Abstract
Euforia, a supplement containing a variety of natural ingredients, is widely used as an antioxidant and anti-inflammatory formula. It is not approved by the US Food and Drug Administration and its side effects are unknown. We report a 45-year-old woman with limited systemic sclerosis who presented with jaundice and marked elevation of serum transaminases. One month before, she started taking Euforia juice. A liver biopsy disclosed submassive hepatocellular necrosis with histopathological changes consistent with toxic hepatitis. The patient's symptoms resolved with cessation of Euforia. Six months later, she persisted with abnormal liver function tests, but these resolved 18 months after discontinuation of Euforia. The mechanism by which Euforia causes liver injury is unknown. Some ingredients contained in this supplement (green tea, Aloe vera, noni and goji) are linked to hepatic injury. To our knowledge, this is the first report of hepatotoxicity associated with Euforia.
Background
The utilisation of dietary supplements has continually increased in recent years. According to a recent report, sales of herbal supplements in the USA increased more than 3% in 2010, now totalling more than $5 billion annually.1 The potential side effects of these preparations as well as the lack of safety regulations may have serious consequences.2 Lately, several cases of hepatotoxicity associated with the use of herbal medicines have been reported, ranging from mild elevation of liver enzymes to fulminant liver failure requiring liver transplantation.3 Euforia (Nuverus International, Irving, Texas, USA) is a popular supplement of fruits and herbal concentrates widely used as an antioxidant and anti-inflammatory agent. Although not approved by the US Food and Drug Administration, extensive media advertising has increased its popularity and the company now claims to have millions of users. Herein, we report a 45-year-old woman with scleroderma who developed severe hepatotoxicity associated with the use of Euforia.
Case presentation
A 45-year-old woman with hypothyroidism and recently diagnosed limited systemic sclerosis, treated with levothyroxine and nifedipine, was hospitalised owing to acute liver injury. One month prior to admission, she had been taking two ounces of Euforia juice daily. She was not taking over-the-counter drugs and had no history of alcohol intake or illicit drug use. On physical examination, she was febrile (101.8°F) and had marked jaundice and scleral icterus. She had abdominal tenderness in the right upper quadrant associated with voluntary guarding. There was no ascites, visceromegaly or stigmata of chronic liver disease. She had sclerodactyly and skin induration up to the forearms. Raynaud's phenomenon was observed.
Investigations
Laboratory tests revealed marked elevation of serum transaminases and bilirubin levels. The initial and subsequent liver tests are summarised in table 1. Serological tests for hepatitis A, B and C were negative. Antinuclear antibodies were positive at 1:320 (speckled pattern). Antismooth muscle and anti-mitochondrial antibodies were negative. Abdominal CT exhibited mild hepatomegaly, diffuse fatty infiltration, periportal oedema and minimal dilation of right and left main ducts with normal calibre of the common hepatic duct and common bile duct.
Table 1.
Summary of liver tests
| Test | Day 1 | Day 7 | Follow-up 2 weeks | Follow-up 6 months | Follow-up 18 months |
|---|---|---|---|---|---|
| AST (U/l) | 1169 | 517 | 1368 | 149 | 44 |
| ALT (U/l) | 837 | 490 | 906 | 109 | 30 |
| ALP (U/l) | 134 | 121 | 117 | 203 | 158 |
| GGT (U/l) | 138 | – | – | – | – |
| Albumin (g/l) | 29 | 23 | 31 | 28 | 37 |
| TBil (μmol/l) | 302.6 | 237.7 | 429.2 | 30.8 | 11.9 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransaminase, TBil, total Bilirubin; GGT, gammaglutamyl transpeptidase.
During the hospitalisation serum transaminases and bilirubin levels decreased, and she was discharged home by day 7. The patient discontinued the use of Euforia. On her follow-up visit, 2 weeks later, she persisted with jaundice and serum transaminases that were higher than baseline parameters (table 1). A liver needle biopsy showed submassive necrosis with predominantly plasma cell infiltration with occasional eosinophils consistent with drug-induced hepatitis (figure 1). There was no histological evidence of autoimmune hepatitis.
Figure 1.
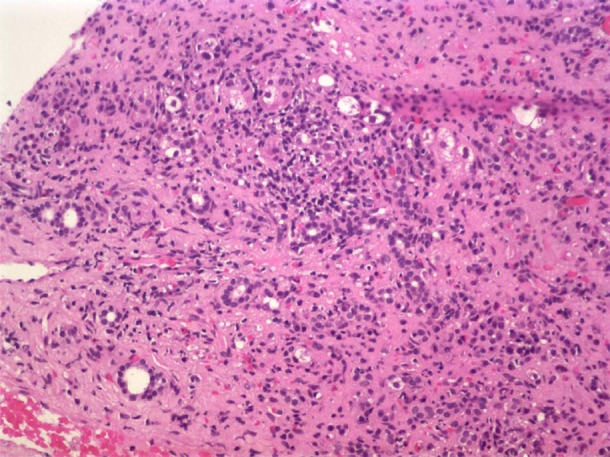
Liver biopsy shows submassive confluent coagulative necrosis, presenting collapse of the liver parenchyma and grouping of portal triads. The surviving periportal hepatocytes present ballooning at the periphery. There is presence of a predominantly lymphoplasmacytic infiltrate with occasional eosinophils surrounding the portal triads, with extension of hepatocyte injury to the mid-zonal areas of the lobule and associated sinusoidal lymphocytosis.
Outcome and follow-up
The patient gradually improved and had no relapses. Further work-up, during follow-up, showed negative liver–kidney microsomal antibodies and hypergammaglobulinaemia with IgG levels of 2765 mg/dl (540–1822 mg/dl). At 6 months, she remained with mild elevation of aminotransaminases, mild hyperbilirubinaemia and hypoalbuminaemia but these resolved 18 months after discontinuation of Euforia (table 1).
Discussion
We present a case of acute hepatic injury associated with Euforia which, to our knowledge, represents the first report of hepatotoxicity associated with this natural preparation. Our patient developed severe liver injury that slowly improved after cessation of Euforia. The temporal relationship between Euforia intake and liver dysfunction, and the exclusion of other causes of acute hepatitis suggested toxic hepatitis in this case. Furthermore, histological changes were confirmatory of acute drug-induced hepatotoxicity.
There are no specific tests or diagnostic criteria for herb-induced hepatic injury; however, other diagnostic tools may be utilised. The most commonly used is the semiqualitative Roussel Uclaf Causality Assessment Method of the Council for International Organizations of Medical Sciences scale, which correlates a causing agent and toxic liver injury.4 The total score is categorised as ‘highly probable’, ‘probable’, ‘possible’, ‘unlikely’ or ‘excluded’. According to this scale, our patient had a score of 6 which correlates with a ‘probable’ diagnosis of Euforia-induced liver injury.
Euforia contains a variety of herbs and fruits including acai berry, mangosteen, Aloe vera, resveratrol, curcumin, black seed (Nigella sativa), blueberry, pomegranate, green tea, noni (Morinda citrifolia) and goji berry. Because of the worldwide use, for centuries, of the individual ingredients contained in Euforia, this formula has been perceived as safe. Nevertheless, several cases of hepatotoxicity following the consumption of dietary supplements containing green tea, Aloe vera, noni and goji have been reported.5–8 Thus, liver injury related to Euforia could be related to one or more of its individual components.
There are three types of acute liver injury induced by drugs or herbs: hepatocellular, cholestatic and mixed type.9 Our case was characterised by the hepatocellular type as confirmed by histopathological findings. The two proposed pathogenic mechanisms of drug-induced liver disease are idiosyncratic and direct toxicity.10 An immunoallergic mechanism could be responsible for the liver injury observed in our patient as suggested by the presence of eosinophils in the periportal space seen in the biopsy. Furthermore, hypersensitivity to one of the Euforia ingredients, Aloe vera, has been previously reported in humans.11 Although severe liver damage has been associated with hypersensitivity reactions, the chronicity of this damage, after dechallenge, makes this type of idiosyncratic mechanism unlikely in our case. On the other hand, green tea, another ingredient of Euforia, has been associated with direct liver injury attributable to its polyphenols content. Polyphenols are the main chemical components of unfermented tea (up to 20%); of which, catechins, particularly epigallocatechin gallate (EGCG), have been linked to liver injury.12 Experiments performed in rat liver cells show that high concentrations of green tea extracts are toxic; this cytotoxicity appears to be related to EGCG.13 Another potential toxic component of Euforia is noni. This product by itself contains multiple putative active ingredients of which anthraquinones are the most likely to be hepatotoxic. Anthraquinones in other herbal remedies have been reported to produce oxygen-derived free radicals resulting in depletion of intracellular reduced glutathione, decreased mitochondrial membrane potential, and cell death.14 Finally, the consumption of goji berries was recently reported as the cause of toxic hepatitis, but the exact mechanism of liver injury is unknown.8 Taken together, we hypothesise that the hepatic damage induced by Euforia could be attributed to the concomitant use of these components.
Other components of Euforia may cause liver injury but a definite association has not been established. Mangosteen has been found to be both substrate and inhibitor for multiple hepatic cytochomre P450 isoforms.15 Curcumin accelerates liver injury and liver cellular oedema in mice with injured liver.16 Pomegranate, a rich source of several chemicals such as pectin, tannins, flavonoids and anthocyanins, has been found to inhibit human CYP2C9 activity affecting the hepatic metabolism of various drugs.17 Resveratrol has in vitro and in vivo effects on various cytochrome P450 isoenzymes as well. All these components through their interactions with various cytochrome P450 isoforms, especially when taken in high doses, can potentially lead to reduced first-pass metabolism resulting in higher systemic exposure to certain co-administrated cytochrome P450 substrates. Therefore, patients who ingest high doses of these supplements combined with medications may be at risk of having clinically relevant drug interactions. Conversely, the remaining components of Euforia, black seed, blueberry and acaiberry, have not been associated with alterations of liver homeostasis.
The fact that our patient has an underlying autoimmune disease makes the diagnosis of drug-induced hepatitis more challenging because patients with systemic sclerosis may have hepatic involvement. However, liver disease is rare in scleroderma.18 In a postmortem series of 57 systemic sclerosis patients, D'Angelo et al19 found histological liver damage in only 8.8% of cases Furthermore, application of the diagnostic scoring system for autoimmune hepatitis (AIH) in patients, with systemic sclerosis, is to some extent unreliable. Based on the simplified diagnostic scoring system developed by Hennes et al,20 our patient has a ‘probable’ diagnosis of AIH. However, this score takes into consideration the presence of antinuclear antibodies (ANA) and hypergammaglobulinaemia which are commonly seen in patients with systemic sclerosis. ANA are present in more than 90% of systemic sclerosis patients,21 and elevated levels of serum IgG have been reported in up to 56% of patients (with levels more than 1600 mg/dl).22 Therefore, we may not rely on this scoring system to make the diagnosis of AIH in patients with systemic sclerosis. Besides, the development of AIH in these patients is extremely rare with only few cases reported to date.23
AIH and drug-induced liver injury may be clinically indistinguishable. These conditions are mediated by immunological reactions and thus have considerable resemblance in clinical and histopathological features. Recently, an international consensus was done with the purpose of creating more uniform criteria to define and characterise the spectrum of clinical syndromes that constitute drug-induced liver injury. According to this consensus, more than 80% of patients with idiopathic AIH have a relapse within 1 year in the absence of adequate immunosuppression, whereas those with drug-induced AIH have no relapse.24 Our patient had no relapses within the 18-month period after discontinuation of the offending product. During this period, she did not receive immunosuppressive therapy. Timely diagnosis and proper management are critical for both conditions. Early immunosuppressive therapy typically controls disease activity in patients with idiopathic AIH and can lead to disease remission. On the other hand, prompt identification and discontinuation of the offending drug halts ongoing liver injury in patients with drug-induced hepatitis. However, even after withdrawal of the causative agent, the liver injury may be persistent beyond 3 months of follow-up in hepatocellular liver injury,24 as in our patient. Failure to properly treat either of those conditions could result in clinically devastating acute or chronic outcomes.
In conclusion, careful history taking, laboratory findings, and histopathology are needed to diagnose drug-induced hepatitis. Since patients usually do not regard dietary supplements as ‘true’ medicines, they may fail to disclose it when physicians query medication history. Physicians should keep in mind that dietary supplements can cause hepatotoxicity, and should educate their patients about this risk. These products should be used with caution in patients with pre-existing liver disease and in those with predisposition of liver involvement such as patients with autoimmune connective tissue diseases, like our case. This case highlights the potential hepatotoxicity of Euforia as well as of its individual components.
Learning points.
Euforia, a supplement containing a variety of natural ingredients, is widely used as an antioxidant and anti-inflammatory formula.
Some ingredients contained in this formula (green tea, Aloe vera, noni and goji) are linked to hepatic injury.
We report a middle-aged woman with scleroderma who developed severe hepatotoxicity associated with the use of Euforia.
Herbal supplements should be used with caution in patients with pre-existing liver disease and in those with predisposition of liver involvement such as patients with autoimmune connective tissue diseases.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Blumenthal M, Lindstrom A, Lynch ME, et al. Herb sales continue growth—up 3.3% in 2010. HerbalGram 2011;90:64–7. [Google Scholar]
- 2.Boullata JI, Nace AM. Safety issues with herbal medicine. Pharmacotherapy 2000;20:257–69. [DOI] [PubMed] [Google Scholar]
- 3.Stedman C. Herbal hepatotoxicity. Semin Liver Dis 2002;22:195–206. [DOI] [PubMed] [Google Scholar]
- 4.Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323–30. [DOI] [PubMed] [Google Scholar]
- 5.Mazzanti G, Menniti-Ippolito F, Moro P, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol 2009;65:331–41. [DOI] [PubMed] [Google Scholar]
- 6.Ha Na Y, Dong JK, Young MK, et al. Aloe-induced toxic hepatitis. J Korean Med Sci 2010;25:492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadlbauer V, Fickert P, Lackner C, et al. Hepatotoxicity of NONI juice: report of two cases. World J Gastroenterol 2005;11:4758–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco M, Monmany J, Domingo P, et al. Autoimmune hepatitis triggered by consumption of Goji berries. Med Clin (Barc) 2012;139:320–1. [DOI] [PubMed] [Google Scholar]
- 9.Chae HB. Clinical features and diagnosis of drug induced liver injury. Korean J Hepatol 2004;10(Suppl 1):7–18. [Google Scholar]
- 10.Kang DY. Pathologic features of toxic and drug induced liver injury. Korean J Hepatol 2004;10(Suppl 1):19–29. [Google Scholar]
- 11.Lee EG, Kwon SH, Kim SH, et al. A case of hypersensitivity associated with oral aloe agent. J Asthma Allergy Clin Immunol 2003;23:833–6. [Google Scholar]
- 12.Bruneton J. Pharmacognosy, phytochemistry, medicinal plants, 2nd edn Paris: Lavoisier, 1999. [Google Scholar]
- 13.Galati G, Lin A, Sultan AM, et al. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med 2006;40:570–80. [DOI] [PubMed] [Google Scholar]
- 14.Bironaite D, Ollinger K. The hepatotoxicity of rhein involves impairment of mitochondrial functions. Chem Biol Interact 1997;103:35–50. [DOI] [PubMed] [Google Scholar]
- 15.Foti RS, Pearson JT, Rock DA, et al. In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract. Drug Metab Dispos 2009;37:1848–55. [DOI] [PubMed] [Google Scholar]
- 16.Zhao HL, Song CH, Chai OH. Negative effects of curcumin on liver injury induced by alcohol. Phytother Res 2012;26:1857–63. [DOI] [PubMed] [Google Scholar]
- 17.Nagata M, Hidaka M, Sekiya H, et al. Effects of pomegranate juice on human cytochrome P450 2C9 and tolbutamide pharmacokinetics in rats. Drug Metab Dispos 2007;35:302–5. [DOI] [PubMed] [Google Scholar]
- 18.Schlenker C, Halterman T, Kowdley KV. Rheumatologic disease and the liver. Clin Liver Dis 2011;15:153–64. [DOI] [PubMed] [Google Scholar]
- 19.D'Angelo WA, Fries JF, Masi AT, et al. Pathologic observations in systemic sclerosis (scleroderma): a study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969;46:428–40. [DOI] [PubMed] [Google Scholar]
- 20.Hennes EM, Zeniya M, Czaja AJ, et al. International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169–76. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol 2010;37:42–53. [DOI] [PubMed] [Google Scholar]
- 22.Usha, Gupta V, Singh RG, et al. Immunological alterations in scleroderma. J Indian Acad Clin Med 2001;2:185–8. [Google Scholar]
- 23.Rodrigues CE, Borges CL, de Carvalho JF. Diffuse systemic sclerosis and autoimmune hepatitis: a unique association. Clin Rheumatol 2010;29:799–801. [DOI] [PubMed] [Google Scholar]
- 24.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89:806–15. [DOI] [PubMed] [Google Scholar]


