Abstract
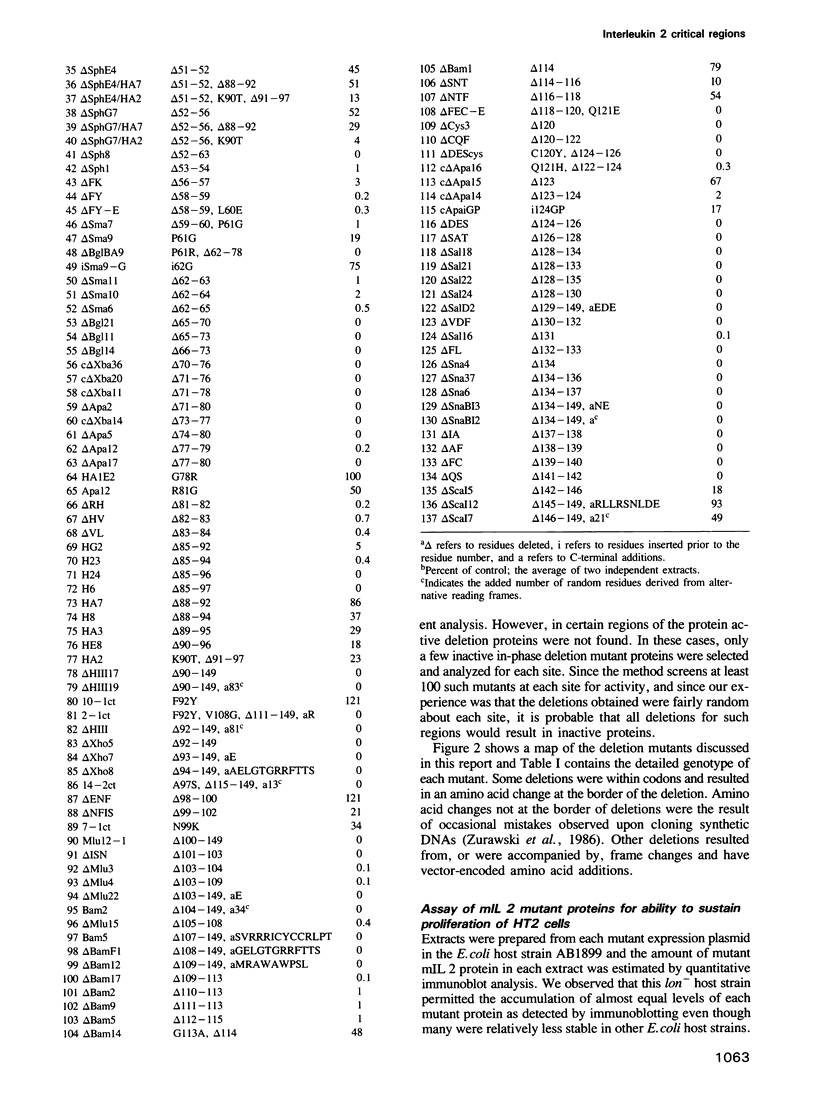
We have analyzed structure--function relationships of the protein hormone murine interleukin 2 by fine structural deletion mapping. A total of 130 deletion mutant proteins, together with some substitution and insertion mutant proteins, was expressed in Escherichia coli and analyzed for their ability to sustain the proliferation of a cloned murine T cell line. This analysis has permitted a functional map of the protein to be drawn and classifies five segments of the protein, which together contain 48% of the sequence, as unessential to the biological activity of the protein. A further 26% of the protein is classified as important, but not crucial, for the activity. Three regions, consisting of amino acids 32-35, 66-77 and 119-141 contain the remaining 26% of the protein and are critical to the biological activity of the protein. The functional map is discussed in the context of the possible role of the identified critical regions in the structure of the hormone and its binding to the interleukin 2 receptor complex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandhuber B. J., Boone T., Kenney W. C., McKay D. B. Crystals and a low resolution structure of interleukin-2. J Biol Chem. 1987 Sep 5;262(25):12306–12308. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Kosen P. A., Kuntz I. D., Epstein L. B., Ciardelli T. L., Smith K. A. Structure-activity studies of interleukin-2. Science. 1986 Oct 17;234(4774):349–352. doi: 10.1126/science.3489989. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Ju G., Collins L., Kaffka K. L., Tsien W. H., Chizzonite R., Crowl R., Bhatt R., Kilian P. L. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987 Apr 25;262(12):5723–5731. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Liang S. M., Thatcher D. R., Liang C. M., Allet B. Studies of structure-activity relationships of human interleukin-2. J Biol Chem. 1986 Jan 5;261(1):334–337. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Yokota T., Kastelein R., Zurawski S. M., Arai N., Takebe Y. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4). J Immunol. 1987 Mar 15;138(6):1813–1816. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Reeves R., Spies A. G., Nissen M. S., Buck C. D., Weinberg A. D., Barr P. J., Magnuson N. S., Magnuson J. A. Molecular cloning of a functional bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3228–3232. doi: 10.1073/pnas.83.10.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Mosmann T. R., Benedik M., Zurawski G. Alterations in the amino-terminal third of mouse interleukin 2: effects on biological activity and immunoreactivity. J Immunol. 1986 Nov 15;137(10):3354–3360. [PubMed] [Google Scholar]
- Zurawski S. M., Pope K., Cherwinski H., Zurawski G. Expression in Escherichia coli of synthetic human interleukin-1 alpha genes encoding the processed active protein, mutant proteins, and beta-galactosidase fusion proteins. Gene. 1986;49(1):61–68. doi: 10.1016/0378-1119(86)90385-9. [DOI] [PubMed] [Google Scholar]





