Abstract
A 47-year-old Turkish female patient was diagnosed with tuberculosis of the sacro-iliac joints and terminal ileum. She developed a severe adverse drug reaction while taking first-line tuberculosis therapy consisting of isoniazid, pyrazinamide and rifampicin as Rifater and ethambutol. Within 5 min of ingestion she developed pruritic rash, angioedema and breathing difficulties, resulting in an A&E admission. The tuberculosis therapy was discontinued. Intradermal and oral challenge tests for rifampicin were conducted but abandoned early on due to reactions which included audible wheeze, vomiting, throat pain and violent rigours. Clinical manifestations were swiftly treated with appropriate medications. This resulted in a change to the tuberculosis treatment regime, where streptomycin, isoniazid, ethambutol and pyrazinamide were given for 2 months and isoniazid and ethambutol for 12 months. Allergic reactions to rifampicin are rare and should be distinguished from flushing due to pyrazinamide. Prompt diagnosis and treatment by clinicians can be life saving.
Background
Tuberculosis infection is becoming increasingly common in the UK. The Health Protection Agency estimates that approximately 9000 cases are reported each year. Standard first-line worldwide drug therapy for Mycobacterium tuberculosis consists of the drugs isoniazid, pyrazinamide, ethambutol and rifampicin. Combination drug therapy is recommended by the WHO to prevent drug resistance. The drugs isoniazid, pyrazinamide and rifampicin are often prescribed as the combined medication; Rifater. Adverse reactions to first-line tuberculosis drug therapy are very rare.1 Pyrazinamide can cause flushing with the first dose. Rifampicin is a semisynthetic broad spectrum antibiotic which is effective in the treatment of mycobacteria.2 The standard treatment regime entails quadruple drug therapy of rifampicin, isoniazid, pyrazinamide and ethambutol for the first 2 months (intense phase) followed by a further 4 months of rifampicin and isoniazid (continuation phase).
This case raises awareness of adverse reactions to first-line tuberculosis therapy constituents and highlights an instance where the use of allergy testing becomes paramount to patient safety and care.
Case presentation
A 47-year-old Turkish female patient first presented to a rheumatologist with a history of lower back pain and radiological findings suggestive of bilateral sacroiliitis. In addition, the patient was also investigated for abdominal pain and anaemia. As part of this investigation, a colonoscopy was performed which showed a mild degree of ulceration in the terminal ileum. A biopsy of this showed evidence of active chronic inflammation. A Mantoux test was performed which showed a positive reaction of 22 mm as well as a Quantiferon Gold in Tube test which was strongly positive at 16.67 IU/ml (cut-off 0.35). Chest x-ray was clear. In addition, blood count results showed that the patient's haemoglobin was 9 g/dl (normal range: 11.5–16.5), mean corpuscular volume was 61.5 fl (normal range: 80–98), serum ferritin was 2 mg/l (normal range: 9–120) and erythrocyte sedimentation rate (ESR) was 49 mm/h (normal range: 3–15). However, renal, liver and bone profiles were normal.
These findings led to a diagnosis of tuberculosis of the sacro-iliac joints and terminal ileum along with iron deficiency anaemia. The patient was later started on iron supplement therapy. Subsequently, the haemoglobin level improved slightly over a period of 2 years and was between 10–11 g/dl. However, the serum ferritin remained low at 2 mg/l.
The patient was started on standard first-line tuberculosis therapy at home; Rifater tablets (constituents; isoniazid, pyrazinamide and rifampicin) and ethambutol. She had no history of allergy or atopy. Approximately 5 min after ingesting the first dose of her medication, she developed a pruritic rash, angioedema localised to the tongue and throat with increased difficulty in breathing. She was taken to the local A&E by the Helicopter Emergency Medical Services team by which time she developed an audible wheeze with some difficulty in breathing in addition to vomiting, abdominal and throat pain, a decrease in blood pressure and fever with violent rigours for which she was managed as detailed below. Subsequently, the patient was referred to the allergy service to establish the cause of this reaction and to query if first-line tuberculosis treatment could be restarted.
Investigations
Rifampicin was chosen as the first drug constituting the standard tuberculosis drug therapy based on an early review of literature.
An intradermal test was arranged in which 1 : 10 000 rifampicin was given intradermally to the left forearm. The result was negative although mild erythema was noted. Blood pressure, peak flow and pulse were constant.
Subsequently, a rifampicin oral challenge was conducted. Three increments of rifampicin at 1%, 4% and 10% of the full dose were administered after which the oral challenge was discontinued due to significant adverse symptoms being noted.
Blood pressure, pulse and peak flow were recorded at 15 min intervals. There was a slight decrease in blood pressure but a more significant decrease in peak expiratory flow rate (340–200 l/m). The other medications were not tested.
Differential diagnosis
The patient had acute bronchospasm with urticaria and angioedema likely to be drug induced (figure 1).
Figure 1.
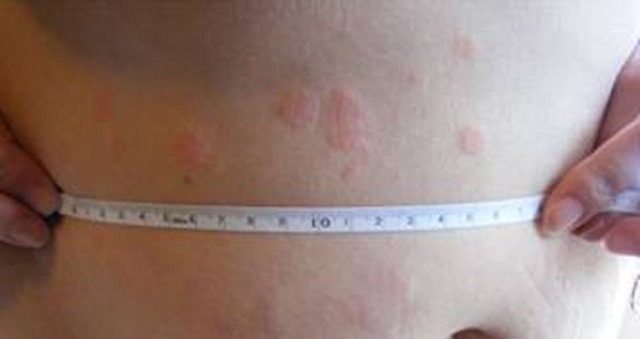
Urticaria is a common clinical manifestation of an adverse drug reaction. Illustrative image, not from the case described, with permission.
Treatment
The clinical manifestations of the patient were swiftly treated with oral prednisolone (30 mg), cetirizine (10 mg) and nebulised salbutamol (2.5 mg). Cyclizine (50 mg) and paracetamol (1 g) were also administered later. The patient was monitored and discharged the same day after resolution of the symptoms.
Outcome and follow-up
Confirmation of immediate hypersensitivity to rifampicin resulted in a change of treatment regime where rifampicin was substituted for intramuscularly administered streptomycin.
The complete modified treatment regime entailed quadruple drug therapy of pyrazinamide, isoniazid, ethambutol and streptomycin for the first 2 months (intense phase) followed by a further 12 months of isoniazid and ethambutol (continuation phase).
Following treatment, the patient underwent a repeat colonoscopy. A biopsy of the ileum showed no significant abnormality and a repeat ESR has come down to less than 15 mm/h.
The patient continues to avoid rifampicin constituent products.
Discussion
Clinical manifestations of rifampicin allergy typically occur as serum sickness and mild cutaneous reactions have been observed in 0.5–5% of patients. However, a major adverse effect such as anaphylactic shock is rare (6/30 000 reports of possible allergic reactions to rifampicin).2
There have been very few cases of immediate hypersensitivity to rifampicin published in the literature. However, exact context and clinical manifestations of these cases are principally dissimilar to the case we report here.
Recognition of this case depicting immediate allergy to rifampicin, a component of first-line tuberculosis drug therapy is for the benefit of those involved in clinical practice and research. It is important that health professionals involved in the treatment of tuberculosis are aware that severe allergic reactions do occur to the medications they commonly use and that allergy testing is available in the NHS.
Learning points.
Severe allergic reactions to rifampicin, although extremely rare, do occur.
Allergy reactions should be distinguished from immediate flushing due to pyrazinamide.
Prompt recognition of this complication and institution of control measures are vital for patient safety.
Allergy testing is helpful towards confirming the likely cause of a given adverse reaction when drug combinations are given.
Allergy testing is available in the NHS through referrals to allergy clinics.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Vervloet D, Pradal M, Brinbaum J, et al. Drug allergy. France: Corlet imprimeur, 2007. [Google Scholar]
- 2.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003;167:2472–7. [DOI] [PubMed] [Google Scholar]


