Abstract
Gelastic epilepsy or laughing seizures have been historically related to children with hypothalamic hamartomas. We report three adult patients who had gelastic epilepsy, defined as the presence of seizures with a prominent laugh component, including brain imaging, surface/invasive electroencephalography, positron emission tomography, and medical/surgical outcomes. None of the patients had hamartoma or other hypothalamic lesion. Two patients were classified as having refractory epilepsy (one had biopsy-proven neurocysticercosis and the other one hippocampal sclerosis and temporal cortical dysplasia). The third patient had no lesion on MRI and had complete control with carbamazepine. Both lesional patients underwent resective surgery, one with complete seizure control and the other one with poor outcome. Although hypothalamic hamartomas should always be ruled out in patients with gelastic epilepsy, laughing seizures can also arise from frontal and temporal lobe foci, which can be surgically removed. In addition, we present the first case of gelastic epilepsy due to neurocysticercosis.
Keywords: Gelastic epilepsy, Gelastic seizures, Epilepsy surgery, Neurocysticercosis
1. Introduction
The term “gelastic epilepsy” derives from the Greek word gelos (laughter) and was introduced in 1957 by Daly and Mulder. It is used to name seizures characterized by sudden laughter attacks, out of social context, without any particular emotion like joy or happiness [1]. In 1971, Gascon and Lombroso suggested as diagnostic criteria the presence of stereotyped laughter episodes in the absence of external triggers that can be associated with other epileptic manifestations, ictal or interictal discharges on the electroencephalogram (EEG), and absence of other conditions that could explain the pathologic laughter [2]. The vast majority of gelastic epilepsy series reported include only children, and they have found an association with hypothalamic hamartomas, a lesion that therefore must be always ruled out in these patients. However, other lesions and localizations have also been reported, mainly in other adult patients [3], [4], [5]. Here, we describe three patients with gelastic seizures (GS) with no evidence of hypothalamic hamartomas.
1.1. Case 1
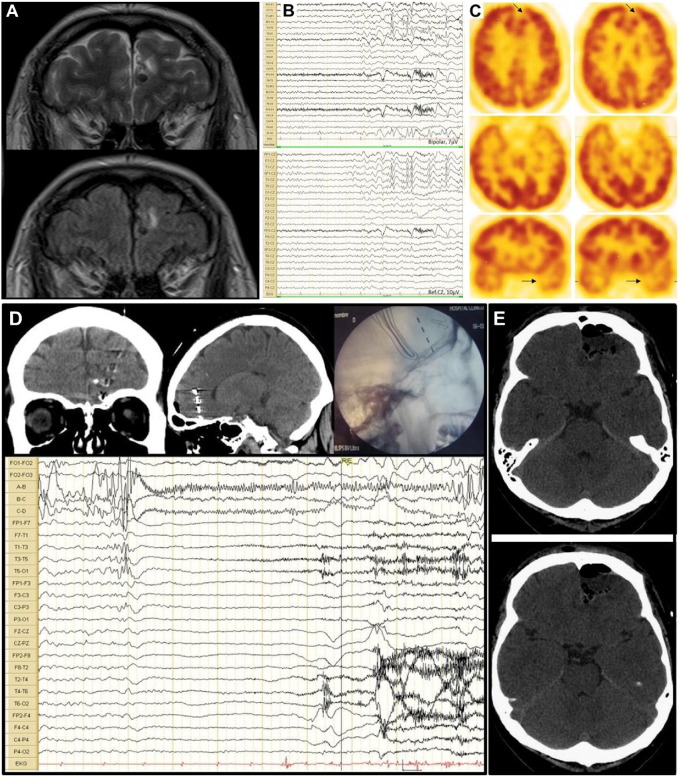
A 35-year-old man with a history of neurocysticercosis was treated with antiparasitic drugs as a teenager. Since the age of 29 years, he had almost daily spells characterized by sudden unmotivated laughter attacks which were described by his relatives since the patient was not aware. In addition, he had occasional seizures which started with forced head version towards the right, followed by right upper and lower limb jerks, and finally secondary generalization. He received carbamazepine, topiramate, and levetiracetam with poor response. Magnetic resonance imaging (MRI) showed upper right and inferior left mesial frontal lobe nodular lesions, consistent with calcified neurocysticercosis (Fig. 1A). Five GS were recorded on scalp video-EEG monitoring, all of them showing ictal patterns arising from the left anterior temporal lobe (Fig. 1B). Positron emission tomography (PET) was performed, showing left mesial frontal and anterior temporal hypometabolism (Fig. 1C). Neuropsychological evaluation showed bilateral frontotemporal alterations with left hemisphere predominance. Invasive recordings were performed with left frontal deep electrodes and foramen ovale electrodes, and ictal and interictal activities arising from the left mesial frontal region was demonstrated (Fig. 1D). Resection of the left frontal pole, which included the lesion, was performed (Fig. 1E), and the biopsy showed cysticercosis. The patient has been seizure-free since the surgery (5 years of follow-up) and under no medication for 3 years (ILAE class I).
Fig. 1.
MRI, scalp EEG, PET and invasive EEG monitoring of case 1.
A. MRI T2-weighted and FLAIR showing neurocysticercosis lesions in both frontal lobes. B. Surface EEG recording showing left anterior temporal onset of gelastic seizure on bipolar montage and referenced to Cz. C. FDG-PET showing left mesial frontal and temporal hypometabolism (arrows). D. Depth electrode EEG recording showing left mesial frontal onset of the gelastic seizure (electrodes A–D vs electrodes on both foramen ovale FO1–2). E. Head CT showing left frontal pole resection.
1.2. Case 2
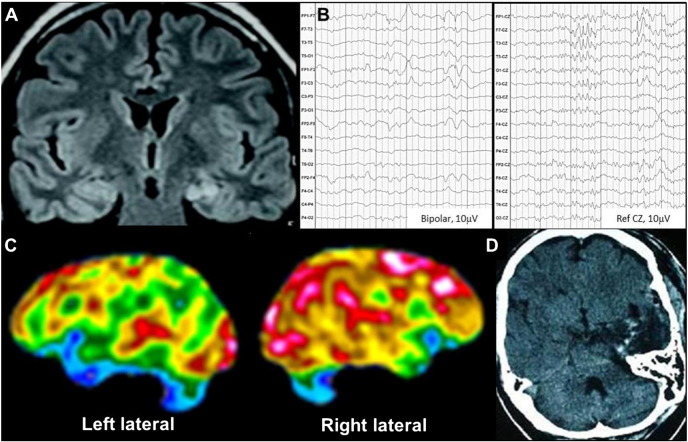
A 45-year-old man with epilepsy since the age of 14 years had seizures characterized by sudden laughter attacks, associated with feelings of joy and happiness, which lasted about 30 s, occasionally associated with disconnection followed by one to two minutes of postictal confusion. He had up to 5 episodes per day in spite of multiple antiepileptic treatments (carbamazepine, phenobarbital, levetiracetam, and topiramate). His brain MRI showed left mesial temporal sclerosis (Fig. 2A); surface EEG recordings showed left frontotemporal interictal epileptiform discharges (Fig. 2B); two gelastic seizures with left frontotemporal ictal onset were recorded. Fluorodeoxyglucose Positron Emission Tomography showed left anteromesial temporal hypometabolism (Fig. 2C). Neuropsychological evaluation showed bilateral mesial and anterior temporal alterations with predominance of the left hemisphere. Left amygdalohippocampectomy was performed (Fig. 2D), and the biopsy showed hippocampal sclerosis associated with cortical dysplasia. The patient remained seizure-free for a year, and seizures relapsed afterwards, with a 50% reduction compared to presurgical average (ILAE class IV).
Fig. 2.
MRI, surface EEG and PET of Case 2.
A. MRI FLAIR showing left mesial temporal sclerosis. B. Surface EEG recording showing left frontotemporal interictal discharges (bipolar and CZ reference montage). C. FDG-PET showing left mesial temporal hypometabolism. D. Head CT showing left temporal pole resection with amygdalohippocampectomy.
1.3. Case 3
A 42-year-old man had no relevant medical history. At 15 years old, he experienced brief laughter attacks, apparently unmotivated, that was perceived as strange behavior by his family. Gelastic epilepsy was diagnosed, and the patient was placed on carbamazepine; the treatment was stopped after a seizure-free period of 6 years. Five years after the medication withdrawal, he began again with episodes of unmotivated laughter, with no emotional correlate, associated with disconnection. Routine EEGs and brain MRI were normal. Carbamazepine was restarted with complete control of the spells except during a few poor adherence periods.
2. Discussion
Gelastic seizures are seen in less than 1% of all epilepsies [6], and they are mainly associated with hypothalamic hamartomas in children. Usually, these seizures begin during infancy, even in the neonatal period, with a progressive course which may include other focal or generalized seizures. Surface EEG is normal in most cases. Surgical or radiosurgical treatment of the hamartoma produces a dramatic improvement [7]. In addition, these children may also have precocious puberty and cognitive impairment [8]. Invasive EEG recordings and electrical stimulation have shown that gelastic seizures originated from the hypothalamic hamartomas, although they can arise from other lesions, like tumors, malformations of cortical development, tuberous sclerosis, and postinfectious foci [4]. To the best of our knowledge, this is the first report of neurocysticercosis related to GS.
In patients who have gelastic seizures without an impairment of consciousness, laughter is described as unmotivated or emotion-free, which could be interpreted as a dissociation between the motor and emotional components of laughter, although the physiological basis of joy and laughter are not fully understood. Arroyo et al. [9] described their experience with three patients. One of them had a lesion in the left superior mesial frontal region and experienced GS not accompanied by joy. Ictal subdural recordings showed that the seizures began in the left anterior cingulate gyrus. The other two patients had complex partial seizures originating from the temporal lobe; in these cases, electrical stimulation of the fusiform and parahippocampal gyri produced bursts of laughter accompanied by a feeling of mirth. The authors concluded that the anterior cingulate region is involved in the motor aspects of laughter, while the basal temporal cortex is involved in the processing of joy. These findings are also consistent with a case described by Coria et al. [10] in a patient who had a right lateral basal temporal lesion and experienced joy associated with gelastic seizures.
There are few publications on GS with frontal foci. Intracranial recordings and cortical electrical stimulation have shown involvement of the mesial and lateral superior frontal, cingulate and orbitofrontal gyri [3]. Interestingly, in a case studied by Chassagnon et al. [11] with stereo-EEG, a nonlesional patient became seizure-free after radiofrequency stimulation, resulting in two distinct lesions in the left superior frontal and cingulate gyri.
In our first patient, GS were associated with motor signs but not with emotion, therefore suggesting a frontal lobe origin. Although the surface EEG showed left anterior temporal ictal onset, both the MRI and PET showed left mesial frontal and anterior temporal changes. Invasive EEG recordings localized the left mesial frontal lobe as the ictal onset area, which was assumed to be the probable epileptogenic area, which was confirmed by the good surgical outcome.
In the second case, the structural and functional neuroimaging and the surface EEG were consistent with a temporal origin of the seizures. This patient had, during the seizure, a sense of joy, similar to the cases reported by Iwasa et al. [12] which originated from the right or left mesial–basal temporal regions. Our patient initially reached seizure-freedom for a year, although a long-term reduction of 50% compared to presurgical status was finally reached. This was probably due to an incomplete resection of a cortical dysplasia associated with the hippocampal sclerosis.
Unlike the previous cases, our last patient had unremarkable imaging or EEG findings and responded well to antiepileptic drugs. Although there were no ictal recordings that could confirm the topographical origin of the seizures, a hypothalamic onset is unlikely. Since the patient has had complete seizure control with antiepileptic drugs, further studies were not indicated.
3. Conclusions
Although gelastic epilepsy has been associated with hypothalamic hamartomas in children, other localizations, including frontal and temporal lobe foci, are more frequent among adult patients [4], [5]. Gelastic epilepsy is usually refractory, and surgery may be a useful tool, as reported in two of our cases.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Daly D.D., Mulder D.W. Gelastic epilepsy. Neurology. 1957;7:189–192. doi: 10.1212/wnl.7.3.189. [DOI] [PubMed] [Google Scholar]
- 2.Gascon G.G., Lambroso C.T. Epileptic (gelastic) laughter. Epilepsia. 1971;12:63–76. doi: 10.1111/j.1528-1157.1971.tb03916.x. [DOI] [PubMed] [Google Scholar]
- 3.Unnwongse K., Wehner T., Bingaman W., Foldvary-Schaefer N. Gelastic seizures and the anteromesial frontal lobe: a case report and review of intracranial EEG recording and electrocortical stimulation case studies. Epilepsia. 2010;51:2195–2198. doi: 10.1111/j.1528-1167.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 4.Kovac S, Diehl B, Wehner T, et al. Gelastic seizures: incidence, clinical and EEG features in adult patients undergoing video-EEG telemetry. Epilepsia, 56: e1–e5. doi: http://dx.doi.org/10.1111/epi.12868. [DOI] [PubMed]
- 5.Savasta S., Budetta M., Spartà M.V., Carpentieri M.L., Trasimeni G., Zavras N. Gelastic epilepsy without hypothalamic hamartoma: three additional cases. Epilepsy Behav. 2014;37:87–90. doi: 10.1016/j.yebeh.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Assal G., Majdalani A., Gautier J.C. Epilepsie gelastique. Probable hamartome diencephalique. Rev Neurol. 1993;149:291–293. [PubMed] [Google Scholar]
- 7.Parvizi J., Le S., Foster B.L., Bourgeois B., Riviello J.J., Prenger E. Gelastic epilepsy and hypothalamic hamartomas: neuroanatomical analysis of brain lesions in 100 patients. Brain. 2011;134:2960–2968. doi: 10.1093/brain/awr235. [DOI] [PubMed] [Google Scholar]
- 8.Berkovic S.F., Arzimanoglou A., Kuzniecky R., Harvey A.S., Palmini A., Andermann F. Hypothalamic hamartoma and seizures: a treatable epileptic encephalopathy. Epilepsia. 2003;44:969–973. doi: 10.1046/j.1528-1157.2003.59102.x. [DOI] [PubMed] [Google Scholar]
- 9.Arroyo S., Lesser R.P., Gordon B., Uematsu S., Hart J., Schwerdt P. Mirth, laughter and gelastic seizures. Brain. 1993;116:757–780. doi: 10.1093/brain/116.4.757. [DOI] [PubMed] [Google Scholar]
- 10.Coria F., Bahillo Marcos E., Moral Blanco M., García Gutiérrez P., Ortiz Sáenz de Santa María R. Late onset isolated gelastic epilepsy secondary to entrapment of the right temporal horn. Neurologia. 2000;15:204–207. [PubMed] [Google Scholar]
- 11.Chassagnon S., Minotti L., Kremer S., Foldvary-Schaefer N., Verceuil L., Hoffmann D., Benabid A.L. Restricted frontomesial epileptogenic focus generating dyskinetic behavior and laughter. Epilepsia. 2003;44:859–863. doi: 10.1046/j.1528-1157.2003.60802.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwasa H., Shibata T., Mine S., Koseki K., Yasuda K., Kasagi Y. Different patterns of dipole source localization in gelastic seizure with or without a sense of mirth. Neurosci Res. 2002;43:23–29. doi: 10.1016/s0168-0102(02)00012-3. [DOI] [PubMed] [Google Scholar]