Abstract
A patient with a bulky inoperable stage IIIC melanoma involving the left axilla and neck from a primary of the left medial elbow received vemurafenib as neo-adjuvant treatment. Based on the molecular analysis, BRAF V600E mutation was present. After 4 months of vemurafinib treatment, the tumours shrank to less than 50% of original clinical size and allowed the surgeons to perform a left modified radical neck dissection and left radical axillary dissection. Pathological analysis of specimen revealed viable metastatic cells only in 1 of 40 nodes resected in the neck and axillary dissection, accounting for over 98% pathological response. Other lymph nodes had a mixture of foamy histiocytic inflammatory reaction fibrosis and islands of necrotic tissues. After recovery from surgery, vemurafenib was resumed and continued for 6 months. He remained disease free 6 months after surgery.
Background
Survival and outcome of malignant melanoma is limited in patients with unresectable melanoma.1–4 Many modalities of adjuvant and some neoadjuvant therapy have been tested to achieve a longer disease-free survival. Recent advances in molecular targeting treatment of melanoma have shown a promising prospect in treating the locally advanced or distant disease.
Vemurafenib is a potent inhibitor of the BRAF V600E mutation, which has been recently approved by Food and Drug Administration (FDA) for the treatment of metastatic melanoma. It is developed to target specific genetic aberrations (mitogen-activated protein kinase pathway (MAPK)), which normally regulates cell growth, proliferation and differentiation.5 BRAF mutation can be found in 50–60% of patients with cutaneous melanoma.6 7 Of these patients, 74–90% are V600E and 16–29% are V600K.8 BRAF V600E mutation results in elevated kinase activity and subsequently phosphorylation and stimulation of downstream endogenous phosphorylated extracellular signal-regulated kinase (ERK) in the former group compared with tumour-carrying BRAF wild type.9
In patients with stage IV and some advanced stage IIIC melanoma carrying BRAF V600E mutation, vemurafenib has been proved to be an effective therapy in 40—60% of patients in an adjuvant setting.5 7 10 This case presentation is to show that this inhibitor is an effective treatment for inoperable stage IIIC patients in a neo-adjuvant setting.
Case presentation
A 59-year-old man presented to our clinic with an extensive left neck and left axillary mass for 4 months. His primary melanoma was diagnosed on left medial elbow/distal upper arm, after biopsy of a mole done on 07/20/08, showing it to be a melanoma with Breslow thickness of 1.4 mm, close to peripheral and deep surgical margins, Clark level IV, 1 mitosis/mm2 and mild tumour-infiltrating lymphocytes. There was no ulceration, nor regression. He was originally treated with wide local excision on 8/29/2008 in his local surgical centre with no selective sentinel or regional lymph node dissection. Pathological analysis of the wide local excision revealed no evidence of residual melanoma.
Three months after this surgery, he developed nausea, vomiting, shortness of breath and finally he was admitted in January 2009 with diagnosis of endocarditis, most likely secondary to a dental procedure. His endocarditis resolved with antibiotic treatment. Subsequently, in April 2011, he felt a fast-growing mass in his left axilla. After initial workup, he underwent an excision of the most distal component of axillary mass on 06/09/2011 which was reported as a lymph node of 8×6.5×5 cm in diameter, entirely replaced by metastatic melanoma, (S-100 positive, HMB-45 focally positive, Ki-67 positive (50%)). After surgery, he developed antibiotic-resistant skin redness around the incision site including the left chest wall and axilla, consistent with tumor inflammation.
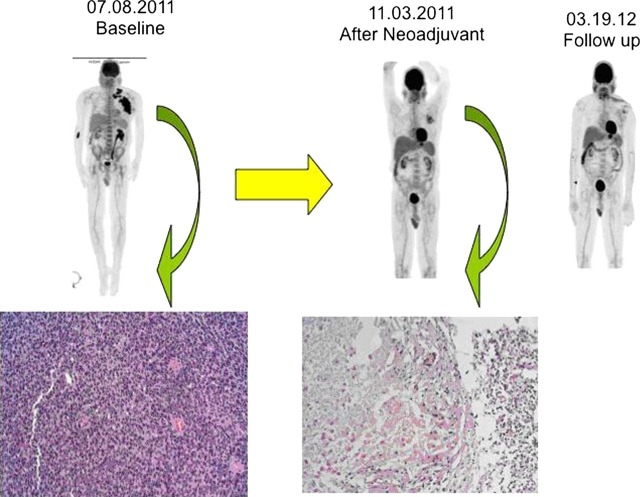
At the time of his presentation to our clinic, he had a multilobulated 15 cm fixed mass in the left axilla which expanded the whole width of axillary fossa from anterior border of latissimus dorsi muscle to pectoralis muscles and was associated with bulky fixed extensions posterior to pectoralis major and minor muscles in the axillary region and towards the base of the neck (figure 1). There was an area of skin erythema around the excisional biopsy scar which had remained unchanged despite antibiotic therapy. No other mass or lymphadenopathy was appreciated in the right neck, contralateral axilla or bilateral inguinal chains.
Figure 1.
A 53-year-old patient had an unresectable metastatic melanoma of the left neck and left axilla on 7/8/2011. Biopsy showed typical appearance of patient's melanoma with cytology markedly atypical with brisk mitotic activity (H&E 200×). The patient was treated with vemurafenib (960 mg every 12 h) with excellent resolution of the tumour as shown on the positron emission tomography (PET)/CT scan of 11/03/2011. He then underwent left radical neck and axillary lymph node dissection (12/2/2011). Pathology showed nodes after treatment replaced by granulomatous and histiocytic reaction with no tumour except for a focal 1 mm residual tumour in 1 out of 40 nodes (H&E 200×). Follow-up after surgery with PET CT on 03/19/2012 and continuous vemurafenib showed no evidence of disease with focal activity consistent with postoperative changes.
Positron emission tomography (PET)/CT imaging (7/8/2011) revealed an intensely hypermetabolic mass in the left axilla and left supraclavicular area without evidence of internal organ metastasis. MRI of the brain (7/8/2011) showed no evidence of brain metastasis. White blood cell count was 18.3, haemoglobin 16.2, haematocrit 47.1, platelet count 247 000 with aspartate aminotransferase 33, alanine aminotransferase 37, alkaline phosphatase 104 and total bilirubin 0.2, lactate dehydrogenase (LDH) 156 (120−243 U/l).
The patient was considered to have inoperable stage IIIC melanoma of the left upper extremity with extensive metastases to the left axillary and supraclavicular lymph nodes, and possible adhesions to the chest wall, pectoralis muscles, vascular structures or all of them.
Treatment
The patient's case was presented and reviewed by the multidisciplinary Tumor Board in the Center for Melanoma Research and Treatment at California Pacific Medical Center, San Francisco and in the presence of unresectable tumour in both left axilla and left side of the neck, it was decided to evaluate the status of BRAF mutation which was performed and was shown to carry BRAF V600E mutation. As a result, the patient received systemic therapy of vemurafenib in order to induce tumour shrinkage prior to surgical resection. He was initiated on 960 mg every 12 h for 1 month from 8/9/2011, but due to side effects such as fever and intractable joints pain, the dosage was reduced to 720 mg twice a day. After 4 months of neo-adjuvant therapy with vemurafenib, a dramatic clinical response was observed and verified by PET/CT scan (figure 1). Furthermore, all surrounding skin erythema had resolved.
On 12/2/2011, the patient was taken to the operating room and a left radical axillary lymph node dissection including level I, II and III with a Petit's manoeuvre (resection of pectoralis minor) and a left modified radical neck dissection of II, III, IV and V levels with sparing of the spinal accessory nerve, SCM muscle and the internal jugular vein were carried out simultaneously. The patient tolerated the surgery well and was discharged 4 days after surgery.
Outcome and follow-up
Pathological analysis of the specimen of the radical neck dissection could detect a total of 1 mm isolated viable melanoma cells in only 1 of 25 resected lymph nodes: of which 13 showed a mixture of marked foamy histiocytic inflammatory reaction, fibrosis and islands of necrotic tumour consistent with regression of prior metastatic melanoma cells. The left radical axillary lymph node dissection of all levels of I, II and III, revealed an 8 cm necrotic and fibrotic mass with a mixed chronic inflammatory infiltrates and foreign body giant cell reaction; without any viable tumour cells. Examination of other 14 resected axillary lymph nodes showed necrotic and partially hyalinised foci, consistent with prior tumour deposits, but with no viable tumour identified. Vemurafinib was resumed after left axillary and neck dissection resection and he has remained relapse free 6 months after surgery.
Discussion
Cytotoxic therapy for stage III (A−C) melanoma with a high dose of interferon with or without chemotherapy has been evaluated by some studies in a neo-adjuvant setting and had shown to have some limited promising results11–14 with overall clinical response rate of 26–55% and complete pathological response rate of 11–15%. However, only a minority of the patients participated in these studies had >4 lymph node involvement and none of them were considered as bulky or unresectable tumours. Bulky inoperable stage IIIC melanoma patients were generally treated as a stage IV melanoma and were considered with poor prognosis.15 16 There is another study currently being conducted by Tarhini et al,17 which evaluates the effect of ipillimumab as an anti-CTAL agent in a neo-adjuvant setting in stage IIIB-C cutaneous melanoma. The preliminary results presented in ASCO 2011 were encouraging and reported that 81% of treated patients remained disease free with a median follow-up of 7.9 months.
BRAF MP kinase inhibitors represent a major change in treatment approach for advanced melanoma patients. In a recent study conducted by Chapman et al,5 it was demonstrated that vemurafenib improved both progression-free and overall survival in patients with stage IIIC or IV metastatic melanoma who carried BRAF V600E mutation. The same study demonstrated that 48% of the patients treated with vemurafenib had detectable objective response compared to 5% clinical improvement in dacarbazine arm. Also, all patients with stage III in vemurafenib arm had some degree of tumour shrinkage, thus, an improved local tumour control. Recently, the use of another BRAF inhibitor (Dabrafenib) has been associated with improved rates of progression-free survival and overall survival among patients with BRAF-mutated melanoma even in the presence of brain metastasis.18 19
The effectiveness of vemurafenib in a neo-adjuvant setting has been demonstrated by this case presentation and verified by both remarkable clinical tumour shrinkage and by pathological evidence of necrosis of tumour cells in all but one lymph node. To our knowledge, this is the first case of administering vemurafenib in a neo-adjuvant setting in unresectable stage IIIC melanoma with a confirmed pathological response.
In the era of personalised medicine, molecular and genomic profiling has become a critical step in an approach to specific targeted therapy. This case report illustrates an oncogene-targeted neo-adjuvant approach, which may be the basis of selecting appropriate individualised patient treatment in the near future.
Learning points.
Vemurafinib is an important antimelanoma agent.
Patients with metastatic melanoma should have BRAF V600E determination to be qualified for vemurafinib treatment as only 50% of patients show mutation.
For inoperable bulky melanoma with positive BRAF V600E mutation, neo-adjuvant therapy is recommended to reduce the tumour burden for resection.
Footnotes
Funding: Dr Servando Cardona-Huerta is a visiting professor supported by a scholarship from the Consorcio de Universidades Mexicanas (CuMEX) and the Universidad Autonoma de Nuevo Leon, Mex. Dr Shih-Tsung Cheng is a visiting scholar sponsored by Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
Competing interests: None.
Patient consent: Obtained.
References
- 1.McLoughlin JM, Zager JS, Sondak VK, et al. Treatment options for limited or symptomatic metastatic melanoma. Cancer control. J Moffitt Cancer Center 2008;15:239–47. [DOI] [PubMed] [Google Scholar]
- 2.Feun LG, Gutterman J, Burgess MA, et al. The natural history of resectable metastatic melanoma (stage IVA melanoma). Cancer 1982;50:1656–63. [DOI] [PubMed] [Google Scholar]
- 3.Brand CU, Ellwanger U, Stroebel W, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma. An analysis of related prognostic factors. Cancer 1997;79:2345–53. [PubMed] [Google Scholar]
- 4.Wong SL, Coit DG. Role of surgery in patients with stage IV melanoma. Curr Opin Oncol 2004;16:155–60. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platz A, Egyhazi S, Ringborg U, et al. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol 2008;1:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47. [DOI] [PubMed] [Google Scholar]
- 8.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol Off J Am Soc Clin Oncol 2011;29:1239–46. [DOI] [PubMed] [Google Scholar]
- 9.Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol/Oncol Clin North Am 2009;23:529–45, ix. [DOI] [PubMed] [Google Scholar]
- 10.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs P, Anderson C, Pearlman Net al. A phase II study of neoadjuvant biochemotherapy for stage III melanoma. Cancer 2002;94:470–6. [DOI] [PubMed] [Google Scholar]
- 12.Lewis KD, Robinson WA, McCarter M, et al. Phase II multicenter study of neoadjuvant biochemotherapy for patients with stage III malignant melanoma. J Clin Oncol Off J Am Soc Clin Oncol 2006;24:3157–63. [DOI] [PubMed] [Google Scholar]
- 13.Buzaid AC, Colome M, Bedikian A, et al. Phase II study of neoadjuvant concurrent biochemotherapy in melanoma patients with local−regional metastases. Melanoma Res 1998;8:549–56. [DOI] [PubMed] [Google Scholar]
- 14.Tarhini AA, Pahuja S, Kirkwood JM. Neoadjuvant therapy for high-risk bulky regional melanoma. J Surg Oncol 2011;104:386–90. [DOI] [PubMed] [Google Scholar]
- 15.Tarhini AA, Frankel P, Margolin KA, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage IV melanoma of cutaneous or uveal origin. Clin Cancer Res Off J Am Assoc Cancer Res 2011;17:6574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher WS, Daniels DS, Sondak VK, et al. Evaluation of cisplatin and DTIC in inoperable stage III and IV melanoma. A Southwest Oncology Group study. Am J Clin Oncol 1993;16:359–62. [DOI] [PubMed] [Google Scholar]
- 17.Tarhini A, Edington H, Butterfield LH, et al. Neoadjuvant ipilimumab in patients with stage IIIB/C melanoma: immunogenicity and biomarker analysis. 2011 ASCO Annual Meeting. Chicago, IL, USA. [Google Scholar]
- 18.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107–14. [DOI] [PubMed] [Google Scholar]
- 19.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]