Abstract
The treatment of complex fistulae-in-ano is challenging and often includes a number of operations due to high rates of recurrence. We report the successful treatment of three consecutive patients with long-standing cryptoglandular fistula-in-ano with a novel combination of mucosal advancement flap and adipose-tissue derived regenerative cells (ADRCs) from the stromal vascular fraction (SVF) obtained from a simple lipoaspiration procedure, using Celution technology. There was no operative morbidity; one patient who had a colostomy for faecal diversion has since undergone restoration of bowel continuity. All thee fistulae remain healed at 2–3-year follow-up. Lipofilling of cryptoglandular fistulae-in-ano with ADRC-enhanced lipofilling appears feasible and safe, and may add to the range of procedures that can be used to treat this difficult problem.
Background
‘Complex’ fistulae-in-ano are those fistulae that can not be treated with a laying-open procedure,1 due to the risk of incontinence posed by division of a portion of the sphincter complex.2 Common aetiologies of complex fistulae are cryptoglandular infection, Crohn's disease and iatrogenic causes. Aim of the surgical management of fistula-in-ano is to eradicate or obliterate the fistulous tract while maintaining continence. Following treatment of underlying sepsis and drainage of associated collections, definitive management strategies include both surgical closure with a mucosal advancement flap,3 ligation of intersphincteric fistula tract,4 or video-assisted anal fistula treatment,5 and the use of biological fillers including fibrin glue6 or fistula plugs.7 Recently, Garcia-Olmo et al8 combined fistula glue with in vitro cultured adipose tissue-derived mesenchymal stem cells (MSC), achieving initial success in more than 70% but, in the group of patients with initial closure, only 58% long-term closure.9 In this paper, we describe a novel technique using autologous transplantation of adipose-tissue derived regenerative cells (ADRCs) from lipoaspirate using the Celution system, without need for in-vitro cell enhancement.
Case presentation
Patient 1
A 49-year-old otherwise fit male patient, who had a longstanding history of persistent crypto-glandular posterior horse-shoe trans-sphincteric fistula-in-ano, which initially presented with recurrent ischio-rectal abscesses 6 years earlier. After initial attempts to treat the fistula with simple partial laying-open procedures, recurrent deep abscess formation in the ischio-rectal fossa required the placement of a seton for drainage and the formation of a laparoscopic-assisted diversion loop colostomy in 3 years prior to ADRC treatment. Assessment for underlying Crohn's disease was negative, and ongoing sepsis and recurrence of ischio-rectal collections continued to preclude definitive treatment. Following further operative assessment, partial laying-open of the septic foci and placement of a seton in the remaining fistula tract between the external opening at 9 o'clock in Lithotomy position and the internal opening in the upper anal canal at 6 o'clock resulted in clinical control of the sepsis 1 year prior to the ADRC treatment.
Patient 2
A 46-year-old female patient with a background of Chronic Obstructive Airways Disease, Peptic Ulcer Disease and Wolf-Parkinson-White syndrome, who had been diagnosed with a left-sided ischio-rectal abscess almost 3 years prior to ADRC treatment; following initial drainage of the abscess, a high trans-sphincteric fistula-in-ano was confirmed and symptomatically treated with a long-standing lose seton 2 years prior to ADRC treatment.
Patient 3
A 55-year-old otherwise fit male patient, who presented initially with a large right-sided ischio-rectal abscess 3 years prior to ADRC treatment. Following initial drainage of the abscess, re-examination underanaesthesia failed to find a complete fistula-in-ano, and a peri-anal sinus was excised. However, recurrence of symptoms prompted imaging with MRI, which confirmed the presence of a high-signal trans-sphincteric tract from the 9 o'clock position externally to a posterior internal opening with horse-shoe extension to the left side. With subsequent insertion of a seton, the left sided extension healed both clinically and radiologically, leaving the right-sided fistula persisting when ADRC treatment was initiated.
Investigations
Patient 1
Prior to ADRC treatment, the patient's fistulating disease was repeatedly evaluated both with assessment under general anaesthesia and MRI. The latter repeatedly confirmed the presence of persistent posterior fistula tracts with ongoing sepsis.
Patient 2
The patient's fistula was repeatedly examined under anaesthesia, together with changes of the indwelling seton drain. An MRI 2 years prior to ADRC treatment confirmed a high-signal tract from the left ischio-rectal fossa at the 3 o'clock position to the internal opening in the upper anal canal; at this point, following drainage of the initial abscess and prior to the insertion of seton, no collections were found.
Patient 3
The patient was repeatedly examined under anaesthesia, and had three preoperative MRI scans that demonstrated persistence of a right-sided fistula, but resolution of the extension into the left ischio-rectal fossa.
Differential diagnosis
All patients had causes other than cryptoglandular infection sufficiently excluded (ie, Crohn's disease and tuberculosis).
Treatment
Following informed consent to participate in a locally funded feasibility study, approved by our institution's research and development department and the regional ethics committee on the basis of the evidence published by Garcia-Olmo,8 all three patients were routinely assessed for operative fitness. In all cases, absence of acute sepsis and infected collections was confirmed by clinical and radiological assessment prior to the procedure. The procedure for all patients can be outlined as follows.
Lipoaspiration
Under local or general anaesthesia, a manual liposuction procedure is carried out through two small bilateral flank incisions. Initially, approximately 200 ml of a solution containing 1000 ml of normal saline solution, 2 ml epinephrine 1 : 1000, 50 ml of 1% lidocaine and 1500 units hyaluronidase is injected to allow tumescence of fat with minimal blood loss. A hollow blunt-tipped canula size 3–4 is moved rapidly back-and-forth through the anterior adipose compartment of the abdominal wall to disrupt the fatty stromal tissue and obtain approximately 300–400 ml raw lipoaspirate. Following this, the harvest site in the abdominal wall is covered with a pressure dressing for 10 days to reduce haematoma formation.
Celution technique
The raw lipoaspirate is transferred into the tissue collection chamber of the Celution 800/CRS system (figure 1; Cytori Therapeutics Inc, San Diego, California, USA), washed to remove free blood and lipid, and digested with the proprietary enzyme reagent Celase 835/CRS (Cytori Therapeutics Inc) to release the stromal vascular fraction (SVF). The SVF is concentrated by short centrifugation and automated wash cycles to obtain the ADRC fraction, which is then taken out of the automated 90–120 min long cycle of the Celution system and mixed with the supernatant lipid fraction of the lipoaspirate to a volume of 20–50 ml depending on the estimated required volume for injection and obliteration of the fistulous tract(s).
Figure 1.
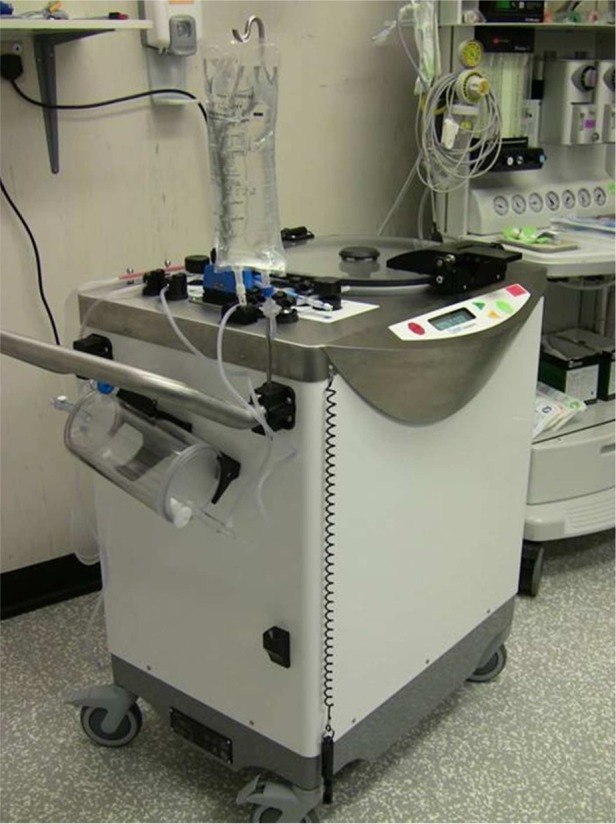
Celution 800/CRS system.
Autologous cell-transplant to fistula-in-ano
All patients are reanaesthetised and placed in lithotomy position. Fistula tracts are identified, and debrided or excised with minimal tissue loss. The internal opening(s) are closed, usually with mucosal advancement flaps. The previously prepared 5 ml ADRC solution is combined with 20–30 ml of raw lipoaspirate and injected, employing a criss-cross lattice technique, into the fistula(e) and surrounding tissues to achieve maximal tissue density and filling of adjacent tissue spaces (figure 2). The external opening is obliterated by peri-orifice tissue bulking injection.
Figure 2.
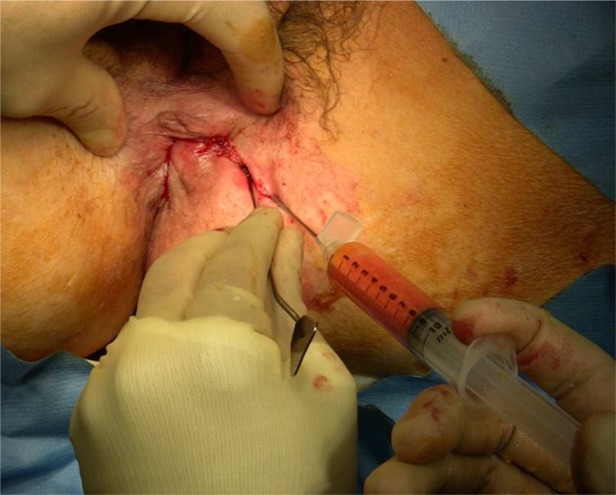
Injection of adipose-tissue derived regenerative cells with fresh lipoaspirate into the peri-fistular tissue.
Postoperative care
Postoperatively, patients are discharged from inpatient care as indicated, and are advised to keep the abdominal pressure dressing in place for 10 days when it is removed at first outpatient review. Patients routinely receive laxatives and adequate analgesia, and were followed up at regular intervals (10 days following surgery, then 6-weekly intervals for of 3 months and 3-monthly intervals thereafter to 1 year), using clinical examination and—as required—MRI for assessment of progress. The fistulae were considered healed if the external and internal orifices were closed, with no clinical and/or radiological evidence of ongoing sepsis or collection.
Outcome and follow-up
Patient 1
At 3 months from ADRC treatment, the patient underwent examination under anaesthesia, with minor curettage of site of the internal opening. After reassessment at 6 months, the patients underwent reversal of his colostomy 10 months following ADRC procedure. The fistula remains healed 30 months postsurgery, with no incontinence.
Patient 2
At 4 months from ADRC treatment, the fistula was clinically healed; this was confirmed by MRI scan at 1-year follow-up. There was no sign of faecal incontinence.
Patient 3
At 5 months from ADRC treatment, the fistula appeared completely healed on clinical examination (figure 3); MRI at 9 months confirmed absence of collections or fistulae, although a thin transsphincteric high-signal area remained visible. However, the patient remains clinically asymptomatic, although occasional minor incontinence for liquid has been recorded.
Figure 3.
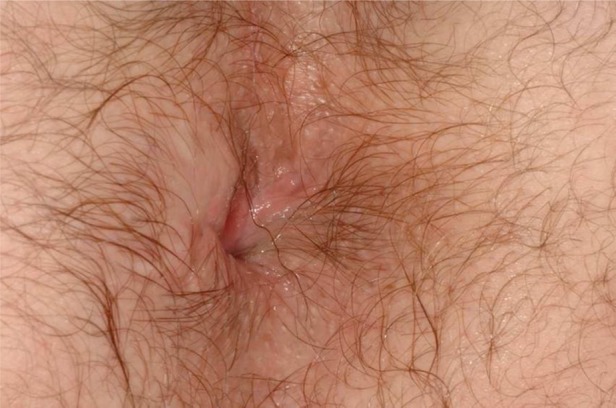
Patient 3: final result after 5 months.
Discussion
In recent years, advances in the field of regenerative medicine have made a large array of new treatments available for the management of many medical and surgical conditions. Adult MSC offer the possibility of extensive tissue renewal,10 with a substantial proportion of these cells found in human adipose tissue (so-called adipose tissue-derived stem cells, ADSC).11 ADSC have the ability to differentiate into a large variety of cell types, including connective, endothelial, muscle and scar tissue.12 Whereas ADSC can be isolated and cultured in-vitro from the SVF of liposuction-harvested adipose tissue, which is processed in a sequence of washing, enzymatic lipolysis and filtration, it has been shown that the non-cultured SVF itself contains a rich population consistent of endothelial progenitor cells, tissue macrophages and smooth muscle cells,13 now referred to as ADRC. It is estimated that ADRC amount to approximately 1% of all nucleated cells in the lipoaspirate, more than 100-fold the yield obtained from bone marrow aspiration.13
The technique reported here is a modification of that described by Garcia-Olmo,14 who used a combination surgical closure of the internal opening and injection of fistula glue with in-vitro expanded ADSCs. It was developed based on our own experience in the field of ADRC-enhanced reconstructive lipomodelling for postsurgical soft-tissue defects in the breast, which is now a recognised15 and trialled16 technique that has a high level of patient safety due to its autologous and real-time application. We used the automated process offered by the Celution system to obtain the SVF of the lipoaspirate; the cells of the SVF are available immediately for autotransplantation. The Celution system is a closed system using single-use disposables, thus significantly reducing the risk of contamination and mutation posed by a lengthy process of in-vitro cell culture and enhancement. While the Celution process is comparatively more costly than other treatment options (overall cost is estimated at around £4500/€5400/$7100, with £1450/€1750/$2300 attributable to the additional disposables), the increasing availability of the Celution system for plastic and reconstructive surgery and the potential long-term benefit and cost-savings for patients who otherwise require additional subsequent treatments are likely to offset some of these costs.
The success seen in our initial three patients may indicate that there is a possible role for the use of ADRC in combination with surgical intervention for complex fistulae-in-ano; however, larger comparative studies are required to evaluate this treatment further.
Learning points.
Treatment of complex fistulae-in-ano is challenging and no overall successful treatment has been described.
A combination of mucosal advancement flap with autologous treatment with adipose-tissue derived regenerative cells from the adipose stromal vascular fraction may add to the range of procedures available for the treatment of complex fistulae-in-ano.
Further evaluation of this technique is required in larger studies, also including patients with other underlying pathology, such as inflammatory bowel disease.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg 1976;63:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Aguilar J, Belmonte C, Wang WD, et al. Anal fistula surgery: factors associated with recurrence and incontinence. Dis Colon Rectum 1996;39:723–9. [DOI] [PubMed] [Google Scholar]
- 3.Hyman NH. Endoanal advancement flap repair for complex anorectal fistulas. Am J Surg 1999;178:337–40. [DOI] [PubMed] [Google Scholar]
- 4.Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol 2009;13:237–40. [DOI] [PubMed] [Google Scholar]
- 5.Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol 2011;15:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel ME, Chiu YS, Russell TR, et al. Autologous fibrin glue in the treatment of rectovaginal and complex fistulas. Dis Colon Rectum 1993;36:447–9. [DOI] [PubMed] [Google Scholar]
- 7.Corman ML. The surgisis AFP anal fistula plug: report of a consensus conference. Colorectal Dis 2007;10:17–20. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Olmo D, Herreros D, Pascual M, et al. Treatment of enterocutaneous fistula in Crohn's disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis 2009;24:27–30. [DOI] [PubMed] [Google Scholar]
- 9.Guadalajara H, Herreros D, De-La-Quintana P, et al. Long-term follow-up of patients undergoing adipose-derived adult setm cell administration to treat complex perianal fistulas. Int J Colorectal Dis 2012;27:595–600. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1990;284:143–7. [DOI] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211–28. [DOI] [PubMed] [Google Scholar]
- 12.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 2005;54:132–41. [DOI] [PubMed] [Google Scholar]
- 13.Lin K, Matsubara Y, Masuda Y, et al. Characterisation of adipose tissue-derived cells isolated with the Celution system. Cytotherapy 2008;10:417–26. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005;48:1416–23. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Clinical Excellence (NICE). Breast reconstruction using lipomodelling after breast cancer treatment—IP 417. 2012. [Google Scholar]
- 16.Perez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative caell (ADRC)-enhanced fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol 2012;38:382–9. [DOI] [PubMed] [Google Scholar]


