Abstract
Cadherin-catenin complexes are critical for the assembly of cell-cell adhesion structures known as adherens junctions. In addition to the mechanical linkage of neighboring cells to each other, these cell-cell adhesion protein complexes have recently emerged as important sensors and transmitters of the extracellular cues inside the cell body and into the nucleus. In the past few years, multiple studies have identified a connection between the cadherin-catenin protein complexes and major intracellular signaling pathways. Those studies are the main focus of this review.
Keywords: cell-cell interactions, cadherin, adherin junctions, cell-cell adhesion, catenin, intracellular signaling pathways
Introduction
The ability of cells to communicate and adhere to each other represents an ultimate prerequisite for the formation and maintenance of a multicellular organism. By sensing their microenvironment, cells can decide whether to continue or stop proliferating, change shape, accept a new identity, move out of the neighborhood, or simply cease to exist. How do the external signals get transmitted inside and prompt the cells to respond accordingly? In the past several years, cadherin-catenin protein complexes emerged as important regulators of morphogenesis and adult tissue homeostasis, linking cell-cell adhesion to multiple major signaling networks. In this short review, we will focus on the most recent studies that address the mechanisms and the functional relevance of the cadherin-mediated intracellular signaling.
Adherens junctions: structural organization and association with the actin cytoskeleton
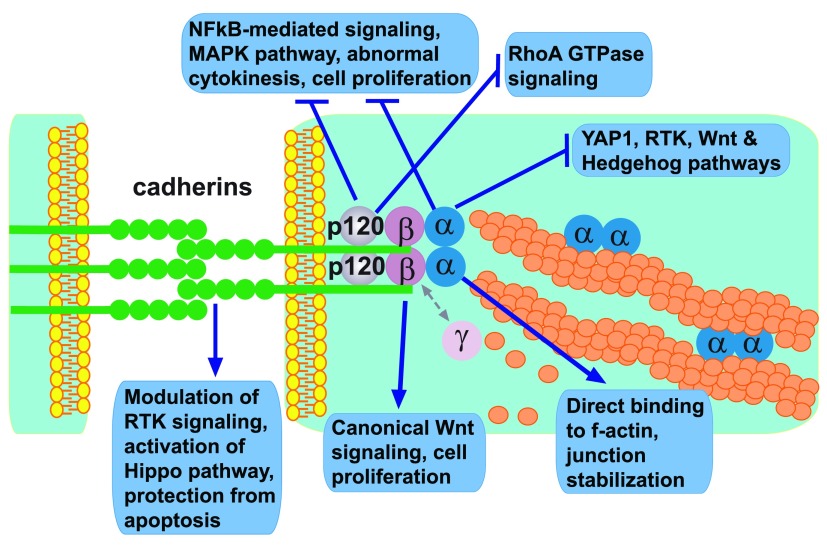
Cadherin-catenin complexes comprise the core of a specialized type of adhesion junction named an adherens junction (AJ) ( Figure 1). Among the family of classic cadherins, which includes E (epithelial)-, N (neural)-, P (placental)-, VE (vascular-endothelial)-, R (retinal)-, and K (kidney)-cadherins, E-cadherin is the most frequently employed in the formation of AJs in epithelial cells. To initiate the adhesion process, extracellular domains of cadherins engage in the Ca 2+-dependent homophilic trans-interaction with identical cadherin molecules on an adjacent cell, while their cytoplasmic tails bind to p120- and β- (or its homolog γ-) catenin proteins. In turn, β-catenin interacts with α-catenin, which contains an actin-binding domain and physically links AJ complexes to the actin cytoskeleton 1, 2. Interaction between the actomyosin cytoskeleton and the AJs is prominently regulated by the mechanical forces and Rho-family of small GTPases (covered in detail in 3– 6). This regulation is necessary for proper tissue morphogenesis and is highly dynamic, facilitating not only the coupling but also the detachment of cadherin-catenin complexes from actomyosin cytoskeleton, allowing cell-cell separation, cell sorting, and cell migration.
Figure 1. Cadherin-catenin complexes and their role in regulation of major intracellular signaling pathways.
The diagram depicts protein members of the adherens junctions clustered at the plasma membranes of two juxtaposed cells and summarizes their individual roles in the intricate network of intracellular signaling pathways. Note that, despite their unique structural features and separate functions, both cadherins and catenins often work in concert and may also participate in the regulation of the same signaling pathway though via a distinct mechanism. Abbreviations: MAPK, mitogen-activated protein kinase; NFκB, nuclear factor-kappa-B; RTK, receptor tyrosine kinase; YAP1, yes-associated protein 1.
Cadherin-mediated intracellular signaling has a pivotal role in contact inhibition of cell proliferation
The ability of cadherins to transmit signals from the extracellular microenvironment inside the cell body is likely a direct consequence of their adhesive function, which stimulates clustering of cadherin molecules involved in AJ formation. In cell culture experiments, formation of a confluent cell monolayer results in prominent clustering of cadherin-catenin molecules at the AJs. This clustering not only strengthens cell-cell adhesion but also provides important cues for apical-basal cell polarization and significantly influences the downstream signaling events (for review, see 3, 5, 7). It was noticed a long time ago that formation of a confluent cell monolayer results in cell cycle withdrawal 8. This phenomenon is known as “contact inhibition of cell proliferation” 7. Re-expression of E-cadherin in human epithelial cancer cell lines that lack E-cadherin expression or disruption of E-cadherin with neutralizing antibodies in cell lines that maintained endogenous E-cadherin demonstrated that cadherin-mediated cell-cell adhesion plays a pivotal role in execution of contact inhibition of cell proliferation 9. Similarly, activation of cadherin-catenin-mediated cell-cell adhesion by re-expression of α-catenin in a carcinoma line that was missing endogenous α-catenin resulted in retardation of cell proliferation 10. A negative impact of E-cadherin expression on tumor progression was also revealed in genetic mouse experiments in vivo 11. Since restoration of cadherin-catenin-mediated cell-cell adhesion results in prominent changes in cell morphology and re-establishment of apical-basal cell polarity, these early experiments were unable to determine whether cadherin clustering plays a direct or indirect role in negative regulation of cell proliferation. This question was later addressed by elegant experiments in Dr. Gumbiner’s laboratory, which demonstrated that clustering of cellular cadherins by E-cadherin-coated extracellular beads is sufficient to induce proliferation inhibitory signaling, thus directly implicating cadherin clustering in cell signaling events 12.
Cadherin-catenin adhesion and growth factor receptor signaling pathways
How do cadherins exert their signaling functions? Multiple signaling molecules are located at the cell-cell contact sites in direct proximity to the AJ complexes. Many growth- and proliferation-promoting signaling pathways are initiated at the cell surface by receptor-type tyrosine kinases (RTKs). Cadherins can physically interact with several RTKs and they prominently impact their signaling abilities. For example, E-cadherin associates with epidermal growth factor receptor (EGFR) and negatively regulates its kinase activity 12– 14. Tumor-suppressor protein neurofibromatosis type 2 (NF2 or Merlin) promotes association between E-cadherin and EGFR, links EGFR to the cortical actin cytoskeleton, and blocks its internalization, which is necessary for EGFR activation and signaling 15, 16. Loss of Merlin in mouse liver results in prominent activation of EGFR signaling, expansion of progenitors, and development of liver cancer 17. In addition to EGFR, E-cadherin can also negatively impact signaling of other RTKs, including ErbB2, insulin-like growth factor receptor (IGFR), and c-Met 14. Similar to E-cadherin in epithelial cells, VE-cadherin in endothelial cells interacts with vascular-endothelial growth factor receptor 2 (VEGFR2) and negatively regulates its mitogen-activated protein kinase (MAPK) signaling by preventing the clathrin-dependent internalization of VEGFR2 and promoting the association between VEGFR2 and tyrosine phosphatase PTPRJ, which dephosphorylates and inactivates VEGFR2 18, 19.
It is important to note that in some cases cadherins can promote growth factor receptor signaling. For example, N-cadherin stimulates fibroblast growth factor receptor signaling by preventing ligand-induced receptor internalization 20. Both E-cadherin and VE-cadherin can promote PI3-kinase (PI3K) signaling and protect cells from apoptotic cell death 21, 22. VE-cadherin associates with the transforming growth factor-beta (TGF-β) receptor complex and potentiates cell proliferation inhibitory TGF-β signaling events 23.
β- and p120-catenins and the direct line of communication between cell-cell junctions and transcriptional regulation of gene expression
By acting at the plasma membrane, cadherins are ideally positioned to attract and retain their cytoplasmic partners, thus modulating their activation, stability, or nuclear accumulation or a combination of these.
This is important because some of these intracellular proteins are pivotal signaling molecules in their own right. For example, β-catenin is a very potent transcriptional co-activator and a key member of the canonical Wnt signaling pathway (for review, see 24– 26). The levels of cytoplasmic β-catenin available for signaling are tightly controlled by the activity of the β-catenin-destruction protein complex, which is inhibited by activation of Wnt signaling 24, 25. Sequestration of β-catenin at the cell junctions can attenuate its ability to enter the cell nucleus and participate in transcriptional regulation. Indeed, multiple studies demonstrated that the loss of cadherin-mediated cell adhesion can promote β-catenin release and signaling 26. The exact relationship between cadherin-mediated adhesion and β-catenin signaling is highly complex and context-dependent. In some cases, not only do cadherins not inhibit but they actually potentiate the β-catenin signaling pathway (for review, see 27).
Similarly to β-catenin, cadherins can sequester at the plasma membrane and prevent cytoplasmic accumulation of another member of AJs, p120-catenin (for review, see 28). p120-catenin binds to the transcriptional repressor KAISO and inhibits its function 29– 31. In addition, p120-catenin is a potent regulator of Rho-family GTPases and the nuclear factor-kappa-B (NFκB) signaling pathway 28, 32. p120-catenin is critical for stabilization of cadherin-catenin complexes and formation of AJs, and this function is likely to be responsible for its tumor-suppressor function in squamous cell carcinoma (SCC), which was revealed by genetic loss-of-function experiments in mice 33.
α-catenin and regulation of cellular signal transduction pathways
α-catenin is crucial for AJ formation because it is necessary for the direct linkage of cadherin-catenin complexes at the membrane with the actin cytoskeleton 34. Although there are three α-catenin genes in mammalian genomes (alpha E-catenin CTNNA1, alpha N-catenin CTNNA2, and alpha T (testis)-catenin CTNNA3), most epithelial cells express only one α-catenin ( CTNNA1), and the knockout of this gene is usually sufficient for the complete loss of AJ function and loss of cell polarity 35, 36. This is different from inactivation of E-cadherin or β-catenin, which may often have redundant functions in the AJs because of the expression of other cadherins and γ-catenin. Notably, this is not the case in the adult heart, where inactivation of all expressed alpha-catenins ( Ctnna1 and Ctnna3) does not cause a severe cell adhesion defect comparatively to N-cadherin knockout mice 37, 38.
Similar to p120 catenin ( Ctnnd1), genetic loss-of-function experiments in mice revealed prominent tumor-suppressor activity of epithelial α-catenin ( Ctnna1), as epidermal stem cell-specific deletion of α-catenin in mice results in the development of SCC tumors 35, 39, 40. Like p120-catenin, α-catenin has been linked to NFκB signaling pathway in skin 39 and in E-cadherin-negative basal-like breast cancer cells 41, where it interacts with and stabilizes IκBα by preventing its ubiquitylation and association with proteasomes 41. In addition to its critical role in cell-cell adhesion, via direct interaction with the dynactin protein complex, α-catenin can regulate dynactin-dynein-mediated traffic and integrate the microtubule and actin cytoskeletons during intracellular trafficking events 42.
Loss-of-function experiments in vivo and in vitro revealed an important role of α-catenin in regulation of several major signaling networks, including Ras-MAPK 35, canonical Wnt 27, 43, and Hedgehog 44 pathways. Since α-catenin acts as a tumor suppressor in skin epidermis, our laboratory performed a small interfering RNA (siRNA) screen for genes necessary for this function in keratinocytes, which revealed a connection between α-catenin and yes-associated protein 1 (YAP1), a pivotal target of the Hippo signal transduction pathway 40. The connection between cadherin-catenin proteins and the Hippo pathway components has been demonstrated by multiple studies and we will discuss these findings in more detail 45– 47 (see below).
Meet the Hippo: the new darling of the cadherin signaling
First identified in Drosophila, the Hippo signaling pathway is evolutionarily conserved and functions as a key regulator of organ size and tumorigenesis by inhibiting cell proliferation and promoting (and, in some cases, inhibiting) apoptotic cell death (for review, see 48, 49). In vertebrates, the core of the canonical Hippo pathway consists of two sequentially acting sets of kinases, MST1/2 and LATS1/2 (Hippo and Warts in Drosophila), and several associated co-activators and scaffold proteins. The MST1/2 kinases phosphorylate and activate LATS1/2, which in turn phosphorylates the growth-promoting transcriptional co-activator YAP1 (Yorkie in Drosophila) and its homolog TAZ (also known as WWTR1), leading to their cytoplasmic retention. When the Hippo pathway is inhibited, YAP1 translocates to the nucleus, where it binds multiple transcriptional factors and promotes their transcriptional activity 48, 49. It is important to note that, in addition to the canonical Hippo signaling pathway, YAP1/TAZ nuclear localization and activity can be regulated independently from MST1/2 and LATS1/2 45, 50, 51. In both Drosophila and mammalian model systems, the Hippo signaling is exquisitely sensitive to changes in the actin cytoskeleton or cellular tension which functions as a pivotal regulator that integrates and transmits upstream signals to the Hippo signal transduction pathway (for review, see 49, 52). Increase in F-actin and actomyosin contractility blocks Hippo signaling and prominently activates Yorkie/YAP1/TAZ 51, 53.
For a long time, it remained largely unknown whether extracellular cues play any role in activating the Hippo pathway in mammals. The identity of the upstream transmembrane receptors responsible for transmitting the external signals inside the cell was undetermined. Elegant experiments in Dr. Guan’s laboratory identified G-protein-coupled receptors as important upstream regulators of Hippo signaling in mammalian cells 54. The evidence that the nuclear localization and activity of YAP1 are inversely correlated with cell density 55 pointed in the direction of the cell-cell junctions as potential upstream regulators of the Hippo signaling pathway. Indeed, it was recently demonstrated that E-cadherin homophilic binding at the cell surface in mammalian MDA-MB-231 cells is sufficient to control the subcellular localization of YAP1 independently of other cell interactions 46. In addition, two recent studies using primary mouse keratinocytes revealed that α-catenin can bind to YAP1 and sequester it in the cytoplasm, thus modulating the level of YAP1 phosphorylation and its activity 40, 45 (for review, see 56, 57). Importantly, there was an inverse correlation between α-catenin levels and nuclear YAP1 localization in both cultured keratinocytes and human SCC tumors, indicating that α-catenin may act as an inhibitor of YAP1 both in vitro and in vivo 40. Of interest, although Ca 2+ depletion, which abolishes cadherin homophilic interactions, triggered translocation of YAP1 into the nucleus, the depletion of E/P-cadherin or β-catenin in cultured keratinocytes did not affect the cellular localization of YAP1 45, pointing at the possibility that the expression of other cadherins and catenins might be sufficient to maintain AJs in E/P-cadherin or β-catenin knockdown keratinocytes.
In addition to α-catenin, β-catenin interacts with YAP1 and these proteins prominently impact each other’s nuclear localization and activity 47, 58, 59. Constitutive activation of β-catenin in human cancer cells results in the formation of a β-catenin-YAP1-TBX5 transcriptional complex, which is essential for cancer cell survival 60.
In Drosophila, the Hippo pathway can be regulated by multiple upstream transmembrane modules, which include atypical cadherins Dachsous and Fat (for review, see 61). Recently, another AJ protein, Echinoid, was shown to activate Hippo signaling via its physical interaction with and stabilization of the Hippo-binding partner Salvador 62. This interaction is triggered by cell-cell contacts and requires the dimerization of Echinoid cytoplasmic domain. It is of interest to mention that, although there is no known Echinoid homolog in mammals, this protein is able to interact with Drosophila E-cadherin, thus contributing to the formation and maintenance of AJs 63. Overall, although there are a lot of similarities between Drosophila and mammalian Hippo signaling pathways, at least some of the upstream regulators may be quite different 64. Drosophila Yorkie is missing the C-terminal PDZ-binding motif, which is necessary for the connection between YAP1/TAZ and tight junction (TJ) proteins in mammalian cells. Although α-catenin is a potent negative regulator of YAP1 in mammalian cells 38, 40, 45, 46, 65, it is a positive regulator of Yorkie in Drosophila 66, 67. While E-cadherin is a cell autonomous-positive regulator of Hippo pathway in mammalian cells 46, it is a cell autonomous-negative regulator of Hippo in Drosophila 67. Fat4, the mammalian ortholog of Drosophila fat gene, does not regulate the Hippo pathway in mouse liver, the organ highly sensitive to changes in the canonical Hippo signaling pathway 64. However, mammalian FAT4 and Dachsous cadherins appear to negatively regulate YAP1 in neural progenitor cells 68, 69, indicating that at least some of the important connections in Hippo signaling may be tissue- and species-specific.
As discussed above, one of the ways for cadherins to regulate contact inhibition of cell proliferation is by antagonizing the activity of a variety of RTKs, including the EGFR. Interestingly, changes in RTK activity may indirectly impact Hippo signaling. For example, it was recently demonstrated that, in immortalized mammary cells, EGF treatment triggers the nuclear accumulation of YAP1 through activation of PI3K and phosphoinositide-dependent kinase (PDPK1) and this is largely independent of AKT signaling 70. Interestingly, in Drosophila, EGF signaling also inhibits the Hippo pathway but through a different mechanism, which uses MAPK and the inhibitor of Warts, Jub 71. Taken together, those findings point at the important connection between AJs, mitogenic factor pathways, and growth-inhibitory Hippo signaling. Of note, the Drosophila Jub was also shown to associate with α-catenin in a cytoskeleton tension-dependent manner, thus linking the actomyosin cytoskeleton, regulation of Hippo pathway activity, and AJs 66.
In addition to the AJs, cadherin-mediated adhesion plays an important role in the formation of TJs and the apical-basal cell polarity domains. In turn, the polarity complex proteins can interact with structural components of both AJs and TJs, thus potentially centralizing the regulation of several signaling pathways (for review, see 72), although it is possible that the AJs and cell polarity regulate the Hippo signaling via multiple, genetically separable mechanisms 67. The TJ-associated proteins angiomotin and angiomotin-like 1 and 2 directly interact with YAP1/TAZ, localize them to the cytoplasm and TJs, and negatively regulate their transcriptional activity 73– 76. Remarkably, at least in some cases, angiomotin proteins promote YAP1 activity by antagonizing YAP1-LATS2 interaction and increasing YAP1 dephosphorylation and translocation to the nucleus 77. Interestingly, via its interaction with Merlin, angiomotin can localize to the AJs and facilitate AJ-specific recruitment and activation of LATS 78. In both Drosophila and mammals, Merlin promotes Hippo signaling by targeting LATS to the cell membrane 79. However, since angiomotin proteins are missing in the Drosophila genome, the angiomotin-mediated localization and activation of LATS at the AJs are likely to be species-specific, and this may potentially explain the differences in AJ-mediated regulation of YAP1 between Drosophila and mammalian model systems.
Future directions
The unique aspect of cadherin-mediated signaling is that the clustering of cadherin molecules is mediated by the direct cell-cell contacts. This enables cells to identify and map the positions of their immediate neighbors, helping to integrate individual cells into the tissues not only at physical but also at biochemical levels. Although we are continually learning about novel aspects of cadherin-mediated signaling, it is clear that the unifying picture is still not within reach. Knowledge remains highly fragmented with distinct and frequently seemingly opposite findings generated in different model organisms, tissues, or cell culture conditions. Future studies are clearly necessary to accumulate more data in the hope that the sheer quantity of information will inevitably result in a qualitative change in our understanding of how individual cells use their cell-cell adhesion structures to coordinate their behavior in building and homeostatic maintenance of multicellular organisms.
Abbreviations
AJ, adherens junction; E, epithelial; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; N, neural; NFκB, nuclear factor-kappa-B; P, placental; PI3K, PI3-kinase; RTK, receptor tyrosine kinase; SCC, squamous cell carcinoma; TGF-β, transforming growth factor-beta; TJ, tight junction; VE, vascular-endothelial; VEGFR2, vascular endothelial growth factor receptor 2; YAP1, yes-associated protein 1.
Funding Statement
Work on this article was supported in part by National Institutes of Health grants CA179914 and CA188452.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v1; ref status: indexed
References
- 1. Stepniak E, Radice GL, Vasioukhin V: Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1(5):a002949. 10.1101/cshperspect.a002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Capaldo CT, Farkas AE, Nusrat A: Epithelial adhesive junctions. F1000Prime Rep. 2014;6:1. 10.12703/P6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maître J, Heisenberg CP: Three functions of cadherins in cell adhesion. Curr Biol. 2013;23(14):R626–33. 10.1016/j.cub.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Takeichi M: Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397–410. 10.1038/nrm3802 [DOI] [PubMed] [Google Scholar]
- 5. Priya R, Yap AS: Active tension: the role of cadherin adhesion and signaling in generating junctional contractility. Curr Top Dev Biol. 2015;112:65–102. 10.1016/bs.ctdb.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 6. Röper K: Integration of cell-cell adhesion and contractile actomyosin activity during morphogenesis. Curr Top Dev Biol. 2015;112:103–27. 10.1016/bs.ctdb.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 7. McClatchey AI, Yap AS: Contact inhibition (of proliferation) redux. Curr Opin Cell Biol. 2012;24(5):685–94. 10.1016/j.ceb.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 8. Eagle H, Levine EM: Growth regulatory effects of cellular interaction. Nature. 1967;213(5081):1102–6. 10.1038/2131102a0 [DOI] [PubMed] [Google Scholar]
- 9. St Croix B, Sheehan C, Rak JW, et al. : E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27 KIP1. J Cell Biol. 1998;142(2):557–71. 10.1083/jcb.142.2.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watabe M, Nagafuchi A, Tsukita S, et al. : Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127(1):247–56. 10.1083/jcb.127.1.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perl AK, Wilgenbus P, Dahl U, et al. : A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–3. 10.1038/32433 [DOI] [PubMed] [Google Scholar]
- 12. Perrais M, Chen X, Perez-Moreno M, et al. : E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18(6):2013–25. 10.1091/mbc.E06-04-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Hoschuetzky H, Aberle H, Kemler R: Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127(5):1375–80. 10.1083/jcb.127.5.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian X, Karpova T, Sheppard AM, et al. : E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23(8):1739–48. 10.1038/sj.emboj.7600136 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Cole BK, Curto M, Chan AW, et al. : Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28(4):1274–84. 10.1128/MCB.01139-07 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Curto M, Cole BK, Lallemand D, et al. : Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177(5):893–903. 10.1083/jcb.200703010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Benhamouche S, Curto M, Saotome I, et al. : Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24(16):1718–30. 10.1101/gad.1938710 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Grazia Lampugnani M, Zanetti A, Corada M, et al. : Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161(4):793–804. 10.1083/jcb.200209019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Lampugnani MG, Orsenigo F, Gagliani MC, et al. : Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174(4):593–604. 10.1083/jcb.200602080 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Suyama K, Shapiro I, Guttman M, et al. : A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer cell. 2002;2(4):301–14. 10.1016/S1535-6108(02)00150-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Pece S, Chiariello M, Murga C, et al. : Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274(27):19347–51. 10.1074/jbc.274.27.19347 [DOI] [PubMed] [Google Scholar]
- 22. Carmeliet P, Lampugnani MG, Moons L, et al. : Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–57. 10.1016/S0092-8674(00)81010-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Rudini N, Felici A, Giampietro C, et al. : VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 2008;27(7):993–1004. 10.1038/emboj.2008.46 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Clevers H, Nusse R: Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 25. Clevers H: Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 26. Heuberger J, Birchmeier W: Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. 10.1101/cshperspect.a002915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCrea PD, Maher MT, Gottardi CJ: Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol. 2015;112:129–96. 10.1016/bs.ctdb.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schackmann RC, Tenhagen M, van de Ven RA, et al. : p120-catenin in cancer - mechanisms, models and opportunities for intervention. J Cell Sci. 2013;126(Pt 16):3515–25. 10.1242/jcs.134411 [DOI] [PubMed] [Google Scholar]
- 29. Daniel JM, Reynolds AB: The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19(5):3614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park JI, Kim SW, Lyons JP, et al. : Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8(6):843–54. 10.1016/j.devcel.2005.04.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Spring CM, Kelly KF, O'Kelly I, et al. : The catenin p120 ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Exp Cell Res. 2005;305(2):253–65. 10.1016/j.yexcr.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 32. Perez-Moreno M, Davis MA, Wong E, et al. : p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124(3):631–44. 10.1016/j.cell.2005.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Stairs DB, Bayne LJ, Rhoades B, et al. : Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer cell. 2011;19(4):470–83. 10.1016/j.ccr.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Buckley CD, Tan J, Anderson KL, et al. : Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346(6209):1254211. 10.1126/science.1254211 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Vasioukhin V, Bauer C, Degenstein L, et al. : Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104(4):605–17. 10.1016/S0092-8674(01)00246-X [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Nemade RV, Bierie B, Nozawa M, et al. : Biogenesis and function of mouse mammary epithelium depends on the presence of functional alpha-catenin. Mech Dev. 2004;121(1):91–9. 10.1016/j.mod.2003.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kostetskii I, Li J, Xiong Y, et al. : Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96(3):346–54. 10.1161/01.RES.0000156274.72390.2c [DOI] [PubMed] [Google Scholar]
- 38. Li J, Gao E, Vite A, et al. : Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res. 2015;116(1):70–9. 10.1161/CIRCRESAHA.116.304472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobielak A, Fuchs E: Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci U S A. 2006;103(7):2322–7. 10.1073/pnas.0510422103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silvis MR, Kreger BT, Lien WH, et al. : α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4(174):ra33. 10.1126/scisignal.2001823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piao H, Yuan Y, Wang M, et al. : α-catenin acts as a tumour suppressor in E-cadherin-negative basal-like breast cancer by inhibiting NF-κB signalling. Nat Cell Biol. 2014;16(3):245–54. 10.1038/ncb2909 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Lien W, Gelfand VI, Vasioukhin V: Alpha-E-catenin binds to dynamitin and regulates dynactin-mediated intracellular traffic. J Cell Biol. 2008;183(6):989–97. 10.1083/jcb.200805041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Choi SH, Estarás C, Moresco JJ, et al. : α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 2013;27(22):2473–88. 10.1101/gad.229062.113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Lien WH, Klezovitch O, Fernandez TE, et al. : alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311(5767):1609–12. 10.1126/science.1121449 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Schlegelmilch K, Mohseni M, Kirak O, et al. : Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144(5):782–95. 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Kim NG, Koh E, Chen X, et al. : E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108(29):11930–5. 10.1073/pnas.1103345108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Azzolin L, Panciera T, Soligo S, et al. : YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70. 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Barry ER, Camargo FD: The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25(2):247–53. 10.1016/j.ceb.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 49. Yu FX, Guan KL: The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–71. 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leung CY, Zernicka-Goetz M: Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nat Commun. 2013;4:2251. 10.1038/ncomms3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dupont S, Morsut L, Aragona M, et al. : Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Gaspar P, Tapon N: Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. 10.1016/j.ceb.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 53. Aragona M, Panciera T, Manfrin A, et al. : A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–59. 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Yu F, Zhao B, Panupinthu N, et al. : Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–91. 10.1016/j.cell.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Zhao B, Wei X, Li W, et al. : Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–61. 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Robinson BS, Moberg KH: Cell-cell junctions: α-catenin and E-cadherin help fence in Yap1. Curr Biol. 2011;21(21):R890–2. 10.1016/j.cub.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eckert F, Wolff H, Ring J, et al. : Das atypische Fibroxanthom. Hautarzt. 1990;41(1):39–42. [PubMed] [Google Scholar]
- 58. Heallen T, Zhang M, Wang J, et al. : Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–61. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Imajo M, Miyatake K, Iimura A, et al. : A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012;31(5):1109–22. 10.1038/emboj.2011.487 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Rosenbluh J, Nijhawan D, Cox AG, et al. : β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–73. 10.1016/j.cell.2012.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Gumbiner BM, Kim N: The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci. 2014;127(Pt 4):709–17. 10.1242/jcs.140103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yue T, Tian A, Jiang J: The cell adhesion molecule echinoid functions as a tumor suppressor and upstream regulator of the Hippo signaling pathway. Dev Cell. 2012;22(2):255–67. 10.1016/j.devcel.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Wei SY, Escudero LM, Yu F, et al. : Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8(4):493–504. 10.1016/j.devcel.2005.03.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Bossuyt W, Chen CL, Chen Q, et al. : An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene. 2014;33(10):1218–28. 10.1038/onc.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herr KJ, Tsang YN, Ong JW, et al. : Loss of α-catenin elicits a cholestatic response and impairs liver regeneration. Sci Rep. 2014;4:6835. 10.1038/srep06835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rauskolb C, Sun S, Sun G, et al. : Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158(1):143–56. 10.1016/j.cell.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Yang CC, Graves HK, Moya IM, et al. : Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proc Natl Acad Sci U S A. 2015;112(6):1785–90. 10.1073/pnas.1420850112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Cappello S, Gray MJ, Badouel C, et al. : Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat Genet. 2013;45(11):1300–8. 10.1038/ng.2765 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Van Hateren NJ, Das RM, Hautbergue GM, et al. : FatJ acts via the Hippo mediator Yap1 to restrict the size of neural progenitor cell pools. Development. 2011;138(10):1893–902. 10.1242/dev.064204 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Fan R, Kim NG, Gumbiner BM: Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110(7):2569–74. 10.1073/pnas.1216462110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Reddy BV, Irvine KD: Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24(5):459–71. 10.1016/j.devcel.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. White MD, Plachta N: How adhesion forms the early mammalian embryo. Curr Top Dev Biol. 2015;112:1–17. 10.1016/bs.ctdb.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 73. Chan SW, Lim CJ, Chong YF, et al. : Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286(9):7018–26. 10.1074/jbc.C110.212621 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Wang W, Huang J, Chen J: Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286(6):4364–70. 10.1074/jbc.C110.205401 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Zhao B, Li L, Lu Q, et al. : Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51–63. 10.1101/gad.2000111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Varelas X, Samavarchi-Tehrani P, Narimatsu M, et al. : The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19(6):831–44. 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Yi C, Shen Z, Stemmer-Rachamimov A, et al. : The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal. 2013;6(291):ra77. 10.1126/scisignal.2004060 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Hirate Y, Hirahara S, Inoue K, et al. : Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr Biol. 2013;23(13):1181–94. 10.1016/j.cub.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Yin F, Yu J, Zheng Y, et al. : Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154(6):1342–55. 10.1016/j.cell.2013.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation