Abstract
Background
The effects of gestational supplementation with fish oil on risks for gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH), and pre-eclampsia (PE) have not been confirmed. In this study, a meta-analysis was performed to evaluate the effect of fish oil supplementation on these gestational complications.
Material/Methods
Randomized controlled human trials that investigated the effects of fish oil supplementation in pregnant women were identified by a systematic search of Medline, Embase, and Cochrane’s Library, and references of related reviews and studies up to December 2014. Relative risks (RRs) for GDM, PIH, and PE were the outcomes of interest. Fixed-effects or random-effects models were applied according to the heterogeneity.
Results
Thirteen comparisons from 11 published articles, including more than 5000 participants, were included. The results showed that fish oil supplementation was not associated with reduced risks for GDM (RR=1.06, 95% confidence interval [CI]: 0.85–1.32, p=0.60), PIH (RR=1.03, 95% CI: 0.89–1.20, p=0.66), or PE (RR=0.93, 95% CI: 0.74–1.16, p=0.51). No statistically significant heterogeneity was detected for the comparison of each outcome. The effects of fish oil on these gestational complications were consistent between women with low-risk and high-risk pregnancies.
Conclusions
Gestational supplementation with fish oil during the second or third trimester of pregnancy is not associated with reduced risks for GDM, PIH, or PE. Other possible benefits of fish oil supplementation during pregnancy warrant further evaluation.
MeSH Keywords: Diabetes, Gestational; Fish Oils; Pre-Eclampsia
Background
Gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH), and pre-eclampsia (PE) have been recognized as the most common complications during pregnancy and seriously affect the health of both mothers and infants [1,2]. Although the prevalence of GDM and PE varies between different populations according to various diagnostic criteria, it has been noted that the incidences of these gestational complication are increasing worldwide [3,4]. Previous studies have revealed that maternal presence of GDM or PE increases the risk of perinatal adverse outcomes and that these complications can also lead to long-term health problems for mothers and children. Women with a history of GDM have been found to have higher risk for the development of type 2 diabetes mellitus and, more importantly, these women have been shown to be more vulnerable to future vascular lesions [5,6]. As for the children from pregnancies complicated by GDM, recent studies suggested that these children are at higher risk for the development of obesity and metabolic syndrome in their lifetime [7,8]. Similarly, the presence of PE, another serious complication in pregnancy characterized by new-onset hypertension and proteinuria, has also been related to an increased risk for cardiovascular diseases for the mothers [9]. Therefore, there is an urgent need to prevent the incidences of both the diabetic and hypertensive complications during pregnancy [10].
It has been suggested that gestational supplementation with fish oil, which mainly consists of 2 categories of marine omega 3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), may have pluripotential benefits for mothers and children [11,12], including elongation of gestational time and thus a lower risk of preterm delivery [13] and improvements in growth measurements of infants [14], as well as cognitive function in children [15]. Early epidemiologic studies suggested that the dietary composition of n-3 PUFAs during pregnancy may reduce the risk for GDM [16,17]. However, further randomized controlled trials (RCTs) did not support that gestational supplementation with fish oil can reduce the risks for GDM, PIH, or PE. Some of the RCTs included few pregnant women and lacked statistical power, which may have confounded the negative results; therefore, in this study we performed a meta-analysis of relevant RCTs to evaluate the effects of fish oil supplementation on the risks of gestational diabetic and hypertensive complications.
Material and Methods
We performed this meta-analysis according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [18] and the Cochrane Handbook guidelines [19].
Database searching
PubMed, Embase, and the Cochrane Library (Cochrane Center Register of Controlled Trials) were searched systematically for related studies, using the terms “omega-3 fatty acids”, “fish oil”, “fish-oil”, “polyunsaturated fatty acids”, “marine oil”, “eicosapentaenoic acid”, “docosahexaenoic acid”, “DHA”, “EPA” paired with “pregnant”, “maternal”, “prenatal”, “pregnancy”, or “gestation”, with the limitation of studies in humans. The final search was performed on December 25, 2014. We also manually searched references of the original and review articles for possible related studies.
Study selection
Studies were included if they met all of the following criteria: 1) published as a full-length article in English only; 2) reported as an RCT; 3) recruited pregnant women who were assigned to either an oral fish oil treatment group or a control group (no treatment or placebo), or they had a concomitant intervention for which the effects of fish oil could be separated; 4) fish oil was administered orally for at least 2 weeks; and 5) reported the incidence of at least 1 of the following events: GDM, PIH, or PE, or the incidence of the events could be calculated. The definitions and diagnostic criteria of GDM, PIH, and PE were in accordance with the original studies [20].
Data extraction and quality assessment
Two of the authors (Bing Chen and Xinran Ji) independently performed the literature search, data extraction, and quality assessment according to the inclusion criteria. Discrepancies were resolved by consensus. Data regarding study design characteristics (parallel or crossover, blind, or open-label), locations of the studies, characteristics of the included pregnant women, numbers of the participants, components of the fish oil (doses of DHA and EPA) and controls, and of the gestational time (in weeks) of supplementation initiation were extracted. The corresponding authors of the original articles were contacted for unreported data. We used the 7 domains of the Cochrane Risk Of Bias tool to evaluate the quality of the included studies, which include criteria concerning aspects of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other potential threats to validity [19].
Statistical analyses
Dichotomous data were analyzed using risk ratios (RRs) with 95% confidence intervals (CIs). Heterogeneity among the included studies was tested using Cochrane’s Q test, and significant heterogeneity was considered if the p value was <0.10. The I2 statistic, indicating the percentage of total variation across studies that is due to heterogeneity rather than chance [21], was also determined, and a value of I2 >50% suggested significant heterogeneity [22]. Fixed-effects models were applied if no significant heterogeneity was detected by Cochrane’s Q test, whereas random-effects models were used if there were significant heterogeneity across studies. We defined women from the general population to have low-risk pregnancies, while women with a history of preterm delivery, PIH, or intrauterine growth retardation and women with current double gestation were considered to have high-risk pregnancies as defined by the original studies. Subgroup analyses were performed to evaluate the impact of pregnancy risk on the outcomes. Additionally, publication bias was detected with Egger regression test [23] and visual inspection of the symmetry of the funnel plots. Statistical significance was considered at p<0.05. We used RevMan software (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (Version 12.0; Stata Corporation, College Station, TX) for the process of meta-analysis and statistical analysis.
Results
Search results
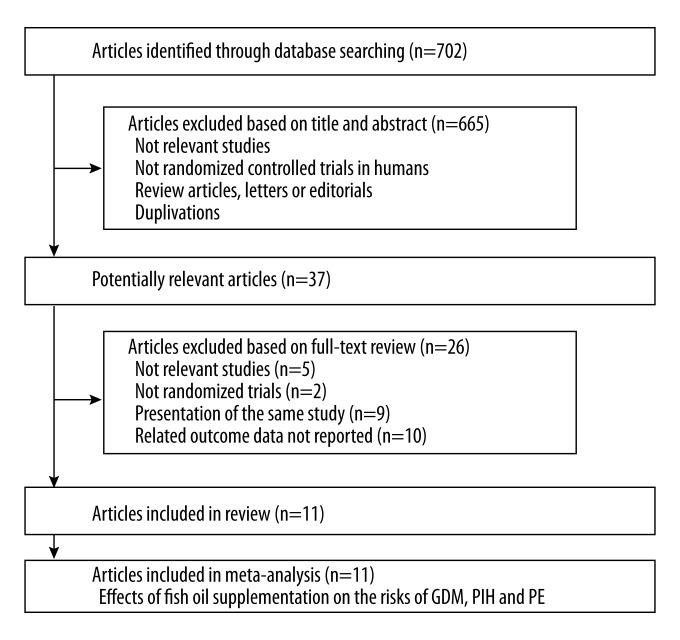
The literature screening process is outlined in Figure 1. Briefly, a total of 702 published articles were obtained in the initial database search, and 665 were excluded, mainly because they were not relevant to the objective of the study. Of the 37 potentially relevant articles, 11 articles [24–34] met the inclusion criteria for the current meta-analysis. Twenty-six articles were excluded because 5 of them were not relevant studies, 2 were not RCTs, 9 were duplicated publications, and 10 did not report related outcome data.
Figure 1.
Flow diagram of the study selection procedure.
Study characteristics
The general characteristics of the included studies are listed in Table 1. One article [27] reported 4 prophylactic trials and 2 therapeutic trials, of which 2 trials reported data of PIH (Olsen 2000-Earl PIH and Olsen 2000-twins) and 2 trials reported data of PE (Olsen 2000-PE and Olsen 2000-twins). These trials were included separately. Another trial [33] included 2 study arms in which the pregnant women were supplied mainly with DHA (Mozurkewich 2013-DHA) or EPA (Mozurkewich 2013-EPA), and these 2 study arms were compared to the controls separately as 2 independent studies. The sample size of the control group was therefore equally distributed to the 2 study arms to overcome a unit of analysis error as recommended by the Cochrane’s Handbook [19]. Therefore, 13 comparisons from 11 published articles were included in the current meta-analysis, all of which were performed in Western countries. Four articles included women who were considered to have high-risk pregnancies [25–27,31], while the other 7 articles included women with low-risk pregnancies who were generally healthy [24,28–30,32–34]. The dose of fish oil ranged from 200 to 4950 mg/d, with that for DHA from 0 to 2070 mg/d and that for EPA from 0 to 3000 mg/d. Supplementation with fish oil was initiated from the 14th to the 33rd weeks of gestation. No severe adverse events that were thought to be related to fish oil supplementation were reported by any included RCTs.
Table 1.
Characteristics of included studies.
| Author year | Study design | Country | Participants | Number of participants | FO dose (mg/d) | DHA dose (mg/d) | EPA dose (mg/d) | Control | Initiation week (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Olsen 1992 | R, SB | Denmark | Single pregnancy women | 397 | 2200 | 920 | 1280 | Olive oil or no treatment | 30 |
| Bulstra-Ramakers 1995 | R, DB, PC | Netherlands | Pregnant women with history of IUGR | 63 | 3000 | 0 | 3000 | Coconut oil | 14 |
| Onwude 1995 | R, DB, PC | UK | High-risk women for PIH and IUGR | 232 | 2700 | 1080 | 1620 | Air-filled capsule | 27 |
| Olsen 2000-prophylaxis | R, DB, PC | Multicenter in Europe | Pregnant women with history of IUGR, PD, PIH and double pregnancy | 1477 | 2200 | 920 | 1280 | Olive oil | 20 |
| Olsen 2000-prevention | R, DB, PC | Multicenter in Europe | High-risk women for PE and IUGR | 142 | 4950 | 2070 | 2880 | Olive oil | 33 |
| Smuts 2003a | R, DB, PC | USA | Healthy pregnant women | 37 | 200 | 200 | 0 | Ordinary eggs | 26 |
| Smuts 2003b | R, DB, PC | USA | Healthy pregnant women | 291 | 200 | 200 | 0 | Ordinary eggs | 26 |
| Goor 2010 | R, DB, PC | Netherlands | Healthy pregnant women | 78 | 250 | 220 | 30 | Soybean oil | 17 |
| Harper 2010 | R, DB, PC | USA | Pregnant women with history of PD | 852 | 2000 | 800 | 1200 | Inert mineral oil | 22 |
| Zhou 2012 | R, DB, PC | Australia | Healthy pregnant women | 2399 | 900 | 800 | 100 | Vegetable oil | 21 |
| Carlson 2013 | R, DB, PC | USA | Healthy pregnant women | 301 | 600 | 600 | 0 | Soybean and corn oil | 20 |
| Mozurkewich 2013-DHA | R, DB, PC | USA | Pregnant women at risk for depression | 79 | 1080 | 900 | 180 | Soy oil | 18 |
| Mozurkewich 2013-EPA | R, DB, PC | USA | Pregnant women at risk for depression | 80 | 1334 | 274 | 1060 | Soy oil | 18 |
R – randomized; SB – single blind; DB – double-blind; PC – placebo-controlled; IUGR – intrauterine growth retardation; PD – preterm delivery; PIH – pregnancy-induced hypertension; PE – pre-eclampsia; FO – fish oil; EPA – eicosapentaenoic acid; DHA – ducosahexaenoic acid.
Quality assessment
The results of risks of biases of the included studies as evaluated by the Cochrane assessment tool are shown in Table 2. Ten of the included RCTs were double-blinded placebo-controlled studies [25–34], while the other study was single-blinded [24]. Six studies reported methods of random sequence generation [26,27,31–34], and 7 studies reported details of allocation concealment [24–27,31–33]. Details of withdrawals and dropouts were reported in all studies.
Table 2.
Cochrane risk of bias assessment.
| Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other potential threats | |
|---|---|---|---|---|---|---|---|
| Olsen 1992 | Unclear | Yes | No | Unclear | Yes | Unclear | Unclear |
| Bulstra-Ramakers 1995 | Unclear | Yes | Yes | Unclear | Yes | Unclear | Unclear |
| Onwude 1995 | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear |
| Olsen 2000-prophylaxis | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear |
| Olsen 2000-prevention | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear |
| Smuts 2003a | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Smuts 2003b | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Goor 2010 | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Harper 2010 | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear |
| Zhou 2012 | Yes | Yes | Yes | Unclear | Yes | Unclear | Unclear |
| Carlson 2013 | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Mozurkewich 2013-DHA | Yes | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Mozurkewich 2013-EPA | Yes | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
Yes – low risk of bias; Unclear – uncertain risk of bias; No – high risk of bias.
Effects of fish oil supplementation on incidence of GDM
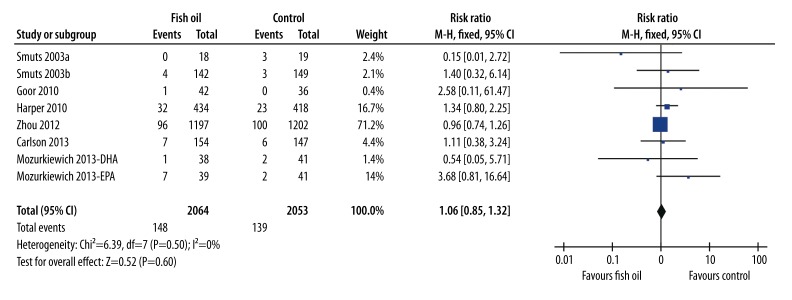
Eight comparisons [28–34] (including 2064 women in the fish oil group and 2053 women in the control group), all in women with low-risk pregnancies, observed the effects of fish oil supplementation on the risk for GDM. The results of pooled analysis with a fixed-effects model showed that fish oil supplementation was not related to a reduced risk of GDM (RR=1.06, 95% CI: 0.85–1.32, p=0.60; Figure 2). No evidence of significant heterogeneity was detected (Cochrane’s Q test: p=0.50; I2=0%).
Figure 2.
Forest plot from meta-analysis of risk ratio (RR) of gestational diabetes mellitus for pregnant women randomized to a fish oil or control group.
Effects of fish oil supplementation on incidence of PIH
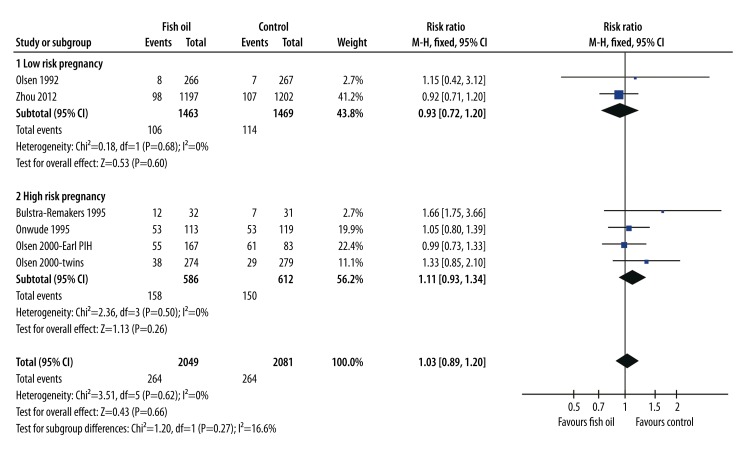
Meta-analysis of 6 comparisons [24–27,32], including 2049 women in the fish oil group and 2081 in the control group, showed that fish oil supplementation was not associated with a reduced incidence of PIH (RR=1.03, 95% CI: 0.89–1.20, p=0.66; Figure 3). No evidence of significant heterogeneity was detected (Cochrane’s Q test: p=0.62; I2=0%). Moreover, subgroup analyses indicated that the results were consistent between women with low-risk (RR=0.93, 95% CI: 0.72–1.20, p=0.60) and high-risk (RR=1.11, 95% CI: 0.93–1.34, p=0.26) pregnancies.
Figure 3.
Forest plot from meta-analysis of risk ratio (RR) of pregnancy-induced hypertension for pregnant women (low-risk and high-risk) randomized to a fish oil or control group.
Effects of fish oil supplementation on incidence of PE
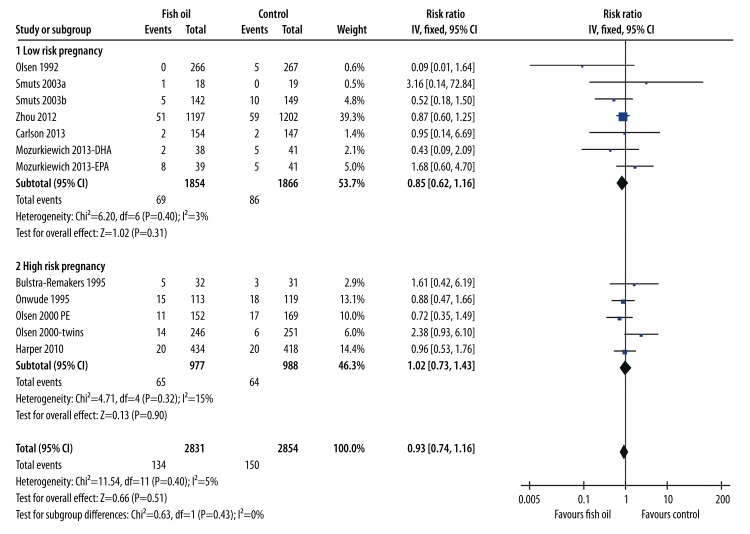
The results of pooling the 12 comparisons [24–29,31–34] (including 2831 women in the fish oil group and 2854 women in the control group) did not support a protective role of fish oil supplementation on the risk of PE (RR=0.93, 95% CI: 0.74–1.16, p=0.51; Figure 4). No evidence of significant heterogeneity was detected (Cochrane’s Q test: p=0.40; I2=5%). Moreover, subgroup analyses indicated that the results were consistent between women with low-risk (RR=0.85, 95% CI: 0.62–1.16, p=0.31) and high-risk (RR=1.02, 95% CI: 0.73–1.43, p=0.90) pregnancies.
Figure 4.
Forest plot from meta-analysis of risk ratio (RR) of pre-eclampsia for pregnant women (low-risk and high-risk) randomized to a fish oil or control group.
Publication bias
The funnel plots for the effects of fish oil supplementation on the risks for GDM, PIH, and PE were symmetrical on visual inspection, suggesting no significant publication biases (figures not shown). Egger’s significance tests also did not indicate the existence of publication biases (for GDM, p=0.28; for PIH, p=0.18; for PE, p=0.37).
Discussion
In this meta-analysis, by pooling the results of all available RCTs, we found that gestational supplementation with fish oil during the second or third trimester of pregnancy is not associated with a reduced risk for GDM, PIH, or PE. These results are consistent in both low-risk and high-risk pregnancies. The potential benefits of fish oil supplementation during pregnancy on other maternal outcomes should be evaluated in future studies.
Although there have been a few meta-analyses aiming to evaluate the effects of gestational supplementation with fish oil on the incidence complications during pregnancy [14,35–37], ours, to the best of our knowledge, is the largest meta-analysis including all available RCTs to date. The results of some recently published high-quality RCTs [33,34] have rarely been included in previous meta-analyses. Although the results of previous meta-analyses also did not support a preventative role for gestational fish oil supplementation against GDM, PIH, or PE, the chances that the negative results were retrieved due to the limited numbers of included studies and participants could not be totally excluded. Our meta-analysis, on the other hand, which includes 11 studies and about 5000 participants (Table 3) indicated that fish oil supplementation during pregnancy is not associated with reduced risks of gestational diabetic or hypertensive complications.
Table 3.
Scale comparison of meta-analyses regarding the roles of n-3 PUFA on GDM, PIH, and PE.
| No. of comparisons (No. of participants) | |||
|---|---|---|---|
| GDM | PIH | PE | |
| Makrides 2006 | – | 5 (1831) | 4 (1683) |
| Szajewska 2006 | 2 (328) | – | 2 (328) |
| Horvath A 2007 | – | 3 (645) | 2 (295) |
| Imhoff-Kunsch 2012 | – | 5 (1831) | 4 (1683) |
| Current one | 8 (4117) | 6 (4130) | 12 (5685) |
n-3 PUFA – omega-3 polyunsaturated fatty acids; GDM – gestational diabetes mellitus; PIH – pregnancy-induced hypertension; PE – pre-eclampsia.
Routine supplementation of DHA or EPA during pregnancy should not be denied based on our results, because gestational fish oil supplementation may have other potential benefits. Accumulating evidence suggests that regular supplementation with fish oil during pregnancy may improve the body measurements of the infants, which may reduce the risk of low birth weight [14]. In addition, a previous meta-analysis suggested that fish oil administered in pregnancy may reduce the rate of preterm birth [13]. Other than growth outcomes, recent studies also raised the possibility that n-3 PUFA supplementation during pregnancy may improve the cognitive or visual development of the children [15]. More importantly, in view of the fact that fish oil has been proved to confer various cardiovascular benefits, possibly through its anti-inflammatory effects [38] and improvement of endothelial function [39], whether gestational supplementation with fish oil can reduce the risk of cardiovascular diseases in mothers and children still needs to be clarified.
Our study has a few limitations that must be considered when interpreting the results. First, fish oil supplementation was initiated during the second or third trimester of pregnancy. Whether early supplementation of fish oil may affect the incidence of GDM, PIH, and PE needs to be determined in the future. Moreover, the baseline body composition and the habitual dietary contents of n-3 PUFAs were not evaluated or controlled in the included studies, which may affect the results of the meta-analysis. In addition, according to the study by Zhou et al. [32], their sample size of more than 2000 women was sufficiently powered to discover a 3% reduction in absolute reduction in the risk of GDM. However, our study did not provide a sample size calculation to determine the statistical power of the current study. Finally, all of the studies were performed in Western countries. Whether gestational fish oil supplementation can benefit women in less developed countries should be studied in the future.
Conclusions
The results of our meta-analysis indicate that gestational supplementation with fish oil during the second and third trimesters of pregnancy is not associated with reduced risks for GDM, PIH, or PE. Further studies are needed to evaluate other potential benefits of gestational fish oil supplementation to mothers and infants.
Footnotes
Source of support: Self financing
Competing interests
The authors declare no competing interests.
References
- 1.Mitanchez D, Burguet A, Simeoni U. Infants born to mothers with gestational diabetes mellitus: mild neonatal effects, a long-term threat to global health. J Pediatr. 2014;164(3):445–50. doi: 10.1016/j.jpeds.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6(1):1–7. doi: 10.4021/jocmr1682w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29(1):1–31. [PubMed] [Google Scholar]
- 4.Lopez-Jaramillo P, Garcia RG, Lopez M. Preventing pregnancy-induced hypertension: are there regional differences for this global problem? J Hypertens. 2005;23(6):1121–29. doi: 10.1097/01.hjh.0000170371.49010.4a. [DOI] [PubMed] [Google Scholar]
- 5.Harreiter J, Dovjak G, Kautzky-Willer A. Gestational diabetes mellitus and cardiovascular risk after pregnancy. Womens Health (Lond Engl) 2014;10(1):91–108. doi: 10.2217/whe.13.69. [DOI] [PubMed] [Google Scholar]
- 6.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez-Torres MA. The importance of gestational diabetes beyond pregnancy. Nutr Rev. 2013;71(Suppl 1):S37–41. doi: 10.1111/nure.12070. [DOI] [PubMed] [Google Scholar]
- 8.Hillier TA, Pedula KL, Schmidt MM, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 9.Nerenberg K, Daskalopoulou SS, Dasgupta K. Gestational diabetes and hypertensive disorders of pregnancy as vascular risk signals: an overview and grading of the evidence. Can J Cardiol. 2014;30(7):765–73. doi: 10.1016/j.cjca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Hartling L, Dryden DM, Guthrie A, et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159(2):123–29. doi: 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- 11.Larque E, Gil-Sanchez A, Prieto-Sanchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr. 2012;107(Suppl 2):S77–84. doi: 10.1017/S0007114512001481. [DOI] [PubMed] [Google Scholar]
- 12.Mozurkewich EL, Klemens C. Omega-3 fatty acids and pregnancy: current implications for practice. Curr Opin Obstet Gynecol. 2012;24(2):72–77. doi: 10.1097/GCO.0b013e328350fd34. [DOI] [PubMed] [Google Scholar]
- 13.Salvig JD, Lamont RF. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90(8):825–38. doi: 10.1111/j.1600-0412.2011.01171.x. [DOI] [PubMed] [Google Scholar]
- 14.Imhoff-Kunsch B, Briggs V, Goldenberg T, Ramakrishnan U. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):91–107. doi: 10.1111/j.1365-3016.2012.01292.x. [DOI] [PubMed] [Google Scholar]
- 15.Gould JF, Smithers LG, Makrides M. The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97(3):531–44. doi: 10.3945/ajcn.112.045781. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Storlien LH, Jenkins AB, et al. Dietary variables and glucose tolerance in pregnancy. Diabetes Care. 2000;23(4):460–64. doi: 10.2337/diacare.23.4.460. [DOI] [PubMed] [Google Scholar]
- 17.Bo S, Menato G, Lezo A, et al. Dietary fat and gestational hyperglycaemia. Diabetologia. 2001;44(8):972–78. doi: 10.1007/s001250100590. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. www.cochranehandbookorg. [Google Scholar]
- 20.Ortega-Gonzalez C, Ballesteros A, Casanueva E, et al. Searching for alternative methods of diagnosing gestational diabetes mellitus in a Mexican urban population. Med Sci Monit. 2008;14(12):CR598–603. [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen SF, Sorensen JD, Secher NJ, et al. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339(8800):1003–7. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 25.Bulstra-Ramakers MT, Huisjes HJ, Visser GH. The effects of 3g eicosapentaenoic acid daily on recurrence of intrauterine growth retardation and pregnancy induced hypertension. Br J Obstet Gynaecol. 1995;102(2):123–26. doi: 10.1111/j.1471-0528.1995.tb09064.x. [DOI] [PubMed] [Google Scholar]
- 26.Onwude JL, Lilford RJ, Hjartardottir H, et al. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. Br J Obstet Gynaecol. 1995;102(2):95–100. doi: 10.1111/j.1471-0528.1995.tb09059.x. [DOI] [PubMed] [Google Scholar]
- 27.Olsen SF, Secher NJ, Tabor A, et al. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) Team. BJOG. 2002;107(3):382–95. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 28.Smuts CM, Borod E, Peeples JM, Carlson SE. High-DHA eggs: feasibility as a means to enhance circulating DHA in mother and infant. Lipids. 2003;38(4):407–14. doi: 10.1007/s11745-003-1076-y. [DOI] [PubMed] [Google Scholar]
- 29.Smuts CM, Huang M, Mundy D, et al. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101(3):469–79. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 30.van Goor SA, Dijck-Brouwer DA, Doornbos B, et al. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. Br J Nutr. 2010;103(2):235–42. doi: 10.1017/S0007114509991528. [DOI] [PubMed] [Google Scholar]
- 31.Harper M, Thom E, Klebanoff MA, et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth: a randomized controlled trial. Obstet Gynecol. 2010;115(2 Pt 1):234–42. doi: 10.1097/AOG.0b013e3181cbd60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou SJ, Yelland L, McPhee AJ, et al. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am J Clin Nutr. 2012;95(6):1378–84. doi: 10.3945/ajcn.111.033217. [DOI] [PubMed] [Google Scholar]
- 33.Carlson SE, Colombo J, Gajewski BJ, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97(4):808–15. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozurkewich EL, Clinton CM, Chilimigras JL, et al. The Mothers, Omega-3, and Mental Health Study: a double-blind, randomized controlled trial. Am J Obstet Gynecol. 2013;208(4):313 e311–19. doi: 10.1016/j.ajog.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;(3):CD003402. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83(6):1337–44. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- 37.Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Br J Nutr. 2007;98(2):253–59. doi: 10.1017/S0007114507709078. [DOI] [PubMed] [Google Scholar]
- 38.Xin W, Wei W, Li X. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2012;12:77. doi: 10.1186/1471-2261-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin W, Wei W, Li X. Effect of fish oil supplementation on fasting vascular endothelial function in humans: a meta-analysis of randomized controlled trials. PLoS One. 2012;7(9):e46028. doi: 10.1371/journal.pone.0046028. [DOI] [PMC free article] [PubMed] [Google Scholar]