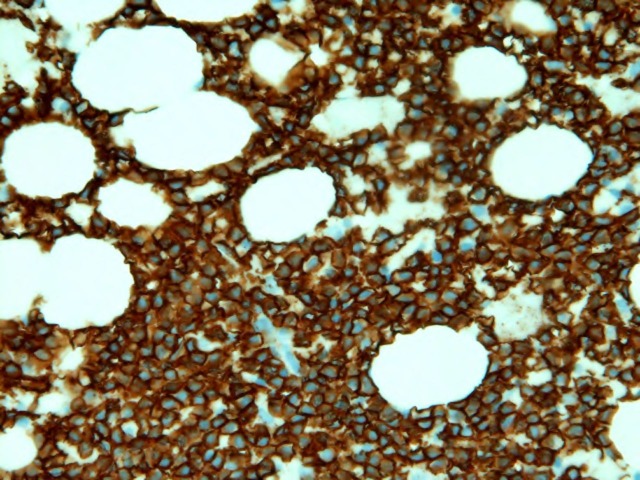
Figure 3.
Bone marrow biopsy. Histological section of the bone marrow biopsy demonstrated diffuse involvement by an atypical B cell infiltrate (CD20 immunohistochemical stain). Flow cytometric analysis demonstrated a hairy cell phenotype with expression of CD103, CD25 and bright CD11c. CD10 was also expressed.
Discussion
HCL is an indolent lymphoproliferative malignancy characterised by infiltration of the bone marrow, liver, spleen and occasionally lymph nodes with malignant B cells with hair-like cytoplasmic projections.9 It was first described as leukaemic reticuloendotheliosis by Ewalt in 1923.10 Bouroncle et al10 in 1958 published a report describing 26 cases and proposed its existence as a distinct haematopathological entity. HCL accounts for 2% of the adult leukaemias, with a 4 : 1 male preponderance.11 12 More than 50% patients with HCL present with haematological complications including anaemia, thrombocytopenia, leucopenia, splenomegaly and infections.9 Of the remaining, a proportion of the patients can present with autoimmune phenomena (vasculitis, skin rash, autoimmune haemolytic anaemia, idiopathic thrombocytopenic purpura, erythema nodosum, pulmonary infiltrates, arthritis and antiphospholipid antibody syndrome).3 4 13 Vasculitis has been reported in 4.5–8% cases who have lymphoproliferative diseases,14 and is responsible for complications including renal disease, pulmonary symptoms, skin ulcers and cerebral vasculitis (similar to our patient).15
The characteristic immunophenotype in HCL comprises of positivity for CD103, CD11c (bright) and CD25. The current case also demonstrated expression of CD10. CD10 expression in hairy cell leukaemia has been reported in a few studies in literature.16 However, there do not seem to be any significant morphological or clinical differences associated with its expression.
Vasculitis was first reported to be associated with HCL in 1976,6 followed by a report by Hasler et al4 in 1995 which described 42 patients. Fain et al2 in 2007 analysed 60 patients with vasculitis associated with all types of malignancies; however, haematological malignancies were most commonly reported. No statistically significant association was established between the type of vasculitis and a particular cancer. There was no temporal relationship between the diagnosis of vasculitis and malignancy (vasculitis diagnosed before cancer in 14 patients, simultaneously in 24 patients and afterwards in 22 patients). This study found only one patient with HCL, but this was attributed to the rarity of HCL in the general population.
The mechanism of the association between vasculitis and HCL has been postulated in studies, but there is no evidence implicating a specific aetiology. Vasculitis maybe the result of a reaction to infection or it may be a paraneoplastic presentation of the leukaemia itself.2 17 Production of cytokines including tumour necrosis factor-α, transforming growth factor-β and various paracrine growth factors by leukaemic cells possibly contributes to the latter. While some studies have related the production of autoantibodies by the polyclonal bystander B cells to autoimmune disease, others have implicated treatment with anticancer drugs (eg, purine analogues) that could lead to a loss of auto regulatory T cells.5 18 The role of neutropenia, impaired function of monocytes and macrophages and infiltration of the reticuloendothelial system by leukaemic hairy cells are other proposed mechanisms.2 This impaired functioning of the reticuloendothelial system may aggravate autoimmune phenomena. For example, vasculitis may be aggravated by impaired clearance of immune complexes formed with tumour antigens. Antibodies to tumour antigens may cross react with molecules present on the vascular endothelium (molecular mimicry). Impaired immune surveillance may promote viral invasion followed by the formation of viral immune complexes. Deposition of malignant cells in vascular walls may incite inflammatory damage.7 However, no firm molecular evidence exists in literature to support or quantify the role of each of these potential mechanisms.
Interestingly there have been reports (Musette et al19) which suggest that the genetic sequence of the serine proteases proteinase-3 (PR3-the target antigen in Wegner's granulomatosis related vasculitis) and myeloblastin (mbn—found in HL-60 human leukaemic cells) is similar. This similarity might be responsible for the coexistence of leukaemia and vasculitis in some patients.
Learning points.
While the exact mechanism still remains elusive, the fact that vasculitis can be a harbinger of hairy cell leukaemia needs to be considered. Patients presenting with vasculitis should be evaluated thoroughly with a history, physical examination and pertinent laboratory studies to exclude aetiologies including malignancy.
While testing for malignancy in every leukocytoclastic vasculitis (LCV) would not be cost effective, based on our case and other studies,8 20 we suggest that if vasculitis persists despite treatment with steroids or recurs after initial response, it mandates search for malignancy with more complex imaging or invasive techniques.
Also, as exemplified by our case, if vasculitis is accompanied by atypical features like weight loss and unremitting constitutional signs and symptoms, it should raise a clinician's suspicion for cancer.
In addition, physicians involved in the care of patients with cutaneous LCV must ensure adequate age appropriate screening.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Hayem G, Gomez MJ, Grossin M, et al. Systemic vasculitis and epithelioma. A report of three cases with a literature review. Rev Rhum Engl Ed 1997;64:816–24. [PubMed] [Google Scholar]
- 2.Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum 2007;57:1473–80. [DOI] [PubMed] [Google Scholar]
- 3.Fain O, Guillevin L, Kaplan G, et al. Vasculitis and neoplasms. 14 cases. Ann Med Interne (Paris) 1991;142:486–504. [PubMed] [Google Scholar]
- 4.Hasler P, Kistler H, Gerber H. Vasculitides in hairy cell leukemia. Semin Arthritis Rheum 1995;25:134–42. [DOI] [PubMed] [Google Scholar]
- 5.Tousi B, D'Silva R, Papish S. Systemic vasculitis complicating hairy cell leukaemia treatment with cladribine. Clin Lab Haematol 2002;24:259–60. [DOI] [PubMed] [Google Scholar]
- 6.Hughes GR, Elkon KB, Spiller R, et al. Polyarteritis nodosa and hairy-cell leukaemia. Lancet 1979;1:678. [DOI] [PubMed] [Google Scholar]
- 7.Mertz LE, Conn DL. Vasculitis associated with malignancy. Curr Opin Rheumatol 1992;4:39–46. [PubMed] [Google Scholar]
- 8.Hutson TE, Hoffman GS. Temporal concurrence of vasculitis and cancer: a report of 12 cases. Arthritis Care Res 2000;13:417–23. [DOI] [PubMed] [Google Scholar]
- 9.Kraut EH. Clinical manifestations and infectious complications of hairy-cell leukaemia. Best Pract Res Clin Haematol 2003;16:33–40. [DOI] [PubMed] [Google Scholar]
- 10.Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood 1958;13:609–30.13560561 [Google Scholar]
- 11.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flandrin G, Collado S. Is male predominance (4/1) in hairy cell leukaemia related to occupational exposure to ionizing radiation, benzene and other solvents? Br J Haematol 1987;67:119–20. [DOI] [PubMed] [Google Scholar]
- 13.Mainwaring CJ, Walewska R, Snowden J, et al. Fatal cold anti-i autoimmune haemolytic anaemia complicating hairy cell leukaemia. Br J Haematol 2000;109:641–3. [DOI] [PubMed] [Google Scholar]
- 14.Wooten MD, Jasin HE. Vasculitis and lymphoproliferative diseases. Semin Arthritis Rheum 1996;26:564–74. [DOI] [PubMed] [Google Scholar]
- 15.Lowe J, Russell NH. Cerebral vasculitis associated with hairy cell leukemia. Cancer 1987;60:3025–8. [DOI] [PubMed] [Google Scholar]
- 16.Seckin D, Senol A, Gurbuz O, et al. Leukemic vasculitis: an unusual manifestation of leukemia cutis. J Am Acad Dermatol 2009;61:519–21. [DOI] [PubMed] [Google Scholar]
- 17.Grey MR, Flanagan NG, Kelsey PR. Severe skin rash in two consecutive patients treated with 2-chlorodeoxyadenosine for hairy cell leukaemia at a single institution. Clin Lab Haematol 2000;22:111–13. [DOI] [PubMed] [Google Scholar]
- 18.Musette P, Labbaye C, Dorner MH, et al. Wegener's autoantigen and leukemia. Blood 1991;77:1398–9. [PubMed] [Google Scholar]
- 19.Jasionowski TM, Hartung L, Greenwood JH, et al. Analysis of CD10+ hairy cell leukemia. Am J Clin Pathol 2003;120:228–35. [DOI] [PubMed] [Google Scholar]
- 20.Kermani TA, Warrington KJ, Amin S. Malignancy risk in vasculitis. Ther Adv Musculoskel Dis 2011;3:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]