Abstract
Purpose
Vasomotor responses of retinal arterioles to luminal flow/shear stress and VEGF have a critical role in governing retinal blood flow possibly via nitric oxide synthase (NOS) activation. However, the cellular mechanism for flow-sensitive vasomotor activity in relation to VEGF signaling in retinal arterioles has not been characterized. We used an isolated vessel approach to specifically address this issue.
Methods
Porcine retinal arterioles were isolated, cannulated, and pressurized to 55 cm H2O luminal pressure by two independent reservoir systems. Luminal flow was increased stepwise by creating hydrostatic pressure gradients across two reservoirs. Diameter changes and associated signaling mechanisms corresponding to increased flow and VEGF receptor 2 (VEGFR2) activation were assessed using videomicroscopic, pharmacological, and molecular tools.
Results
Retinal arterioles developed basal tone under zero-flow condition and dilated concentration-dependently to VEGF165. Stepwise increases in flow produced graded vasodilation. Vasodilations to VEGF165 and increased flow were abolished by endothelial removal, and inhibited by pharmacological blockade of VEGFR2, NOS, phosphoinositide 3-kinase (PI3K), calpains, or sirtuin-1 (SIRT1) deacetylase. A VEGF165 antibody blocked vasodilation to VEGF165 but not flow. Immunostaining indicated that VEGFR2 was expressed in the endothelial and smooth muscle layers of retinal arterioles.
Conclusions
Ligand-dependent and ligand-independent activation of VEGFR2 in the endothelium mediates NO-dependent dilations of porcine retinal arterioles in response to VEGF165 and luminal flow/shear stress, respectively. It appears that NOS stimulation via PI3K, calpain proteases, and SIRT1-dependent deacetylation downstream from VEGFR2 activation contributes to these vasodilator responses.
Keywords: retinal arterioles, endothelium, vasodilation, vascular endothelial growth factor, shear stress
Our findings provide direct evidence of endothelium-dependent dilations of retinal arterioles to flow/shear stress and VEGF. Activation of endothelial VEGF receptor 2 in response to these stimuli leads to PI3K/calpain/SIRT1 signaling to promote nitric oxide synthase-mediated dilation.
The retinal microcirculation lacks autonomic innervation so the regulation of retinal blood flow is largely dependent on alteration of vascular tone of the retinal arterioles in response to local mechanical1,2 and chemical3 stimuli. The shear stress generated by the flowing blood in a vessel exerts a tangential force on the endothelial surface, leading to activation of intracellular signal cascades and modulation of vascular function.4 Dilation of resistance arterioles to increased luminal flow or shear stress, termed flow-induced vasodilation, has been indicated to have a key role in the regulation of local blood flow in many organ systems,5 including the retina.2,6 The increase in luminal flow has been shown to directly elicit endothelium-dependent nitric oxide (NO)–mediated dilation of isolated coronary,7 mesenteric,8 and skeletal muscle arterioles.9 We have demonstrated recently that elevated flow also causes dilation of porcine and human retinal arterioles via activation of NO synthase (NOS).10
An important chemical factor produced and released by the endothelium11,12 and neural-glial cells13,14 in the retina under normal and ischemic/hypoxic conditions is VEGF. Although several different sizes of VEGF exon splice variants have been identified, the 165-amino-acid VEGF (VEGF165) is the predominant human isoform.15 It is a multifunctional protein that has been shown to promote hyperpermeability,16 angiogenesis,17 and hypotension18,19 in in vivo studies via activation of its VEGF receptor 2 (VEGFR2; also known as Flk-1 or KDR). The observed hypotensive effect suggests that VEGF165 promotes dilation of arterioles to reduce vascular resistance. In accord, in vitro studies have shown that VEGF165 dilates isolated coronary20–22 and retinal arterioles.23 Interestingly, VEGFR2 has been implicated as one of several mechanosensors triggered by flow/shear stress leading to transduction of mechanical stimuli into intracellular molecular signals in cultured endothelial cells.24–27 However, underlying cellular and molecular signaling events contributing to dilation of retinal arterioles in response to flow and VEGF165 are largely unknown. A better mechanistic understanding of these vasomotor responses is critical, considering the apparent prominent roles of blood flow and VEGF165 dysregulation in the development of retinal diseases, such as diabetic retinopathy3,28 and age-related macular degeneration.29,30 Therefore, in the present study we used an isolated vessel approach in vitro, which excludes neural-glial, metabolic, and humoral influences, to specifically address roles of the endothelium and VEGFR2 signaling in dilations of porcine retinal arterioles to luminal flow/shear stress and VEGF165.
Methods
Animal Preparation
All animal procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Scott & White Institutional Animal Care and Use Committee. Pigs of either sex (age range, 8–12 weeks; weight, 8–12 kg) purchased from Real Farms (San Antonio, TX, USA) were sedated with Telazol (4.4 mg/kg, intramuscularly), anesthetized with 2% to 4% isoflurane, and intubated. The procedure used for harvesting eyes has been described previously.31
Isolation and Cannulation of Microvessels
The techniques used for identification, isolation, cannulation, pressurization, and visualization of the retinal vasculature have been described previously.31 In brief, the isolated retinal arterioles (∼40–60 μm in situ) were cannulated with a pair of glass micropipettes and pressurized to 55 cm H2O intraluminal pressure without flow by two independent pressure reservoir systems.10 Vasomotor activity of isolated vessels was recorded continuously using videomicroscopic techniques throughout the experiments.10,31
Study of Vasomotor Function
Cannulated, pressurized arterioles were bathed in physiological saline solution (PSS)–albumin (0.1%) at 36°C to 37°C to allow the development of basal tone (stable within 90 minutes). The vascular response to increased flow was studied under constant intraluminal pressure using dual-reservoir techniques as described previously.10 In brief, the luminal flow was produced by simultaneously moving the pressure reservoirs in opposite directions of the same magnitude, which generates a pressure gradient (ΔP; range, 10–60 cm H2O) across the length of the vessel without changing intraluminal pressure.7 We have demonstrated previously that the luminal flow is increased linearly with increasing ΔP and the range of mean volumetric flows for ΔP between 0 and 60 cm H2O is 0 to 34.8 nL/s (0–2.1 μL/min),7 corresponding to the range reported in human retinal arterioles < 60 μm in diameter in vivo.32 In another cohort of vessels, concentration-dependent vasomotor responses to human recombinant VEGF165 (10 pM to 0.1 μM; Cell Signaling, Beverly, MA, USA) were evaluated in the absence of flow. Preliminary studies showed that the vasodilations to flow and VEGF165 were reproducible and did not deteriorate at least 30 and 90 minutes after the first exposure to these stimuli, respectively (Fig. 1), so these minimal time frames were followed for all mechanistic studies delineated below. Vessels were exposed to each concentration of agonist or level of flow for 4 to 5 minutes until a stable diameter was maintained.
Figure 1.
Vasodilator responses of isolated and pressurized porcine retinal arterioles to increased flow and VEGF165. (A) Retinal arterioles were exposed to stepwise increases in pressure gradient; that is, flow, in the absence (Denudation, n = 6) or presence (n = 6) of endothelium, or before and after a 30-minute washout period (Repeat, n = 6) or administration of L-NAME (n = 5). (B) Concentration-dependent vasodilation to VEGF165 was examined in the absence (Denudation, n = 5) or presence (n = 5) of endothelium, or before and after a 90-minute washout period (Repeat, n = 5) or administration of L-NAME (n = 5). *P < 0.05 versus Control.
The following studies were performed to elucidate the possible cellular mechanisms involved in retinal arteriolar dilations to flow and VEGF165. First, the role of endothelium was evaluated in vessels following air bolus injection to remove endothelial cells.33 Vasodilations to agonists were evaluated and compared in intact and denuded vessels from the same animal. The denuded vessels that exhibited normal basal tone, showed no vasodilation to endothelium-dependent vasodilator bradykinin (1 nM),10,33 and showed unaltered response to endothelium-independent vasodilator sodium nitroprusside (10 μM) were accepted for data analysis. Second, the contributions of NOS, VEGFR2, phosphatidylinositol 3-kinase (PI3K), and sirtuin-1 (SIRT1) deacetylase were examined following at least a 30- to 60-minute incubation with their specific inhibitors L-NAME10 (10 μM), SU149827,34 (1 μM; EMD Millipore, Billerica, MA, USA), wortmannin27 (0.1 μM), and EX52735 (5 μM; Bio-Techne/Tocris, Minneapolis, MN, USA), respectively. Third, the contribution of calpains was assessed following a 30-minute incubation with their cognate inhibitors MG13236 (2 μM; Bio-Techne/Tocris) and PD15060637,38 (2 μM; Bio-Techne/Tocris). Finally, vasodilator responses were obtained in another cohort before and after intraluminal administration of a VEGF165 antibody39 (1 μg/mL, sc-57496; Santa Cruz Biotechnology, Dallas, TX, USA).
The vasodilations to bradykinin and sodium nitroprusside also were assessed to test the function of endothelium and vascular smooth muscle, respectively, in the presence of inhibitors delineated above. We have shown previously that L-NAME does not alter dilation of porcine retinal arterioles to sodium nitroprusside.31 In addition, endothelium-dependent NO-mediated vasodilation to SIRT1 activator resveratrol (30 μM)40,41 was examined in the presence of EX527, wortmannin, and MG132.
Western Blot Analysis
Retinal arterioles of similar size to those used for functional studies were isolated and homogenized in lysis buffer. The protein content of each sample was quantified and separated by electrophoresis as described previously.42 For electrophoresis, 5 μg protein were loaded in each lane. Blotting and detection of proteins were done as described previously using a mouse anti-VEGFR2 primary antibody (1:250, sc-393163; Santa Cruz Biotechnology). After incubation with an anti-mouse secondary antibody (1:1000, sc-2005; Santa Cruz Biotechnology), the proteins were visualized via enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Immunohistochemical Analysis
Frozen sections (10-μm thick) of retinal arterioles were fixed in cold acetone for 10 minutes and then immunolabeled with a mouse anti-VEGFR2 antibody (1:100, sc-393163; Santa Cruz Biotechnology) and a goat anti-endothelial NOS (eNOS) antibody (1:100, AF950; R&D Systems, Minneapolis, MN, USA). Images were observed using fluorescence microscopy as reported previously.42
Chemicals
All drugs were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA) except as specifically stated. Sodium nitroprusside, VEGF165, VEGF165 antibody, and L-NAME were dissolved in PSS, whereas SU1498, wortmannin, MG132, PD150606, resveratrol, and EX527 were dissolved in dimethyl sulfoxide (DMSO). Subsequent concentrations of drugs in DMSO were diluted in PSS. The final concentrations of DMSO in the vessel lumen did not exceed 0.05% by volume. The 0.05% DMSO had no significant effect on vessel viability, vasodilator responses, or maintenance of tone (data not shown).
Data Analysis
At the end of each functional experiment, the vessel was relaxed with 0.1 mM sodium nitroprusside in ethylenediaminetetraacetic acid (EDTA, 1 mM)-Ca2+–free PSS to obtain its maximum diameter at 55 cm H2O intraluminal pressure.31 Diameter changes in response to vasodilator agonists and luminal flow were normalized to this maximum vasodilation and expressed as percent maximum dilation. Data are reported as mean ± SEM and n value represents the number of vessels (1 per pig per treatment group) studied. Student's t-test or ANOVA followed by Bonferroni multiple-range test was used to determine the significance of experimental interventions, as appropriate. A value of P < 0.05 was considered significant.
Results
Roles of Endothelium and NOS in Vasodilations to Flow and Agonists
In this study, all vessels (n = 170) developed a similar level of basal tone (constricted to 50 ± 7% of maximum diameter) in the absence of luminal flow. The average resting and maximum diameters of the vessel were 47 ± 9 and 95 ± 13 μm, respectively. The retinal arterioles exhibited graded dilation when the pressure gradient, and thus luminal flow, was increased in a stepwise manner (Fig. 1A). Under control conditions, the highest flow elicited nearly 60% of maximum dilation. In another cohort, vessels exhibited concentration-dependent dilation to VEGF165 with approximately 50% of maximum dilation to the highest concentration of 0.1 μM (Fig. 1B). Disruption of the endothelium did not affect resting basal tone (Control, 47 ± 2% versus Denudation, 51 ± 3%, n = 11, P = 0.24) but abolished vasodilations to flow (Fig. 1A), VEGF165 (Fig. 1B), and bradykinin (Fig. 2A). The vasodilation to sodium nitroprusside (10 μM) was not altered by endothelial removal (Control, 81 ± 6% versus Denudation, 73 ± 4%, n = 6, P = 0.28). In a similar manner as endothelial denudation, exposure to NOS inhibitor L-NAME nearly abolished vasodilations to flow (Fig. 1A), VEGF165 (Fig. 1B), and bradykinin (Fig. 2A) without significantly affecting basal tone (Control, 52 ± 3% versus L-NAME, 50 ± 2%, n = 15, P = 0.18) and vasodilation to sodium nitroprusside (10 μM; Control, 75 ± 3% versus L-NAME, 79 ± 6%, n = 4, P = 0.51).
Figure 2.
Vasodilator responses of isolated and pressurized porcine retinal arterioles to bradykinin and resveratrol. (A) Vasodilation to bradykinin (1 nM) was examined in the absence (Denudation, n = 5) or presence (n = 5) of endothelium, or before and after administration of L-NAME (n = 5), SU1498 (n = 4), wortmannin (n = 5), MG132 (n = 5), PD150606 (n = 5), or EX527 (n = 8). (B) Vasodilation to resveratrol (30 μM) was examined before and after administration of wortmannin (n = 5), MG132 (n = 5), or EX527 (n = 5). *P < 0.05 versus Control.
Roles of VEGFR2, PI3K, Calpain, and SIRT1 in Vasodilations to Flow and Agonists
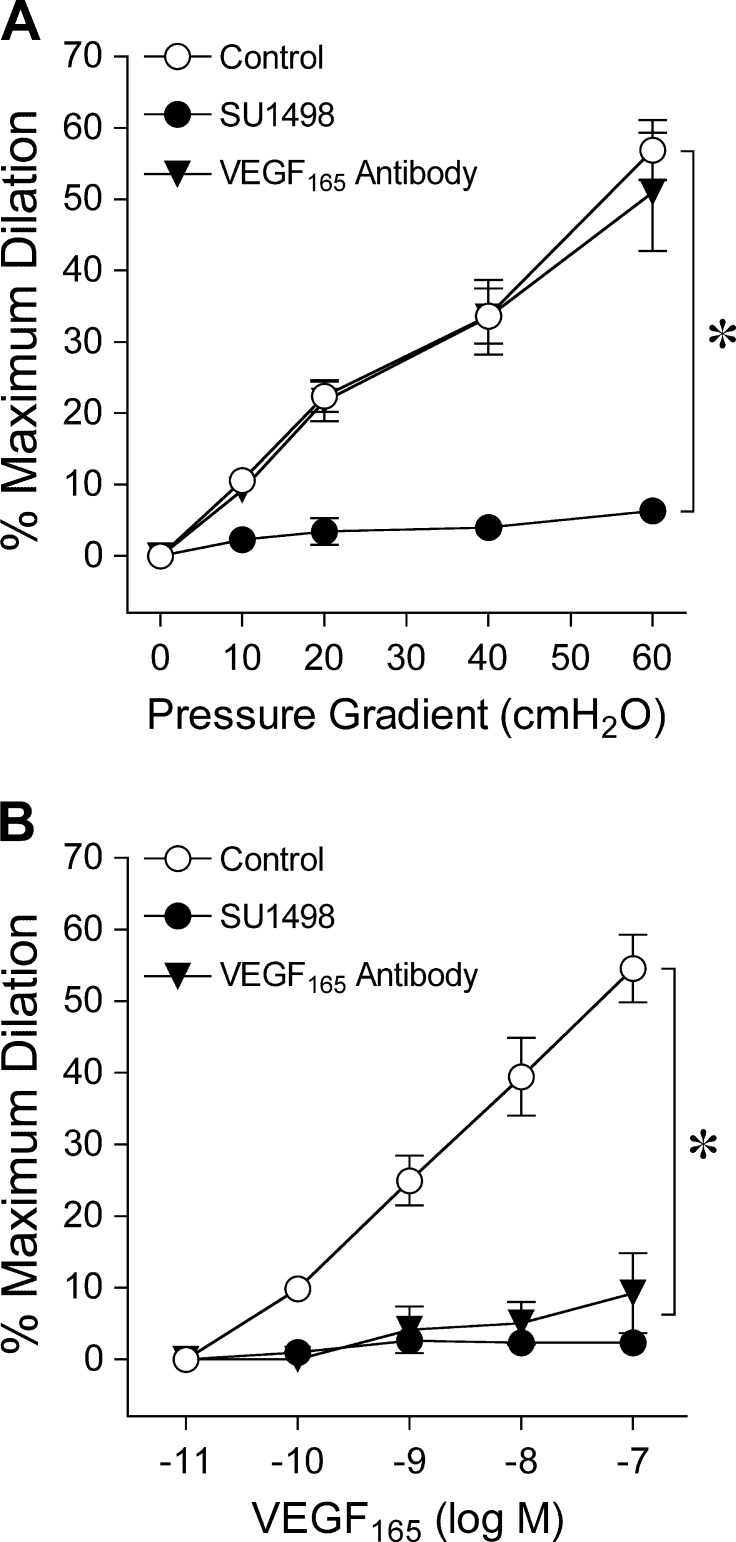
Intraluminal administration of VEGFR2 inhibitor SU1498 abolished vasodilations to flow (Fig. 3A) and VEGF165 (Fig. 3B) but did not affect vasodilation to bradykinin (Fig. 2A) or basal tone (Control, 46 ± 1% versus SU1498, 47 ± 1%, n = 15, P = 0.26). By contrast, luminal exposure to a VEGF165 antibody did not alter flow-induced vasodilation (Fig. 3A) or basal tone (Control, 52 ± 1% versus VEGF165 antibody, 53 ± 1%, n = 10, P = 0.10), whereas vasodilation to VEGF165 was inhibited (Fig. 3B). In the presence of PI3K inhibitor wortmannin, vasodilations to flow (Fig. 4A) and VEGF165 (Fig. 4B) were abolished, while basal tone (Control, 49 ± 1% versus Wortmannin, 50 ± 2%, n = 20, P = 0.32), and vasodilations to bradykinin (Fig. 2A) and resveratrol (Fig. 2B) were maintained. Significant attenuation of vasodilations to bradykinin (Fig. 2A), flow (Fig. 4A), and VEGF165 (Fig. 4B) was observed in the presence of calpain inhibitor MG132, whereas basal tone (Control, 50 ± 2% versus MG132, 51 ± 2%, n = 20, P = 0.73) and vasodilation to resveratrol (Fig. 2B) were unaltered. An additional calpain inhibitor, PD150606, also diminished vasodilator responses to bradykinin (Fig. 2A), flow (Fig. 4A), and VEGF165 (Fig. 4B), without altering basal tone (Control, 50 ± 1% versus PD150606, 50 ± 1%, n = 19, P = 0.91). In another cohort of vessels, the SIRT1 inhibitor EX527 did not influence basal tone (Control, 48 ± 1% versus EX527, 49 ± 1%, n = 24, P = 0.31) but significantly reduced vasodilations to bradykinin (Fig. 2A), resveratrol (Fig. 2B), flow (Fig. 4A), and VEGF165 (Fig. 4B). The vasodilations to the highest flow (i.e., pressure gradient of 60 cm H2O) and concentration of VEGF165 (0.1 μM) in the presence of EX527 were significantly greater than those following wortmannin treatment (ANOVA/Bonferroni multiple-range test). Pharmacological inhibitors wortmannin, EX527, and MG132 had no effect on endothelium-independent vasodilation to NO donor sodium nitroprusside (10 μM; Control, 74 ± 3%; Wortmannin, 70 ± 3%; EX527, 74 ± 7%; MG132, 67 ± 4%), suggesting that the ability of the smooth muscle to relax in response to NO remained intact following these treatments.
Figure 3.
(A) Retinal arterioles were exposed to stepwise increases in pressure gradient; that is, flow, before and after intraluminal administration of SU1498 (n = 6) or VEGF165 antibody (n = 5). (B) Concentration-dependent vasodilation to VEGF165 was examined before and after intraluminal administration of SU1498 (n = 6) or VEGF165 antibody (n = 5). *P < 0.05 versus Control.
Figure 4.
(A) Retinal arterioles were exposed to stepwise increases in pressure gradient; that is, flow, before and after intraluminal administration of wortmannin (n = 5), MG132 (n = 6), PD150606 (n = 5), or EX527 (n = 5). (B) Concentration-dependent vasodilation to VEGF165 was examined before and after intraluminal administration of wortmannin (n = 5), MG132 (n = 4), PD150606 (n = 8), or EX527 (n = 7). *P < 0.05 versus Control; #P < 0.05 EX527 versus Wortmannin.
Protein Expression and Localization of VEGFR2
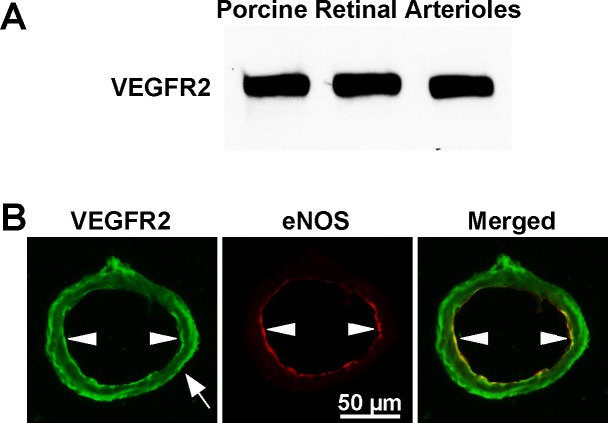
Immunoblot staining revealed expression of VEGFR2 in retinal arterioles (Fig. 5A). For cellular localization of protein, tissue immunofluorescence analysis showed VEGFR2 staining in the endothelial and smooth muscle layers in intact retinal arterioles, with the former showing overlap with eNOS staining in the endothelium (yellow staining in merged image; Fig. 5B).
Figure 5.
(A) Immunoblot detection of VEGFR2 protein expression in retinal arterioles from three pigs. (B) Immunofluorescence detection of VEGFR2 in an isolated porcine retinal arteriole. In the presence of anti-VEGFR2 (green) or anti-eNOS (red) antibodies, high levels of immunostaining were detected in the endothelium for VEGFR2 and eNOS, and in the smooth muscle layer for VEGFR2. The merged image shows overlap staining (orange-yellow) in the endothelial layer. White arrowheads denote endothelial cells and white arrow denotes smooth muscle cells. Data shown are representative of three separate experiments.
Discussion
The present study provides the first direct evidence of endothelium-dependent dilations of retinal arterioles to elevated luminal flow and VEGF165. Our findings also indicated that activation of VEGFR2 in the endothelium of retinal arterioles mediates NO-dependent dilations in response to stimuli via NOS activation. Molecular events downstream from VEGFR2 activation, such as PI3K signaling, calpain protease activity, and SIRT1-dependent deacetylation, appear to be responsible for the NOS activation. The sequential signaling pathway for these vasodilator responses is delineated in Figure 6 and discussed below. These results, which provide new insight into fundamental molecular mechanisms contributing to vasomotor regulation of retinal arterioles by VEGF165 and shear stress, are critical because clinical reports indicate that NO produced from NOS can influence retinal vascular tone and blood flow in humans.43–46
Figure 6.
The proposed signaling pathway involved in VEGF165 and flow/shear stress-induced dilations of retinal arterioles. Evidence from our studies provides support for ligand-dependent and ligand-independent activation of VEGFR2 in the endothelium mediating NO-dependent dilations of retinal arterioles in response to VEGF165 and increased flow/shear stress, respectively. The activation of endothelial VEGFR2 leads to stimulation of PI3K and downstream link to calpain proteases and subsequent SIRT1-dependent deacetylation of NOS for NO production and arteriolar dilation. Bradykinin also can elicit a calpain/SIRT1-dependent vasodilator response, whereas resveratrol activates SIRT1 downstream from calpain leading to NO-mediated vasodilation. Blockade of the proposed signaling pathways by their respective inhibitors is indicated with vertical lines in reference to the direction of the arrows. AB, antibody.
The flow-induced vasodilator response is thought to contribute to local flow regulation by recruiting blood flow to the tissue when metabolic demand is increased (e.g., functional hyperemia) or oxygen supply to the tissue is inadequate (e.g., reactive hyperemia and hypoxia).5 In the clinic, flow-induced dilation of the brachial artery via ultrasound measurement following transient forearm ischemia has been used widely as an index to assess endothelial function47,48 and a diminished response has been reported in type 1 diabetic patients.49–51 Although physiological corroboration of this vascular phenomenon in the retina is lacking, evidence from intact animal studies suggests that hypoxia2 and acute elevation of blood flow secondary to blood pressure elevation6 are likely to elicit flow/shear stress-induced NO-mediated dilation of retinal arterioles. Additional studies have demonstrated that NO is released from cultured bovine retinal endothelial cells subjected to shear stress,52 but the direct functional contribution of the endothelium in flow-induced dilation of retinal microvessels has not been established. To this end, we used an isolated vessel approach in the current study and found that dilation of porcine retinal arterioles to physiological levels of luminal flow32 is abolished by either endothelial disruption or NOS blockade. These interventions also caused similar inhibition of vasodilation to bradykinin, which we have previously established as an endothelium-dependent NO-mediated vasodilator of human and porcine retinal arterioles.10,33 In concert, our current findings support the notion that luminal flow causes endothelium-dependent dilation of retinal arterioles via activation of NOS and consequent NO release.
The retinal vasomotor tone also may be regulated by endogenous chemical substances such as VEGF165. Experimental studies have shown that intravitreal administration of VEGF165 elicits retinal vasodilation in rats,53 rabbits,54 and monkeys.55 In addition, a recent isolated vessel study showed that VEGF (molecular size was not reported) caused dilation of porcine retinal arterioles preconstricted with endothelin-1, although the confounding effect of the preconstrictor on vasomotor function was not assessed.23 At the clinical level, evidence has shown that intravitreal injection of anti-VEGF antibodies (bevacizumab/Avastin or ranibizumab/Lucentis: nonselectively bind and inhibit VEGF isoforms) can significantly reduce retinal arteriolar diameter29,56–59 and retinal blood flow29 in humans with retinal disorders, such as branch retinal vein occlusion56 and neovascular age-related macular degeneration.29,57–59 In colon cancer patients, retinal vasodilation to flickering light, an indication of NOS function in response to retinal metabolic activation,46,60 was significantly reduced by intravenous administration of bevacizumab.61 Conversely, some clinical studies observed that a significant change in retinal arteriolar diameter was not apparent under resting62,63 or flicker-stimulated63 conditions in patients with diabetic macular edema subjected to intravitreal anti-VEGF therapy. Although the reason for these inconsistent clinical findings on anti-VEGF therapy remains unclear, we recently demonstrated a compromised endothelium-dependent, NO-mediated dilation of retinal arterioles in diabetic pigs.64 Because VEGF165 and shear stress exert NO-mediated vasodilation as shown in the present study, the observed absence of a significant vasomotor effect of anti-VEGF therapy in those diabetic patients62,63 is consistent with our findings in diabetic pigs.
In the present study, we found that the retinal arterioles, with basal myogenic tone, were sensitive to VEGF165 with threshold dilation in the high picomolar range. These vasoactive levels are slightly above the mean vitreous VEGF165 concentrations in the range of approximately 5 × 10−13 to 1 × 10−11 M (determined from reported mean values in pg/mL using conversion factor of 40,000 g/mol for VEGF165) obtained from control subjects65–69 in clinical studies of human retinal disease. Although the VEGF165 concentration is expected to be significantly higher at the local retinal vascular wall, its actual level remains to be determined. In fact, the mean vitreous VEGF165 concentrations reported in human retinal pathology (diabetic retinopathy, retinopathy of prematurity, and retinal vein occlusion)65–70 vary from approximately 2 × 10−11 to 2 × 10−9 M and are within the range that elicited retinal arteriolar dilation in the current study. Notably, the 1 × 10−10 to 1 × 10−9 M VEGF165 caused approximately 10% to 20% dilation of retinal arterioles, which would result in a nearly 30% to 110% reduction in resistance to blood flow through an individual vessel in the retinal vascular bed based on Poiseuille's equation (resistance inversely proportional to fourth power of vessel radius).71 This alteration of tone at the level of arterioles < 60 μm in diameter in the retinal microcirculation is likely to contribute significantly to blood flow change, because a marked pressure drop (i.e., 50%) across these vessels was observed in the feline retina.72 In regards to cell signaling, the vasodilator response of retinal arterioles was abolished by endothelial denudation and NOS blockade, supporting the essential role of endothelial NOS in mediating VEGF165-induced vasodilation.
At the level of the resistance vasculature, previous pharmacological evidence suggests that VEGF165 causes dilation of rat coronary arterioles via activation of VEGFR2.22 Similarly, selective blockade of tyrosine phosphorylation and activity of VEGFR2 with SU149834 inhibited dilation of retinal arterioles to VEGF165 in the present study. Interestingly, administration of a VEGFR2 antibody in normal mice caused a significant increase in systemic blood pressure,73 suggesting a tonic regulation of microvascular resistance by endogenous VEGF and VEGFR2 under resting conditions. It is worth noting that the influence of VEGFR2 signaling on vascular tone may extend beyond ligand activation, because VEGFR2 blockade reduces flow-induced dilation of hamster cheek pouch arterioles in vivo27 and of rat coronary arterioles in vitro.22 A comparable mechanism appears to be prevalent in the retinal microcirculation, because VEGFR2 blockade with SU1498 abolished flow-induced dilation of retinal arterioles in the present study. The ability of SU1498 to inhibit dilation of retinal arterioles to VEGF165 without altering dilation to bradykinin receptor activation supports the specific action of this antagonist on VEGFR2. Additional data showing that treatment of retinal arterioles with a VEGF165 antibody inhibited vasodilation to VEGF165 but did not alter flow-induced vasodilation provide inferential evidence that the flow-stimulated VEGFR2 activation is ligand-independent. Evidence for prominent endothelial VEGFR2 protein expression in retinal arterioles was provided by immunohistochemical analysis. Although VEGFR2 expression also was detected in the smooth muscle layer, the lack of vascular reactivity to VEGF165 and shear stress in the absence of endothelium implies its nonvasomotor function, possibly involvement in migration,74 for this cellular receptor. Collectively, our functional and molecular data demonstrated that endothelial VEGFR2 activation mediates ligand-dependent dilation to VEGF165 and ligand-independent dilation to luminal flow in retinal arterioles.
The underlying mechanisms for flow-sensitive vasomotor activity in relation to VEGFR2 signaling in retinal arterioles are largely unknown. A reasonable target to consider was calpains, which are a family of cytoplasmic calcium-dependent proteases that can translocate to the cell membrane upon activation by shear stress75 or VEGF165.76 Our present data showed that blockade of calpains attenuates flow- and VEGF165-induced vasodilations, which provides the first functional evidence for a role of these proteases in vasomotor regulation. Moreover, it appears that the calpain-dependent pathway is triggered in response to other endothelium-dependent agonists, because vasodilation to bradykinin also was diminished by calpain blockade. The two major calpain isoforms are calpain 1 (μ-calpain) and calpain 2 (M-calpain),77 which are blocked by the nonselective calpain inhibitors, MG132 and PD150606, used in the present study. Previous studies in cultured human retinal microvascular endothelial cells reported that VEGF165 treatment caused a greater increase in calpain 2 than calpain 1 activity.78 The contribution of a specific calpain isoform linked to endothelial NO-mediated dilation in retinal arterioles merits future research.
Another signaling molecule that was found to be involved in the vasomotor regulation in the present study was PI3K, which has been suggested to be phosphorylated and activated by VEGFR2 following shear stress26 and VEGF79 stimulation in cultured endothelial cells. We found that PI3K inhibitor wortmannin abolishes dilations of retinal arterioles to VEGF165 and flow, suggesting that these responses are dependent on PI3K signaling. These findings are consistent with previous studies showing that wortmannin blocks NO production in endothelial cells stimulated with VEGF16576 or flow/shear stress.27 However, its relationship with calpain remains unclear. In the current study, the inability of PI3K blockade to inhibit calpain-dependent dilation of retinal arterioles to bradykinin (Fig. 2) suggests that calpain activation is downstream from PI3K in response to VEGF165 and flow (Fig. 6). This idea is supported by earlier evidence showing that shear stress elicited PI3K-dependent activation of calpain 2 in human umbilical vein endothelial cells,80 although its signaling to NOS activation was not studied.
The activity of eNOS can be regulated in part at the post-translational level by acetylation of the enzyme. Accumulating evidence indicates that SIRT1-dependent deacetylation of lysine residues in the calmodulin-binding domain of eNOS leads to increased enzyme activity.41,81 The SIRT1 enzyme is a member of the sirtuin family of nicotinamide adenine dinucleotide-dependent deacetylases, which are involved in regulation of metabolism, stress responses, and senescence.82 In vitro studies have shown that blockade of SIRT1 diminishes endothelium-dependent relaxation of vasculatures.41,83 In addition, exposure of cultured human umbilical vein endothelial cells to laminar shear stress has been shown to promote SIRT1-dependent eNOS deacetylation.81 The present findings underpin the notion that SIRT1 contributes to the transduction of a hemodynamic signal to NO-mediated vasodilation, because selective SIRT1 inhibitor EX52735,84 reduced retinal arteriolar dilation to increased flow. Support for the inhibitory action of EX527 on SIRT1 linked to eNOS activation was provided by the ability of the drug to attenuate vasodilation to resveratrol, a polyphenolic activator of SIRT141,85 that we have previously shown causes endothelium-dependent NO-mediated dilation of porcine retinal arterioles.40 It appears that SIRT1 deacetylation of eNOS may be a general mechanism contributing to regulation of NO production in retinal arterioles, because vasodilations to NO-mediated agonists bradykinin and VEGF165 also were diminished. In addition, the arteriolar dilation to SIRT1 agonist resveratrol remained during calpain blockade, suggesting that SIRT1 activation occurs downstream from calpain in response to VEGFR2-dependent stimuli (Fig. 6). The inability of EX527 to completely prevent vasodilation to the NO-mediated stimuli may be due to a lower than maximally effective concentration of the drug.35 It is worth noting that we were limited to 5 μM EX527 because higher concentrations of this drug contained high levels of solvent DMSO that caused nonspecific inhibition on vasodilator function of retinal arterioles. Nonetheless, our data supported a significant role for SIRT1 in NO-mediated dilation of retinal arterioles in response to chemical and mechanical stimuli, and contributed to the emerging evidence on the protective roles of SIRT1 in ocular disease development.86
In summary, we found that VEGFR2 activation in the endothelium of porcine retinal arterioles leads to NO-mediated dilations in response to VEGF165 and shear stress stimulations. The underlying molecular events downstream from VEGFR2 activation linking to NOS stimulation appear to include a PI3K/calpain/SIRT1 cascade. Prudent evaluation of this signaling pathway in retinal arterioles under pathological conditions is warranted because aberrant endothelial44,64 and arteriolar3,28 functions may contribute to pathogenesis of retinal ischemic diseases.
Acknowledgments
Supported by National Institutes of Health (NIH) National Eye Institute (NEI; Bethesda, MD, USA) Grants R01EY018420 (TWH), R01EY023335 (TWH), and K08EY016143 (RHR), the Retina Research Foundation (LK), the Scott & White Research Foundation Ophthalmic Vascular Research Program (LK), and the Kruse Chair Endowment Fund (LK).
Disclosure: T.W. Hein, None; R.H. Rosa Jr, None; Y. Ren, None; W. Xu, None; L. Kuo, None
References
- 1. Kaya S,, Kolodjaschna J,, Berisha F,, Schmetterer L,, Garhofer G. Comparison of the autoregulatory mechanisms between central retinal artery and posterior ciliary arteries after thigh cuff deflation in healthy subjects. Microvasc Res. 2011. ; 82: 269–273. [DOI] [PubMed] [Google Scholar]
- 2. Nagaoka T,, Sakamoto T,, Mori F,, Sato E,, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest Ophthalmol Vis Sci. 2002. ; 43: 3037–3044. [PubMed] [Google Scholar]
- 3. Pournaras CJ,, Rungger-Brandle E,, Riva CE,, Hardarson SH,, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008. ; 27: 284–330. [DOI] [PubMed] [Google Scholar]
- 4. Davies PF,, Spaan JA,, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005. ; 33: 1714–1718. [DOI] [PubMed] [Google Scholar]
- 5. Davis MJ,, Hill M,, Kuo L. Local regulation of blood flow. : Tuma RF,, Duran WN,, Ley K, Handbook of Physiology; Section 2: The Cardiovascular System; Microcirculation. 2nd ed. Bethesda, MD: The American Physiological Society and Elsevier; 2008: 159–284. [Google Scholar]
- 6. Nakabayashi S,, Nagaoka T,, Tani T,, et al. Retinal arteriolar responses to acute severe elevation in systemic blood pressure in cats: role of endothelium-derived factors. Exp Eye Res. 2012; 103: 63–70. [DOI] [PubMed] [Google Scholar]
- 7. Kuo L,, Davis MJ,, Chilian WM. Endothelium-dependent flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990. ; 259: H1063–H1070. [DOI] [PubMed] [Google Scholar]
- 8. Smiesko V,, Lang DJ,, Johnson PC. Dilator response of rat mesenteric arcading arterioles to increased blood flow velocity. Am J Physiol. 1989. ; 257: H1958–H1965. [DOI] [PubMed] [Google Scholar]
- 9. Koller A,, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994. ; 74: 416–421. [DOI] [PubMed] [Google Scholar]
- 10. Hein TW,, Rosa RH,, Jr,, Yuan Z,, Roberts E,, Kuo L. Divergent roles of nitric oxide and Rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci. 2010; 51: 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan Y,, He T,, Shen Y,, et al. Modeling of diseases of retinal ischemia in vitro: possible participation of autocrine vascular endothelial growth factor signaling. Ophthalmic Res. 2013. ; 49: 90–99. [DOI] [PubMed] [Google Scholar]
- 12. Aiello LP,, Northrup JM,, Keyt BA,, Takagi H,, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995. ; 113: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 13. Shima DT,, Adamis AP,, Ferrara N,, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995. ; 1: 182–193. [PMC free article] [PubMed] [Google Scholar]
- 14. Vinores SA,, Youssri AI,, Luna JD,, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997. ; 12: 99–109. [PubMed] [Google Scholar]
- 15. Takahashi H,, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 2005. ; 109: 227–241. [DOI] [PubMed] [Google Scholar]
- 16. Scheppke L,, Aguilar E,, Gariano RF,, et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J Clin Invest. 2008. ; 118: 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wedge SR,, Ogilvie DJ,, Dukes M,, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002. ; 62: 4645–4655. [PubMed] [Google Scholar]
- 18. Horowitz JR,, Rivard A,, van der Zee R,, et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997. ; 17: 2793–2800. [DOI] [PubMed] [Google Scholar]
- 19. Li B,, Ogasawara AK,, Yang R,, et al. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension. 2002. ; 39: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 20. Laham RJ,, Li J,, Tofukuji M,, Post M,, Simons M,, Sellke FW. Spatial heterogeneity in VEGF-induced vasodilation: VEGF dilates microvessels but not epicardial and systemic arteries and veins. Ann Vasc Surg. 2003. ; 17: 245–252. [DOI] [PubMed] [Google Scholar]
- 21. Fogarty JA,, Muller-Delp JM,, Delp MD,, Mattox ML,, Laughlin MH,, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation. 2004. ; 109: 664–670. [DOI] [PubMed] [Google Scholar]
- 22. LeBlanc AJ,, Shipley RD,, Kang LS,, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008. ; 295: H2280–H2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su EN,, Cringle SJ,, McAllister IL,, Yu DY. An experimental study of VEGF induced changes in vasoactivity in pig retinal arterioles and the influence of an anti-VEGF agent. BMC Ophthalmol. 2012. ; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen KD,, Li YS,, Kim M,, et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999. ; 274: 18393–18400. [DOI] [PubMed] [Google Scholar]
- 25. Shay-Salit A,, Shushy M,, Wolfovitz E,, et al. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A. 2002. ; 99: 9462–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tzima E,, Irani-Tehrani M,, Kiosses WB,, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005. ; 437: 426–431. [DOI] [PubMed] [Google Scholar]
- 27. Jin ZG,, Ueba H,, Tanimoto T,, Lungu AO,, Frame MD,, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003. ; 93: 354–363. [DOI] [PubMed] [Google Scholar]
- 28. Gardiner TA,, Archer DB,, Curtis TM,, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007. ; 14: 25–38. [DOI] [PubMed] [Google Scholar]
- 29. Micieli JA,, Tsui E,, Lam WC,, Brent MH,, Devenyi RG,, Hudson C. Retinal blood flow in response to an intravitreal injection of ranibizumab for neovascular age-related macular degeneration. Acta Ophthalmol. 2012; 90: e13–e20. [DOI] [PubMed] [Google Scholar]
- 30. Burgansky-Eliash Z,, Barash H,, Nelson D,, et al. Retinal blood flow velocity in patients with age-related macular degeneration. Curr Eye Res. 2014. ; 39: 304–311. [DOI] [PubMed] [Google Scholar]
- 31. Hein TW,, Yuan Z,, Rosa RH,, Jr,, Kuo L. Requisite roles of A2A receptors nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci. 2005; 46: 2113–2119. [DOI] [PubMed] [Google Scholar]
- 32. Riva CE,, Grunwald JE,, Sinclair SH,, Petrig BL. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985. ; 26: 1124–1132. [PubMed] [Google Scholar]
- 33. Potts LB,, Bradley PD,, Xu W,, Kuo L,, Hein TW. Role of endothelium in vasomotor responses to endothelin system and protein kinase C activation in porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2013. ; 54: 7587–7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strawn LM,, McMahon G,, App H,, et al. Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996. ; 56: 3540–3545. [PubMed] [Google Scholar]
- 35. Gertz M,, Fischer F,, Nguyen GT,, et al. Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proc Natl Acad Sci U S A. 2013. ; 110: E2772–E2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khoutorsky A,, Spira ME. Activity-dependent calpain activation plays a critical role in synaptic facilitation and post-tetanic potentiation. Learn Mem. 2009. ; 16: 129–141. [DOI] [PubMed] [Google Scholar]
- 37. Wang KK,, Nath R,, Posner A,, et al. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci U S A. 1996. ; 93: 6687–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Autheman D,, Wyder M,, Popoff M,, D'Herde K,, Christen S,, Posthaus H. Clostridium perfringens beta-toxin induces necrostatin-inhibitable calpain-dependent necrosis in primary porcine endothelial cells. PLoS One. 2013. ; 8: e64644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kemeny SF,, Figueroa DS,, Clyne AM. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One. 2013. ; 8: e66176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagaoka T,, Hein TW,, Yoshida A,, Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci. 2007. ; 48: 4232–4239. [DOI] [PubMed] [Google Scholar]
- 41. Mattagajasingh I,, Kim CS,, Naqvi A,, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Nat Acad Sci U S A. 2007. ; 104: 14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hein TW,, Ren Y,, Yuan Z,, et al. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci. 2009. ; 50: 3329–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michelson G,, Warntges S,, Harazny J,, Oehmer S,, Delles C,, Schmieder RE. Effect of NOS inhibition on retinal arterial and capillary circulation in early arterial hypertension. Retina. 2006. ; 26: 437–444. [DOI] [PubMed] [Google Scholar]
- 44. Delles C,, Michelson G,, Harazny J,, Oehmer S,, Hilgers KF,, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004. ; 35: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 45. Polak K,, Dorner G,, Kiss B,, et al. Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol. 2000. ; 84: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dorner GT,, Garhofer G,, Kiss B,, et al. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003. ; 285: H631–H636. [DOI] [PubMed] [Google Scholar]
- 47. Celermajer DS,, Sorensen KE,, Gooch VM,, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992. ; 340: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 48. Meredith IT,, Currie KE,, Anderson TJ,, Roddy MA,, Ganz P,, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996. ; 270: H1435–H1440. [DOI] [PubMed] [Google Scholar]
- 49. Ce GV,, Rohde LE,, da Silva AM,, Punales MK,, de Castro AC,, Bertoluci MC. Endothelial dysfunction is related to poor glycemic control in adolescents with type 1 diabetes under 5 years of disease: evidence of metabolic memory. J Clin Endocrinol Metab. 2011. ; 96: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 50. Ceriello A,, Esposito K,, Ihnat M,, Thorpe J,, Giugliano D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J Clin Endocrinol Metab. 2009. ; 94: 2751–2756. [DOI] [PubMed] [Google Scholar]
- 51. Pemp B,, Weigert G,, Karl K,, et al. Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes Care. 2009. ; 32: 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lakshminarayanan S,, Gardner TW,, Tarbell JM. Effect of shear stress on the hydraulic conductivity of cultured bovine retinal microvascular endothelial cell monolayers. Curr Eye Res. 2000. ; 21: 944–951. [DOI] [PubMed] [Google Scholar]
- 53. Clermont AC,, Aiello LP,, Mori F,, Aiello LM,, Bursell SE. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997. ; 124: 433–446. [DOI] [PubMed] [Google Scholar]
- 54. Edelman JL,, Lutz D,, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005. ; 80: 249–258. [DOI] [PubMed] [Google Scholar]
- 55. Tolentino MJ,, Miller JW,, Gragoudas ES,, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996. ; 103: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 56. Sacu S,, Pemp B,, Weigert G,, et al. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2011. ; 52: 3046–3050. [DOI] [PubMed] [Google Scholar]
- 57. Fontaine O,, Olivier S,, Descovich D,, Cordahi G,, Vaucher E,, Lesk MR. The effect of intravitreal injection of bevacizumab on retinal circulation in patients with neovascular macular degeneration. Invest Ophthalmol Vis Sci. 2011. ; 52: 7400–7405. [DOI] [PubMed] [Google Scholar]
- 58. Papadopoulou DN,, Mendrinos E,, Mangioris G,, Donati G,, Pournaras CJ. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology. 2009. ; 116: 1755–1761. [DOI] [PubMed] [Google Scholar]
- 59. Mendrinos E,, Mangioris G,, Papadopoulou DN,, Donati G,, Pournaras CJ. Long-term results of the effect of intravitreal ranibizumab on the retinal arteriolar diameter in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2013. ; 91: e184–e190. [DOI] [PubMed] [Google Scholar]
- 60. Garhofer G,, Zawinka C,, Resch H,, Huemer KH,, Dorner GT,, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vision Res. 2004. ; 44: 833–838. [DOI] [PubMed] [Google Scholar]
- 61. Reimann M,, Folprecht G,, Haase R,, et al. Anti-vascular endothelial growth factor therapy impairs endothelial function of retinal microcirculation in colon cancer patients – an observational study. Exp Transl Stroke Med. 2013; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soliman W,, Vinten M,, Sander B,, et al. Optical coherence tomography and vessel diameter changes after intravitreal bevacizumab in diabetic macular oedema. Acta Ophthalmol. 2008. ; 86: 365–371. [DOI] [PubMed] [Google Scholar]
- 63. Terai N,, Haustein M,, Siegel A,, Stodtmeister R,, Pillunat LE,, Sandner D. Diameter of retinal vessels in patients with diabetic macular edema is not altered by intravitreal ranibizumab (lucentis). Retina. 2014. ; 34: 1466–1472. [DOI] [PubMed] [Google Scholar]
- 64. Hein TW,, Potts LB,, Xu W,, Yuen JZ,, Kuo L. Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes. Invest Ophthalmol Vis Sci. 2012. ; 53: 7943–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aiello LP,, Avery RL,, Arrigg PG,, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. ; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 66. Funatsu H,, Yamashita H,, Ikeda T,, et al. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002. ; 133: 537–543. [DOI] [PubMed] [Google Scholar]
- 67. Sonmez K,, Drenser KA,, Capone A,, Jr, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008; 115: 1065–1070. [DOI] [PubMed] [Google Scholar]
- 68. Ehlken C,, Rennel ES,, Michels D,, et al. Levels of VEGF but not VEGF(165b) are increased in the vitreous of patients with retinal vein occlusion. Am J Ophthalmol. 2011. ; 152: 298–303. [DOI] [PubMed] [Google Scholar]
- 69. Malecaze F,, Clamens S,, Simorre-Pinatel V,, et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994. ; 112: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 70. Wells JA,, Murthy R,, Chibber R,, et al. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996. ; 80: 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Feke GT,, Tagawa H,, Deupree DM,, Goger DG,, Sebag J,, Weiter JJ. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989. ; 30: 58–65. [PubMed] [Google Scholar]
- 72. Glucksberg MR,, Dunn R. Direct measurement of retinal microvascular pressure in the live, anesthetized cat. Microvasc Res. 1993. ; 45: 158–165. [DOI] [PubMed] [Google Scholar]
- 73. Facemire CS,, Nixon AB,, Griffiths R,, Hurwitz H,, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009. ; 54: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grosskreutz CL,, Anand-Apte B,, Duplaa C,, et al. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999. ; 58: 128–136. [DOI] [PubMed] [Google Scholar]
- 75. Kang H,, Kwak HI,, Kaunas R,, Bayless KJ. Fluid shear stress and sphingosine 1-phosphate activate calpain to promote membrane type 1 matrix metalloproteinase (MT1-MMP) membrane translocation and endothelial invasion into three-dimensional collagen matrices. J Biol Chem. 2011. ; 286: 42017–42026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Youn JY,, Wang T,, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ Res. 2009. ; 104: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goll DE,, Thompson VF,, Li H,, Wei W,, Cong J. The calpain system. Physiol Rev. 2003. ; 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 78. Ma H,, Tochigi A,, Shearer TR,, Azuma M. Calpain inhibitor SNJ-1945 attenuates events prior to angiogenesis in cultured human retinal endothelial cells. J Ocul Pharmacol Ther. 2009. ; 25: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dayanir V,, Meyer RD,, Lashkari K,, Rahimi N. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001. ; 276: 17686–17692. [DOI] [PubMed] [Google Scholar]
- 80. Miyazaki T,, Honda K,, Ohata H. Requirement of Ca2+ influx- and phosphatidylinositol 3-kinase-mediated m-calpain activity for shear stress-induced endothelial cell polarity. Am J Physiol Cell Physiol. 2007. ; 293: C1216–C1225. [DOI] [PubMed] [Google Scholar]
- 81. Chen Z,, Peng IC,, Cui X,, Li YS,, Chien S,, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Nat Acad Sci U S A. 2010. ; 107: 10268–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Finkel T,, Deng CX,, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009. ; 460: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tajbakhsh N,, Sokoya EM. Regulation of cerebral vascular function by sirtuin 1. Microcirculation. 2012. ; 19: 336–342. [DOI] [PubMed] [Google Scholar]
- 84. Solomon JM,, Pasupuleti R,, Xu L,, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006. ; 26: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lakshminarasimhan M,, Curth U,, Moniot S,, Mosalaganti S,, Raunser S,, Steegborn C. Molecular architecture of the human protein deacetylase Sirt1 and its regulation by AROS and resveratrol. Biosci Rep. 2013. ; 33: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mimura T,, Kaji Y,, Noma H,, Funatsu H,, Okamoto S. The role of SIRT1 in ocular aging. Exp Eye Res. 2013. ; 116: 17–26. [DOI] [PubMed] [Google Scholar]