Abstract
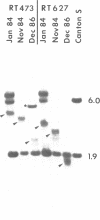
Terminal deficiencies at the tip of the X chromosome can be induced at a high frequency (0.2-0.3%) by irradiating Drosophila females carrying a homozygous mutator (mu-2) with low doses of X-rays. These terminal deficiencies are unstable, since over a period of 3 1/2 years DNA sequences were lost from their distal ends at a rate of 75 bp per generation, presumably due to the absence of a complete wild-type telomeric structure. Breakpoints of these deletions in the 5' upstream regulatory region of the yellow gene, giving rise to a mosaic cuticle pigmentation pattern typical of the y2 type, were used to define the location of tissue-specific cis-acting regulatory elements that are required for body, wing or bristle pigmentation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Jones J., O'Dell M., Thompson R. D., Flavell R. B. A molecular description of telometic heterochromatin in secale species. Cell. 1980 Feb;19(2):545–560. doi: 10.1016/0092-8674(80)90529-2. [DOI] [PubMed] [Google Scholar]
- Berman J., Tachibana C. Y., Tye B. K. Identification of a telomere-binding activity from yeast. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3713–3717. doi: 10.1073/pnas.83.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Biessmann H. Molecular analysis of the yellow gene (y) region of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7369–7373. doi: 10.1073/pnas.82.21.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Carson M. J., Hartwell L. CDC17: an essential gene that prevents telomere elongation in yeast. Cell. 1985 Aug;42(1):249–257. doi: 10.1016/s0092-8674(85)80120-3. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Chia W., Howes G., Martin M., Meng Y. B., Moses K., Tsubota S. Molecular analysis of the yellow locus of Drosophila. EMBO J. 1986 Dec 20;5(13):3597–3605. doi: 10.1002/j.1460-2075.1986.tb04688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison D., Chapman C. H., Wedeen C., Bingham P. M. Genetic and physical studies of a portion of the white locus participating in transcriptional regulation and in synapsis-dependent interactions in Drosophila adult tissues. Genetics. 1985 Jul;110(3):479–494. doi: 10.1093/genetics/110.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. J., Hung M. C., Wensink P. C. Independent control elements that determine yolk protein gene expression in alternative Drosophila tissues. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1396–1400. doi: 10.1073/pnas.82.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M. Genetic control of chromosome breakage and rejoining in Drosophila melanogaster: spontaneous chromosome aberrations in X-linked mutants defective in DNA metabolism. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1377–1381. doi: 10.1073/pnas.76.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Spana C., Corces V. G. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 1986 Oct;5(10):2657–2662. doi: 10.1002/j.1460-2075.1986.tb04548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling D. E., Zakian V. A. Telomere proteins: specific recognition and protection of the natural termini of Oxytricha macronuclear DNA. Cell. 1986 Oct 24;47(2):195–205. doi: 10.1016/0092-8674(86)90442-3. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Thorburn P. C. Healing of broken linear dicentric chromosomes in yeast. Genetics. 1984 Feb;106(2):207–226. doi: 10.1093/genetics/106.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Thorburn P. C., Rogers D. Meiotic and mitotic behavior of dicentric chromosomes in Saccharomyces cerevisiae. Genetics. 1984 Feb;106(2):185–205. doi: 10.1093/genetics/106.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Flavell R. B. Chromosomal structure and arrangement of repeated DNA sequences in the telomeric heterochromatin of Secale cereale and its relatives. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1209–1213. doi: 10.1101/sqb.1983.047.01.136. [DOI] [PubMed] [Google Scholar]
- Kitani T., Yoda K., Okazaki T. Discontinuous DNA replication of Drosophila melanogaster is primed by octaribonucleotide primer. Mol Cell Biol. 1984 Aug;4(8):1591–1596. doi: 10.1128/mcb.4.8.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO J. 1985 Dec 16;4(13A):3489–3499. doi: 10.1002/j.1460-2075.1985.tb04108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig A. J., Petes T. D. Identification of yeast mutants with altered telomere structure. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1398–1402. doi: 10.1073/pnas.83.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Strobel E., Green M. M. mu-2: mutator gene in Drosophila that potentiates the induction of terminal deficiencies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6090–6094. doi: 10.1073/pnas.81.19.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The Behavior in Successive Nuclear Divisions of a Chromosome Broken at Meiosis. Proc Natl Acad Sci U S A. 1939 Aug;25(8):405–416. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The Fusion of Broken Ends of Chromosomes Following Nuclear Fusion. Proc Natl Acad Sci U S A. 1942 Nov;28(11):458–463. doi: 10.1073/pnas.28.11.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash W. G. Patterns of pigmentation color states regulated by the y locus in Drosophila melanogaster. Dev Biol. 1976 Feb;48(2):336–343. doi: 10.1016/0012-1606(76)90095-6. [DOI] [PubMed] [Google Scholar]
- Nash W. G., Yarkin R. J. Genetic regulation and pattern formation: a study of the yellow locus in Drosophila melanogaster. Genet Res. 1974 Aug;24(1):19–26. doi: 10.1017/s0016672300015044. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Forked, gypsys, and suppressors in Drosophila. Cell. 1985 Jun;41(2):429–437. doi: 10.1016/s0092-8674(85)80016-7. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Interactions among the gypsy transposable element and the yellow and the suppressor of hairy-wing loci in Drosophila melanogaster. Mol Cell Biol. 1986 Jan;6(1):47–53. doi: 10.1128/mcb.6.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Laurent M., Delinte K., Van Meirvenne N., Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983 Dec 10;11(23):8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz-Pohl R., Bialojan S. A DNA sequence of Drosophila melanogaster with a differential telomeric distribution. Chromosoma. 1984;89(3):206–211. doi: 10.1007/BF00295001. [DOI] [PubMed] [Google Scholar]
- Roberts P. A. In support of the telomere concept. Genetics. 1975 May;80(1):135–142. doi: 10.1093/genetics/80.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. Isolation of a telomeric DNA sequence from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1041–1046. doi: 10.1101/sqb.1978.042.01.104. [DOI] [PubMed] [Google Scholar]
- Saiga H., Edström J. E. Long tandem arrays of complex repeat units in Chironomus telomeres. EMBO J. 1985 Mar;4(3):799–804. doi: 10.1002/j.1460-2075.1985.tb03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Borst P. Structure of the growing telomeres of Trypanosomes. Cell. 1984 Feb;36(2):459–468. doi: 10.1016/0092-8674(84)90239-3. [DOI] [PubMed] [Google Scholar]
- Walmsley R. W., Chan C. S., Tye B. K., Petes T. D. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984 Jul 12;310(5973):157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- Young B. S., Pession A., Traverse K. L., French C., Pardue M. L. Telomere regions in Drosophila share complex DNA sequences with pericentric heterochromatin. Cell. 1983 Aug;34(1):85–94. doi: 10.1016/0092-8674(83)90138-1. [DOI] [PubMed] [Google Scholar]