Abstract
Background
Premature atherosclerosis in HIV-infected patients is associated with chronic infection by itself and adverse effects of antiretroviral treatment (ART). Extra virgin olive oil (EVOO) has a beneficial effect on the cardiovascular system because of its anti-inflammatory properties.
The objective of this study was to determine whether the consumption of EVOO improves inflammation and atherosclerosis biomarkers in HIV-infected patients receiving ART.
Material/Methods
This randomized, crossover, controlled trial included 39 HIV-positive male participants who consumed 50 mL of EVOO or refined olive oil (ROO) daily. Four participants dropped out of the study. Leukocyte count, erythrocyte sedimentation rate (ESR), high-sensitivity C-reactive protein (hsCRP), interleukin-6, fibrinogen, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, malondialdehyde, glutathione-peroxidase, superoxide dismutase, oxidized LDL and von Willebrand factor were determined before the first and after each of the 2 intervention periods. Intervention and washout periods lasted for 20 and 14 days, respectively.
Results
In participants with >90% compliance (N=30), hsCRP concentrations were lower after EVOO intervention (geometric mean [GM], 1.70 mg/L; 95% confidence interval [CI], 1.15–2.52) compared to ROO administration (GM, 2.92 mg/L; 95% CI, 1.95–4.37) (p=0.035). In participants using lopinavir/ritonavir, ESR and hsCRP concentrations decreased 62% and 151%, respectively, after EVOO administration. In the whole study population (N=35) we found no difference in analyzed biomarkers after EVOO administration.
Conclusions
Our exploratory study suggests that EVOO consumption could lower hsCRP in patients on ART.
MeSH Keywords: Anti-HIV Agents, Atherosclerosis, Inflammation, Olea
Background
Antiretroviral therapy (ART) prolongs life expectancy and improves the quality of life in HIV-infected people. However, persons successfully treated with ART also have an increased risk for developing many chronic conditions (e.g., subclinical atherosclerosis, coronary heart disease (CHD), diabetes mellitus, renal disease, malignancies) [1,2]. CHD and myocardial infarction have been reported in relatively young HIV-infected patients [3] and some reports found an association between increased frequency of myocardial infarction and protease inhibitors (PI) or abacavir use [4,5]. HIV infection is a chronic inflammatory disease that disrupts the endothelial function. It is now understood that inflammation is a major component in the pathogenesis of atherosclerosis [6]. The adverse effects of ART (e.g., high levels of total cholesterol, LDL cholesterol, and triglycerides) could also have an additional influence on accelerated atherosclerosis development. Traditional risk factors such as male sex, older age, and cigarette smoking may contribute to the appearance of cardiovascular risks and disease in HIV-infected patients [7–10].
In the last few decades many studies have been conducted to analyze the influence of some potentially healthy food ingredients [11–13] or vitamin supplements [14] on atherosclerosis biomarkers. Extra virgin olive oil (EVOO) is an ingredient believed for centuries to have a favorable influence on health, although some of its features were better defined only recently [11,12]. EVOO is the main source of dietary fats in some Mediterranean countries. Besides its high content of oleic acid, which is monounsaturated and less prone to lipid peroxidation, EVOO has some other important components such as phenolic compounds [15]. The favorable effect on systolic blood pressure, HDL cholesterol level, and LDL oxidation could be attributed to these phenolic compounds [16–18].
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that is synthesized during tissue damage or infection. In hepatocytes, IL-6 stimulates the synthesis of acute-phase proteins such as C-reactive protein (CRP), fibrinogen, hepcidin, and serum amyloid A [19]. CRP is a pentameric protein molecule whose level during acute infection may increase to 1000 times normal values. Therefore, its main application is for infection and therapy success monitoring. However, with improved sensitivity of the test (e.g., high-sensitivity CRP and hsCRP) it became an early atherosclerosis marker [20].
Glutathione-related metabolism enzymes such as glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) are involved in reactive oxygen species (ROS) processing, making them less toxic. Inadequate removal of ROS results in oxidative stress and subsequent atherogenesis. GSH-Px and SOD are today recognized as oxidative stress biomarkers, found to be disturbed in cardiovascular diseases [21].
Malondialdehyde (MDA) is a final lipid peroxidation product involved in the atherosclerosis process, as shown in animal and human studies [22,23].
Several EVOO intervention studies showed that IL-6 and hsCRP were decreased [24], GSH-Px mRNA was expressed at higher levels, enzyme activity was higher [25], and lipid peroxides (MDA) decreased [16].
We conducted an exploratory study to examine the effect of EVOO on atherosclerosis biomarkers in a group of HIV-infected men undergoing antiretroviral therapy. The primary outcome measures were changes of atherosclerosis biomarkers in HIV-infected patients on antiretroviral regimen after EVOO consumption. Secondary outcome measures were changes in atherosclerosis biomarkers after EVOO consumption in patients treated with PI and abacavir, respectively.
Material and Methods
Participants
We recruited HIV-infected men on a stable antiretroviral regimen (> 6 months) and who were currently receiving 1 of the following combinations: 1) nucleoside/nucleotide analogue reverse transcriptase inhibitors (NRTI/NtRTI) + protease inhibitors (PI) (N=10), 2) NRTI/NtRTI + non-nucleoside reverse transcriptase inhibitors (NNRTI) (N=28), and 3) NNRTI + PI (N=1). The inclusion criteria for enrollment of participants were: HIV-seropositivity, male sex (18 to 75 years of age), being treated with antiretroviral drugs, undetectable HIV RNA viral load for at least 6 months (by ultrasensitive Amplicor HIV-1 Monitor test, version 1.5 (Roche Diagnostics, Indianapolis, USA), and glucose levels within the reference range. We excluded HIV-patients with underlying acute/chronic diseases (e.g., diabetes or kidney diseases), except history of CHD. Four of the 43 eligible patients, who initially agreed to participate, dropped out before the beginning of the study. Finally, 39 participants were included in the study and were interviewed about family history of CHD, vitamin supplements administration, and side effects of ongoing therapy, and were given a 14-point questionnaire on Mediterranean food consumption [26,27]. Clinical examination was performed by doctors and nurses in the Outpatient Department for HIV-infection at the University Hospital for Infectious Diseases. The study was approved by the local institutional Ethics Committee and all participants provided written informed consent.
Study design
This study was registered at ClinicalTrials.gov with ClinicalTrials.gov Identifier: NCT00925795. A single-blind, randomized, crossover, controlled study was conducted between September 2009 and October 2010. Randomization of participants into 2 groups was computer-generated by the principal investigator. Each group consumed EVOO and refined olive oil (ROO) as a placebo, but in different order (Sequence 1: first EVOO; then ROO. Sequence 2: first ROO, then EVOO). Original manufacturers’ declarations were removed from both types of olive oils and EVOO was designated as oil number 1 and ROO as oil number 2. Intervention periods for oil administration lasted 20 days each, with 14 days of interruption (washout period) modeled on similar studies [24]. Daily doses of both oils were 50 mL. Participants received detailed instructions about olive oil consumption. It was emphasized not to cook olive oil, but to pour it over meals. During the washout period, participants were requested to avoid consumption of olives and olive oils of any kind. Compliance with oil consumption was assessed by participant self-report after each intervention period and registered on a visual analogue scale with percentage expression. Participants who self-administered more than 90% of the whole amount of a particular olive oil in 1 sequence were considered compliant.
Laboratory analysis
Before the study, we analyzed a total phenolic content of several different olive oils by the Folin-Ciocalteu method [28], as previously described. We decided to use EVOO and ROO, which contained 236 and 57 mg, respectively, of phenolic compounds per kilogram of oil. EVOO and ROO were originally provided from our market in large amounts. We did not open the bottles and just removed their original labels. Both types of oils were in dark bottles. Before the first, and after both intervention periods, we collected a blood sample in fasting state from all participants. In all 3 samples taken from each participant, we performed laboratory analysis for parameters as follows. Erythrocyte sedimentation rate (ESR), was measured by Westergren method, white blood cell count was measured on a Beckman Coulter LH 750 hematology analyzer (Beckman Coulter, Inc, Brea, California, USA) as a part of complete blood count (CBC). HsCRP concentration was measured by CardioPhase® Siemens using particle-enhanced immunonephelometry on BN ProSpec System® instrument (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). Interleukin-6 (IL-6) concentration was measured by ELISA Quantikine test (R&D Systems Europe, Abingdon, Great Britain). Oxidized LDL (ox-LDL), was measured by ELISA test (DRG Diagnostics GmbH, Marburg, Germany) and malondialdehyde (MDA) was analyzed by the modified thiobarbituric acid reactive substances (TBARS) method originally described by Yagi [29]. Catalytic concentrations of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) were measured in lysate of erythrocytes prepared by centrifuging EDTA blood on 2350 g/10 min, washing erythrocyte pellet with saline, and centrifuging again at 2350 g/5 min. Lysates of erythrocytes were frozen at −80°C until the day of analysis. Catalytic concentration of GSH-Px was measured through glutathione oxidation-reduction reactions by Ransel kit and catalytic concentration of SOD was measured by the rate of inhibition of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride by Ransod SD 125 kit (both kits from Randox Laboratories Ltd., Crumlin, Great Britain). Results for both parameters were expressed per gram of hemoglobin. Serum glucose level was analyzed by glucose oxidase method using a Fisher Diagnostics kit (Thermo Fisher Scientific, Middletown, Virginia, USA). Triglycerides (TG) and total cholesterol (TC) were measured by Thermo reagent kits (Electron Corporation Inc, Victoria, Australia). High-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were determined by standard enzymatic automated methods using Herbos kits (Herbos Diagnostics, Croatia). All of the above biochemistry parameters were analyzed on a Beckman Coulter 400 instrument (Beckman Coulter, Inc, Brea, California, USA). Fibrinogen and von Willebrand factor activity were determined by Multifibren®U reagent and by VWF: RCo assay (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany), respectively. All coagulation assays were measured in citrated plasma samples on a BCS XP Siemens instrument (Siemens Healthcare Diagnostics, Marburg, Germany).
Statistical analysis
Sample size (n=35) was determined by power analysis where α value was 0.05 with power (1-β) >80%. The Kolmogorov-Smirnov test was used to assess the normality of distribution of investigated parameters. For non-normally distributed parameters, data were expressed as median with interquartile range. To determine differences in baseline characteristics of the 2 groups of participants, we used the Mann-Whitney test. Differences in laboratory parameters for subanalysis were determined by Friedman test. The values P<0.05 were considered statistically significant.
Since this was an exploratory study, adjustments for multiple comparisons were not implemented. To assess the effect of period, treatment, and sequence, linear mixed models were used for statistical analysis. Values from linear mixed models are expressed as least-squares means. High-sensitivity CRP values were log-transformed to achieve a normal distribution. The linear mixed model enabled a comparison of atherosclerosis biomarkers results even in incomplete groups of participants (for example, participants who adhered to only 1 olive oil). Statistical analyses were done using MedCalc program, version 12.1.0.0. (MedCalc Software, Mariakerke, Belgium) and SAS program, version 9.1.3, (SAS Institute Inc, Cary, North Carolina).
Results
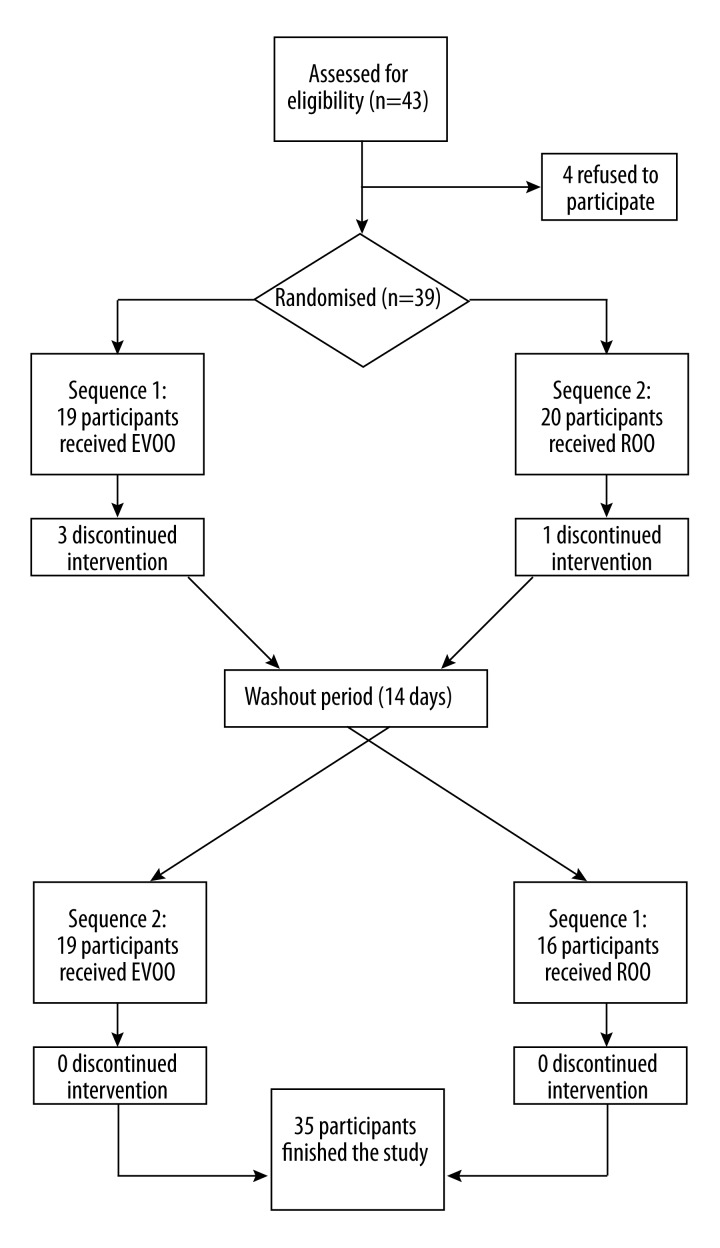
Of 39 randomized participants, 35 finished the study (4 participants did not participate in the second intervention period) (Figure 1). There were no differences in baseline clinical and laboratory parameters by the order of olive oil administration (Table 1).
Figure 1.
Flow of participants through the trial. EVOO – extra virgin olive oil. ROO – refined olive oil.
Table 1.
Baseline clinical and laboratory characteristics of participants by order of olive oil administration.
| Variable | Sequence 1 (N=19) | Sequence 2 (N=20) | p |
|---|---|---|---|
| Glucose (mmol/L) | 5.3 (5.1–6.0) | 5.7 (5.2–6.2) | 0.122 |
| Leukocyte number (×109/L) | 5.4 (4.7–6.5) | 5.6 (5.2–6.7) | 0.339 |
| ESR* (mm/h) | 11 (6–22) | 10 (6–16) | 0.643 |
| hsCRP** (mg/L) | 2.14 (1.06–2.60) | 1.56 (0.97–2.18) | 0.703 |
| Interleukin-6 (ng/L) | 1.83 (1.22–2.17) | 1.77 (1.01–2.99) | 0.633 |
| Fibrinogen (g/L) | 3.3 (2.9–3.5) | 3.1 (2.7–3.7) | 0.757 |
| Total cholesterol (mmol/L) | 5.6 (4.7–6.5) | 5.6 (5.0–6.8) | 0.747 |
| LDL cholesterol (mmol/L) | 3.4 (2.5–3.9) | 3.1 (2.8–4.0) | 0.623 |
| HDL cholesterol (mmol/L) | 1.3 (1.1–1.3) | 1.2 (1.1–1.4) | 0.989 |
| Triglycerides (mmol/L) | 2.3 (1.3–3.3) | 2.0 (1.6–3.1) | 0.888 |
| Malondialdehyde (μmol/L) | 4.02 (3.86–5.38) | 5.68 (4.11–8.01) | 0.068 |
| Glutathione peroxidase (U/g Hb) | 81.6 (58.7–106.0) | 82.9 (64.45–97.60) | 0.822 |
| Superoxide dismutase (U/g Hb) | 1941 (1812–2073) | 1745 (1747–2126) | 0.369 |
| Oxidized LDL (mg/L) | 0.82 (0.57–2.89) | 0.84 (0.21–3.65) | 0.623 |
| vonWillebrand factor (% of activity) | 125 (83–198) | 150 (130–188) | 0.518 |
| CD4+ lymphocyte number (/μL) | 464 (407–584) | 490 (437–692) | 0.286 |
| Puls velocity (beats/min) | 82 (80–96) | 80 (72–86) | 0.231 |
| Systolic blood pressure (mmHg) | 125 (115–140) | 125 (113–140) | 0.804 |
| Body weight (kg) | 74 (69–84) | 77 (73–84) | 0.546 |
| Waist circumference (cm) | 86 (76–95) | 90 (86–94) | 0.347 |
| Body mass index (kg/m2) | 25.2 (23.3–27.9) | 24.3 (23.0–26.0) | 0.311 |
Values are expressed as medians (25th–75th percentile). Sequence 1 = first Extra virgin olive oil, then Refined olive oil. Sequence 2 = first Refined olive oil, then Extra virgin olive oil.
Erythrocyte sedimentation rate,
high sensitivity C-reactive protein.
All participants who finished the study (N=35) had an undetectable plasma HIV1-RNA viral load (<50 copies/mL) at inclusion into the study. After the study (median days from inclusion: 164 days, minimum 63 days, maximum 337 days), all but 1 individual had a plasma HIV1-RNA viral load <50 copies/mL, and the patient with >50 copies/mL had 76 copies/mL. The median CD4 cell count at inclusion was 474 per μL, range 228 to 1648 per μL. The median duration of antiretroviral therapy was 4.7 years, range 0.5 to 12.2 years. There were no clinical events during the study period, and 12 (34%) patients had a past history of clinical AIDS.
The median Mediterranean diet score in our participants was 5 (interquartile range [IQR], 3–7) and was not different by sequence of olive oil administration (median 5 vs. 6; p=0.460). We found no difference in leukocyte count, ESR, hsCRP, IL-6, fibrinogen, TC, HDL cholesterol, TG, MDA, GSH-Px, SOD, ox-LDL, or vWf after EVOO administration in the whole study population. There were also no differences after ROO intervention, except for a lower LDL cholesterol level (p=0.020 for treatment, p=0.006 for period) (Table 2).
Table 2.
Clinical and laboratory characteristics in 35 participants who finished the study after extra virgin olive oil and refined olive oil administration.
| Variable | Post EVOO* (N=35) | Post ROO** (N=35) | Differences of least squares means | p (treatment effect) | p (period effect) | p (sequence effect) |
|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 5.5 (0.1) | 5.5 (0.1) | −0.014 | 0.894 | 0.894 | 0.536 |
| Leukocyte number (x109/L) | 6.2 (0.3) | 6.1 (0.3) | 0.130 | 0.578 | 0.281 | 0.766 |
| ESR*** (mm/h) | 13 (2) | 13 (2) | −0.279 | 0.885 | 0.359 | 0.690 |
| log hsCRP**** (mg/L) | 0.23 (0.08) | 0.33 (0.08) | −0.097 | 0.298 | 0.204 | 0.624 |
| Interleukin-6 (ng/L) | 2.93 (0.54) | 2.01 (0.54) | 0.914 | 0.231 | 0.591 | 0.619 |
| Fibrinogen (g/L) | 3.3 (0.1) | 3.4 (0.1) | −0.083 | 0.569 | 0.868 | 0.402 |
| Total cholesterol (mmol/L) | 5.9 (0.2) | 5.8 (0.2) | 0.082 | 0.471 | 0.566 | 0.588 |
| LDL cholesterol (mmol/L) | 3.3 (0.1) | 3.2 (0.1) | 0.133 | 0.020 | 0.006 | 0.660 |
| HDL cholesterol (mmol/L) | 1.3 (0.0) | 1.3 (0.0) | 0.026 | 0.884 | 0.226 | 0.397 |
| Triglycerides (mmol/L) | 2.8 (0.5) | 3.0 (0.5) | −0.217 | 0.582 | 0.976 | 0.259 |
| Malondialdehyde (μmol/L) | 4.73 (0.26) | 4.40 (0.26) | 0.328 | 0.185 | 0.138 | 0.052 |
| Glutathione peroxidase (U/g Hb) | 83.0 (4.3) | 84.5 (4.3) | −1.521 | 0.455 | 0.043 | 0.443 |
| Superoxide dismutase (U/g Hb) | 1919 (37) | 1965 (37) | −46.941 | 0.125 | 0.571 | 0.717 |
| Oxidized LDL (mg/L) | 1.85 (0.39) | 1.82 (0.39) | 0.033 | 0.787 | 0.024 | 0.759 |
| vonWillebrand factor (% of activity) | 154 (12) | 150 (12) | 4.373 | 0.607 | 0.903 | 0.722 |
| Puls velocity (beats/min) | 81 (2) | 80 (2) | 1.393 | 0.464 | 0.249 | 0.945 |
| Systolic blood pressure (mmHg) | 127 (3) | 130 (3) | −2.860 | 0.208 | 0.971 | 0.565 |
Values are expressed as least squares means (standard error) estimated from linear mixed model with terms for treatment, period and sequence.
Extra virgin olive oil;
refined olive oil;
erythrocyte sedimentation rate;
high sensitivity C-reactive protein.
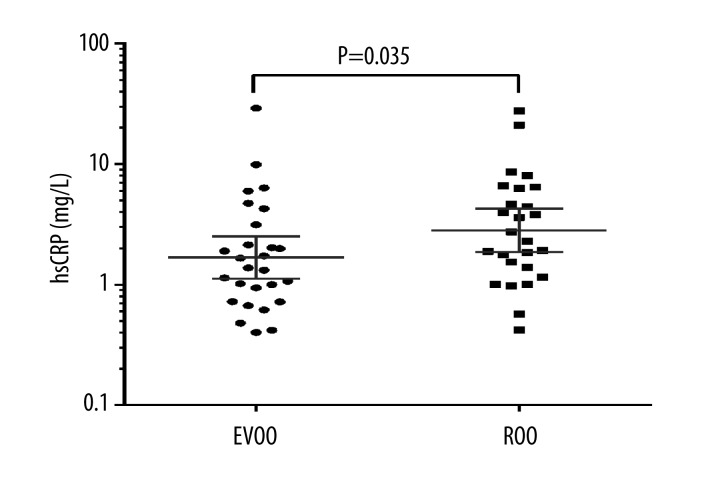
Thirty participants reported consuming olive oils >90% of the time; 27 participants were compliant to EVOO and 26 participants were compliant to ROO administration. Of those 30 individuals, 23 were compliant to both olive oils, 4 were compliant only to EVOO, and 3 were compliant only to ROO. In adherent participants (>90%), we found significantly lower hsCRP concentration after EVOO administration compared to hsCRP concentration after ROO administration (geometric mean [GM], 1.70 mg/L; 95% confidence interval [CI], 1.15–2.52 vs. 2.92 mg/L; 95% CI, 1.95–4.37; p=0.035) (Figure 2).
Figure 2.
High-sensitivity C-reactive protein (hsCRP) values after extra virgin olive oil (EVOO) and refined olive oil (ROO) administration in a crossover study of 30 HIV-infected patients who completed at least 1 intervention period and had > 90% adherence. Data are plotted on a logarithmic scale to reduce positive skewness in the distributions. The geometric mean with 95% confidence intervals is presented (P=0.035 for comparison of EVOO and ROO consumption).
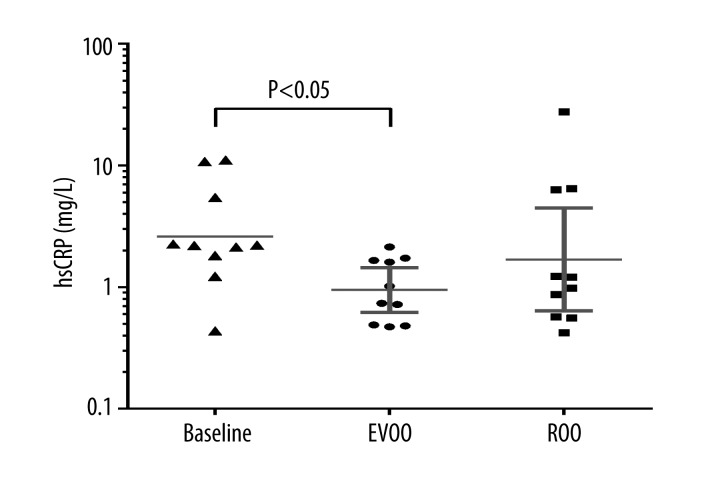
When the effect of olive oil administration was analyzed in 10 participants who received PI lopinavir/ritonavir, EVOO intervention resulted in a 62% lower ESR compared to baseline values (median, 13; IQR, 6–19 vs. median, 21; IQR, 10–36; p=0.040). In the same group of participants, we observed 151% lower hsCRP concentrations after EVOO administration, compared to baseline values (median, 0.88 mg/L; IQR, 0.49–1.66 vs. median, 2.21 mg/L; IQR, 1.82–5.47; p=0.035) (Figure 3). Participants receiving lopinavir/ritonavir had a higher baseline fibrinogen concentration (median, 3.9 g/L; IQR, 3.3–4.4) than participants treated with a NNRTI-based ART (median, 3.0 g/L; IQR, 3.7–3.4) (p=0.004).
Figure 3.
High-sensitivity C-reactive protein (hsCRP) values at baseline and after extra virgin olive oil (EVOO) and refined olive oil (ROO) administration in a crossover study with a subanalysis of 10 patients receiving the protease inhibitor lopinavir/ritonavir. Data are plotted on a logarithmic scale to reduce positive skewness in the distributions. The horizontal line is the geometric mean with 95% confidence interval (P<0.05 for the differences in groups).
There were no statistically significant changes in clinical and laboratory parameters after olive oil administration in 11 participants receiving abacavir.
Discussion
In this study we compared the effect of 2 different olive oils on atherosclerosis biomarkers in HIV-infected patients. There was no difference in biomarkers of atherosclerosis after consumption of EVOO and ROO in the whole study population (N=35). However, participants with compliance >90% to at least 1 olive oil (N=30) had significantly lower hsCRP concentration after EVOO intervention, compared to ROO intervention. There is a possibility that the influence of phenolic compounds was present only in participants with high compliance to EVOO. A decline in hsCRP has also been observed by Fito et al. after 3 weeks of olive oil consumption in non-HIV-infected persons [24].
After EVOO consumption, 10 patients treated with PI had significantly lower ESR and hsCRP when compared to baseline values. Additionally, baseline values of ESR, hsCRP, interleukin-6, and fibrinogen were significantly increased in PI-treated participants (N=10) when compared to non-PI-treated participants (N=28) (results not shown). Although a group of 10 participants is not sufficient to reach firm conclusions, these results suggest a higher inflammation rate in PI-treated participants and a more significant effect of EVOO on their inflammation biomarkers. As ART modifications are relatively frequent in our patient population [30], there was a small group of eligible patients treated for more than 6 months with PI. Of note, the only PI used by our participants was lopinavir/ritonavir. In 2010, the D.A.D. study group reported an association between myocardial infarction and cumulative exposure to indinavir and lopinavir/ritonavir [5]. PI may increase total cholesterol and triglyceride levels and decrease HDL-cholesterol levels as a consequence of longer action of sterol regulatory element-binding protein-1 (SREBP-1) and protein kinase C activation [31]. However, the D.A.D. group authors emphasized that a higher risk of myocardial infarction cannot be explained by lipid changes alone [5].
Madden et al. showed 11% higher fibrinogen concentrations in patients receiving PI compared to patients treated with NNRTI, which may be a consequence of the higher inflammation level related to PI regimen [32]. This assumption is in concordance with our results.
In our participants receiving abacavir, there was no difference in biomarkers after both olive oil consumptions compared to baseline values.
After ROO consumption, we found lower LDL cholesterol concentrations than after EVOO consumption. However, this difference (3.2 vs. 3.3 mmol/L) was not high and has little clinical significance.
The major clinical implication of our study is that EVOO may decrease the inflammation biomarkers in HIV-infected persons (such as hsCRP) during the 3-week period in compliant participants. Other atherosclerosis biomarkers that we analyzed (e.g., IL-6, ox-LDL, SOD, GSH-Px, and MDA) did not change, even in compliant participants. Because HIV-infected persons are immunocompromised, it is possible that a longer intervention period is needed to improve atherosclerosis biomarkers, although this could reduce the compliance of participants.
Our study has limitations. Compliance of our participants to olive oil consumption relied on self-reporting. Non-compliance cannot be ruled out, because we did not measure metabolites of olive oil consumption such as tyrosol and hydroxytyrosol in urine [33]. We also did not measure the content of other olive oil ingredients that could have influenced atherosclerosis biomarkers (e.g., vitamin C and α-tocopherol) [34,35]. Our intervention periods were only 3 weeks long and it is possible that longer duration of intervention periods might have caused a more significant change in atherosclerotic biomarkers. Finally, this was only an exploratory study with a small number of patients.
Conclusions
Overall, we found no difference in biomarkers of inflammation after EVOO administration in our study population. However, in a subanalysis of participants with high compliance to olive oils, hsCRP significantly decreased after EVOO administration. Hence, there could be a protective effect of EVOO consumption on inflammatory markers in HIV-infected patients on stable ART, but larger studies are needed to confirm these findings.
Acknowledgements
The authors thank Ana Mornar, PhD, from the Faculty of Pharmacy and Biochemistry, University of Zagreb, for assistance in olive oil analysis.
Footnotes
Source of support: This study was supported by a grant from the Ministry of Science, Education, and Sports of the Republic of Croatia (108-1080116-0098)
References
- 1.Hasse B, Ledergerber B, Furrer H, et al. Swiss HIV Cohort Study. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–39. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 2.Višković K, Rutherford GW, Sudario G, et al. Ultrasound measurements of carotid intima-media thickness and plaque in HIV-infected patients on the Mediterranean diet. Croat Med J. 2013;54:330–38. doi: 10.3325/cmj.2013.54.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaaqoq AM, Khasawneh FA, Smalligan RD. Cardiovascular complications of HIV-associated immune dysfunction. Cardiol Res Pract. 2015;2015:302638. doi: 10.1155/2015/302638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmberg SD, Moorman AC, Williamson JM, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–48. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 5.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D: A: D) study. J Infect Dis. 2010;201:318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 6.Curtiss LK. Reversing atherosclerosis? N Engl J Med. 2009;360:1144–46. doi: 10.1056/NEJMcibr0810383. [DOI] [PubMed] [Google Scholar]
- 7.Mehta N, Reilly M. Atherosclerotic cardiovascular disease risk in the HAART-treated HIV-1 population. HIV Clin Trials. 2005;6:5–24. doi: 10.1310/HT0W-NX2N-U2BM-7LUU. [DOI] [PubMed] [Google Scholar]
- 8.Galescu O, Bhangoo A, Ten S. Insulin resistance, lipodystrophy and cardiometabolic syndrome in HIV/AIDS. Rev Endocr Metab Disord. 2013;14:133–40. doi: 10.1007/s11154-013-9247-7. [DOI] [PubMed] [Google Scholar]
- 9.Aberg JA. Aging, Inflammation and HIV infection. Top Antivir Med. 2012;20:101–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Begovac J, Dragović G, Višković K, et al. Comparison of four international cardiovascular disease prediction models and the prevalence of eligibility for lipid lowering therapy in HIV infected patients on antiretroviral therapy. Croat Med J. 2015;56:14–23. doi: 10.3325/cmj.2015.56.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estruch R, Ros E, Salas-Salvadó J, et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 12.Medina-Remón A, Tresserra-Rimbau A, Pons A, et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr Metab Cardiovasc Dis. 2015;25:60–67. doi: 10.1016/j.numecd.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Casas R, Sacanella E, Urpí-Sardà M, et al. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS One. 2014;9:e100084. doi: 10.1371/journal.pone.0100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mierzecki A, Kłoda K, Bukowska H, et al. Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors. Med Sci Monit. 2013;19:733–39. doi: 10.12659/MSM.889087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covas MI, Ruiz-Gutierrez V, de la Torre R, et al. Olive oil minor components: evidence to date of health benefits in humans. Nutr Rev. 2006;64:s20–30. [Google Scholar]
- 16.Fito M, Cladellas M, de la Torre R, et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis. 2005;181:149–58. doi: 10.1016/j.atherosclerosis.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Covas MI, Nyyssönen K, Poulsen HE, et al. EUROLIVE Study Group. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med. 2006;145:333–41. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Toledo E, Hu FB, Estruch R, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med. 2013;11:207. doi: 10.1186/1741-7015-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2:288–94. doi: 10.1158/2326-6066.CIR-14-0022. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann O, Li K, Zaczkiewicz M, et al. C-Reactive protein in human atherogenesis: facts and fiction. Mediators Inflamm. 2014;2014:561428. doi: 10.1155/2014/561428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function and diseases. Antioxid Redox Signal. 2011;15:1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Pu H, Ma C, et al. Adiponectin abates atherosclerosis by reducing oxidative stress. Med Sci Monit. 2014;20:1792–800. doi: 10.12659/MSM.892299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho E, Karimi Galougahi K, Liu CC, et al. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1:483–91. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitó M, Cladellas M, de la Torre RH, et al. SOLOS Investigators. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. 2008;62:570–74. doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 25.Massela R, Vari R, D’Archivio M, et al. Extra virgin olive oil phenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J Nutr. 2004;134:785–91. doi: 10.1093/jn/134.4.785. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-González MA, Corella D, Salas-Salvadó J, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41(2):377–85. doi: 10.1093/ije/dyq250. [DOI] [PubMed] [Google Scholar]
- 27.Salas-Salvadó J, Garcia-Arellano A, Estruch R, et al. PREDIMED Investigators. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008;62:651–59. doi: 10.1038/sj.ejcn.1602762. [DOI] [PubMed] [Google Scholar]
- 28.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin & Ciocalteau reagent. Methods in Enzymology. 1999;299:152–78. [Google Scholar]
- 29.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–16. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 30.Perović Mihanović M, Haque NS, Rutherford GW, et al. Toxicity-related antiretroviral drug treatment modifications in individuals starting therapy: A cohort analysis of time patterns, sex, and other risk factors. Med Sci Monit. 2013;19:483–92. doi: 10.12659/MSM.889283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva EF, Bárbaro G. New options in the treatment of lipid disorders in HIV-infected patients. Open AIDS J. 2009;3:31–37. doi: 10.2174/1874613600903010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madden E, Lee G, Kotler DP, et al. Association of antiretroviral therapy with fibrinogen levels in HIV-infection. AIDS. 2008;22:707–15. doi: 10.1097/QAD.0b013e3282f560d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miró-Casas E, Farré Albaladejo M, Covas MI, et al. Capillary gas chromatography-mass spectrometry quantitative determination of hydroxytyrosol and tyrosol in human urine after olive oil intake. Anal Biochem. 2001;294:63–72. doi: 10.1006/abio.2001.5160. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Jimenez F, Alvarez de Cienfuegos G, Badimon L, et al. Consensus report, Jaen (Spain). International conference on the healthy effect of virgin olive oil. Eur J Clin Invest. 2005;35:421–24. doi: 10.1111/j.1365-2362.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 35.Vissers MN, Zock PL, Katan MB. Bioavailability and antioxidant effects of olive phenols in humans: a review. Eur J Clin Nutr. 2004;58:955–65. doi: 10.1038/sj.ejcn.1601917. [DOI] [PubMed] [Google Scholar]