Abstract
The murine MI model is widely recognized in the field of cardiovascular disease, and has consistently been used as a first step to test the efficacy of treatments in vivo1. The traditional, established protocol has been further fine-tuned to minimize the damage to the animal. Notably, the pectoral muscle layers are teased away rather than simply cut, and the thoracotomy is approached intercostally as opposed to breaking the ribs in a sternotomy, preserving the integrity of the ribcage. With these changes, the overall stress on the animal is decreased.
Stem cell therapies aimed to alleviate the damage caused by MIs have shown promise over the years for their pro-angiogenic and anti-apoptotic benefits. Current approaches of delivering cells to the heart surface typically involve the injection of the cells either near the damaged site, within a coronary artery, or into the peripheral blood stream2-4. While the cells have proven to home to the damaged myocardium, functionality is limited by their poor engraftment at the site of injury, resulting in diffusion into the blood stream5. This manuscript highlights a procedure that overcomes this obstacle with the use of a cell-encapsulated hydrogel patch. The patch is fabricated prior to the surgical procedure and is placed on the injured myocardium immediately following the occlusion of the left coronary artery. To adhere the patch in place, biocompatible external fibrin glue is placed directly on top of the patch, allowing for it to dry to both the patch and the heart surface. This approach provides a novel adhesion method for the application of a delicate cell-encapsulating therapeutic construct.
Keywords: Medicine, Issue 100, Myocardial infarction, stem cell therapy, hydrogel, fibrin-based glue, cell-encapsulated patch, hydrogel adhesion
Introduction
A myocardial infarction (MI) is defined as the interruption of blood to a region of the heart caused by the occlusion of a major coronary artery. The damage resulting from an MI is due to the remodeling of the viable heart tissue into non-functional scar tissue, which decreases the ability of the heart or, more specifically, the left ventricle, to beat properly. This results in a decrease in the volume of blood that can be delivered to the body with every heartbeat, known as the stroke volume, and the percentage of blood that is pumped out of the heart with each heartbeat, known as the ejection fraction6. These, along with other diminished functions, increases the strain on the rest of the heart to maintain adequate function. Often, this increased strain can become so severe that it causes a second heart attack, a phenomenon seen in approximately 10% of individuals7.
While medical practices have evolved to treat the immediate aftermath of an MI, no technique has been developed to halt, slow, or reverse the negative side effects of tissue remodeling. Stem cell therapies have emerged as a possible avenue for such a treatment, however, despite their promising potential, stem cells have not proven successful in the clinical setting. One theory for their shortcomings is the inability to ensure the beneficial cells remain at the site of infarction long enough to generate favorable results5. It has been shown that no more than 24% of cells that are simply injected into the site of infarction survived and remained at the damaged site 1 day post-delivery2. A possible prospect for addressing this issue of cell retention is to develop biocompatible hydrogel systems that encapsulate either cells or therapeutics, which can be delivered to the damaged site. The hydrogel of choice in this protocol is a poly(ethylene glycol) dimethacrylate due to its previous use in cell encapsulation procedures, however, any hydrogel capable of encapsulation may be used8. The delivery of the patch directly to the site of injury ensures cell-to-tissue contact over an extended period of time, increasing the length of time the cells can provide beneficial factors to the underlying myocardium.
A bottleneck to the patch approach is the difficulty of adhering the patch to the heart surface. Many groups have overcome this through a variety of techniques, the most prevalent being a simple suture to tie the construct to the heart surface9,10. This has proven successful in a number of cases in which the construct is made of a stiffer material, but fails when attempted on a hydrogel system, due to the high water concentration and delicate nature of the patch construct. To overcome this, we have utilized a fibrin glue external adhesive system that mimics the chemistry of clot formation. Fibrin glue has been used in numerous medical surgeries, including dura tears, bronchial fistulas, and corneal transplantation, highlighting the biocompatibility of the product as a wound sealant11-13. Additionally, fibrin has been used for a variety of cardiac purposes, including surgical treatment of left ventricular ruptures and coronary artery bypass surgeries, however, its use as an adhesion glue for a cardiac patch is not commonly used14-17. A simple formulation of thrombin and fibrinogen results in a biocompatible glue that can be placed directly on the outside of an external cardiac patch, providing a viable adhesion system to ensure patch to heart interaction.
Protocol
Procedure is in full compatibility with IACUC protocol number 13302 and has been approved by the division of animal resources.
1. Instrument Preparation
Autoclave all non-disposable instruments used prior to the surgical procedure to ensure sterility.
Sterilize instruments used multiple times in a session with a glass bead sterilizer between uses.
2. Hydrogel Preparation
Thaw and plate stem cells at least 24 hr prior to creation of the hydrogel construct.
Prepare patches one day prior to the surgical procedure using a stereolithography apparatus (or similar instrument) which has been described in full detail in previous publications8,18.
To create the hydrogel, first generate a computer aided design (CAD) model of the desired patch and export the design to stereolithographic format (STL).
Create a pre-gel solution by dissolving poly(ethylene glycol) dimethacrylate (PEGDMA) in sterile 1x PBS to obtain a 20% w/v solution. Concurrently, dissolve the photoinitiator 1-[4-(2-hydroxy- ethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one in DMSO. Add the photoinitiator to the PEGDMA solution at a final w/v of 0.5%. Directly before crosslinking, add 100 ml of the desired density of cells. Note: A cell density between 2.0 x 105-2.0 x 106 cells/ml is recommended.
Pipette the pre-gel solution into a dish at the center of the SLA platform and run the SLA software with the previously uploaded STL formatted design.
Remove the patches from the apparatus and incubate the hydrogels O/N in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin to allow for cells to adjust to the environment within the patch.
3. Animal Preparation and Oral intubation
Evaluate each mouse prior to the procedure to ensure that the animal is in good health, as determined from their grooming habits and interactions with their cage mates. Note: Female 8-10 week old C57BL/6J mice were used for this procedure, however, any mouse age or type can be used.
Place animals into an anesthetic chamber and expose to 5% isofluorane with a 1 L/min O2 support. Monitor the level of anesthesia by toe pinch reflex.
Once the animal is properly anesthetized, weigh and place them on the intubation stand.
Direct a light source towards the animal’s chest cavity and use forceps to retract the laryngeal surface and expose the vocal cords. Carefully guide a 20 G angiocath between the vocal cords, and smoothly insert into the trachea. Detect proper insertion by a mechanical movement of the chest cavity once the catheter is connected to the small animal ventilator.
Adjust ventilation settings to the weight of the animal based on the manufacturer’s guidelines.
Once intubated, place the animal in the supine position on a heating pad to prevent hypothermia. Ensure that the heating pad does not exceed 40 °C. If necessary, place a barrier between the animal and the heating pad to minimize the chance of burns.
Remove hair from the surgical site through the use of a depilatory cream. Sterilize the site by 3 alternating scrubs of betadine and 75% ethanol to ensure proper sterilization. Place a drape to expose just the surgical site, and place vet ointment on the eyes to prevent dryness during the procedure.
4. Surgical Procedure
The thoracotomy is performed on the left side of the animal, therefore, make a skin incision approximately 1 cm to the left of the sternum, that runs the length of the sternum.
Use forceps to separate the muscle layer from the underlying ribcage in two steps. Observe a delineating line that represents the pectoralis major muscles of the animal. Lift this muscle slightly, separate it from the underlying external oblique muscle and retract medially. Then, free the external oblique muscle from the underlying ribcage in the same manner, and retract laterally, providing an unobscured view of the second, third, and fourth ribs.
Perform the thoracotomy between the third and fourth ribs. Gently lift the fourth rib and use a cauterizer to open the chest cavity between the third and fourth ribs. Place retractors to further open the cavity and expose the heart.
Use forceps to rupture the thin pericardium of the heart.
Ligate the left coronary artery with an 8-0 monofilament nylon suture. Place the suture approximately 4 mm from the apex of the heart, directly below the bottom tip of the left atrium. Determine proper suture placement by a blanching of the ventricular myocardium and an increase in the size of the left atrium after the suture has been tied.
5. Patch Placement and Glue
Keep patches at 37 °C and 5% CO2 conditions until use.
Gently lift the patch using a flat ended spatula and gently place it on the surface of the heart, at the site of infarction. Note: To prevent slipping off the left ventricle, the patch can be held in place by lightly maintaining contact using either the spatula or the tip of a pair of forceps.
Prepare the glue by mixing a 4:1 ratio of fibrinogen to thrombin. Mix the solution by repeated pipetting until it begins to thicken, usually within 1 min after the beginning of the preparation.
Once the solution viscosity reaches the desired level, quickly transfer approximately 10 ml to the patch surface. Note: The clotting time of the fibrin glue is rapid, providing a small window of opportunity for efficient transfer of the glue.
6. Suturing
Perform all closing sutures using a 6-0 monofilament nylon suture.
Close the rib layer with three to four individual interrupted sutures.
Before full closure of the intercostal layer, insert a PE-10 cannula into the incision to evacuate the chest cavity after closure is complete and reestablish proper intrapleural pressure.
Close the pectoral muscles with three to four individual interrupted sutures.
Seal the skin layer with a continuous suture.
Following full closure, attach a 1.0 ml syringe to the end of the cannula and use it to evacuate the chest cavity.
Apply a tissue adhesive to the incision site to reinforce the suture site.
7. Post-surgical Treatment
Give mice an injection of buprenorphine (0.05-1.0 mg/kg) and carprofen (2.2 mg/kg) subcutaneously immediately following the coronary ligation and least 20 min prior to revival. Administer both drugs 6-8 hr after surgery, and then give twice daily for up to three days to control pain and distress.
Continuously monitor the animals until conscious and every hour for the first 4 hr following surgery. Perform a second checkup the night of the surgery (2-4 hr later) to administer a second dose of analgesic.
Monitor the animals daily after the second day following the procedure, until animals appear stable.
8. Analysis of Heart Function and Histology
Perform echocardiographs on the mice 4 weeks following the procedure to determine the extent of damage following the MI.
Anesthetize mice with 4-5% isoflurane within an anesthesia chamber for induction of inhalation anesthesia, and then 2% isoflurane via face mask for maintenance anesthesia.
Clear the imaging area with a depilatory cream and use a small animal ultrasound to obtain a 2D M-mode echocardiography scan of the beating heart and record the functional parameters associated with it.
Euthanize the mice in a carbon dioxide chamber, then collect the hearts from the animals and place in a 10% formalin solution for 24 hr. After 24 hr, transfer the hearts to 70% ethanol and keep at 4 °C until tissue processing.
Process the hearts and embed them in paraffin blocks.
Cut 5 mm sections through the ventricular region of the heart, starting at the apex and ending at the atrium.
Stain sectioned slices with both Masson’s trichrome and hematoxylin and eosin stain using standard protocols.
Representative Results
During the surgical procedure, ligation of the left coronary artery can be identified by a marked blanching downstream of the occluded artery. As a test before tying the knot, the suture can be tightened briefly to check if it is in the appropriate place. Additionally, since occlusion of the artery results in almost instantaneous decrease in the ability of the left atrium to properly beat, the left atrium will enlarge in response to a backflow of blood in the system.
M-mode echocardiography measurements taken as early as 2 days post-infarction show a cessation of left wall movement, indicative of the muscle reconstruction. Qualitative calculations made from the data show a decrease in ejection fraction and stroke volume in the infarcted hearts. At the termination of the experiment, when the hearts are collected for histological purposes, a clear dilation of the left ventricle can be seen, along with a thinning of the left ventricular wall, and the deposition of collagen that denotes scar tissue deposition in place of functioning cardiomyocytes (Figure 1).
Also seen upon histological analysis is the presence of the hydrogel construct thanks to the administration of the fibrin gel (Figure 2). For our purposes, the adhesive system must be viscous enough to allow precise placement control and minimize subsequent runoff into surrounding organs, but malleable enough not to interfere with heart function. Preliminary tests were performed to calculate the viscosity, gelation time, and stiffness of various fibrinogen/thrombin ratios, in order to determine the proper combination that suits our needs. In vivo analysis was performed to test the ability of the fibrin glue to maintain patch-to-tissue adhesion while still allowing for full heart function (data not shown). It should be noted that the fibrin glue did not harm the myocardium, as evidenced by the lack of tissue remodeling or ventricular thinning at the site (Figure 2). The cardiomyocytes remain intact despite the addition of both a cardiac patch and its accompanying glue. Additionally, viability testing confirmed that that the administration of fibrin glue to the external surface of a cell-encapsulating hydrogel patch did not affect cell survival within the patch (Figure 3).
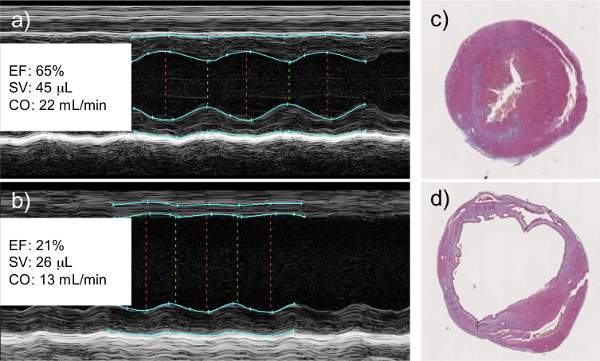 Figure 1: Left Coronary Artery Ligation Results in a Decrease in Cardiac Function as Measured by Echocardiography. M-mode echocardiography of healthy (a) and infarcted (b) hearts. Infarcted hearts show a clear lack of wall movement where viable tissue has been replaced with scar tissue. This correlates with a measureable decrease in left ventricular ejection fraction (EF), cardiac stroke volume (SV), and cardiac output (CO). Histological analysis of healthy (c) and infarcted (d) hearts shows dilation of the left ventricle and thinning of the left ventricular wall of the infarcted myocardium, both signs of tissue remodeling and scar tissue deposition. Histological images are cross sectional slices capturing the left (larger chamber) and right (smaller chamber) ventricles.
Figure 1: Left Coronary Artery Ligation Results in a Decrease in Cardiac Function as Measured by Echocardiography. M-mode echocardiography of healthy (a) and infarcted (b) hearts. Infarcted hearts show a clear lack of wall movement where viable tissue has been replaced with scar tissue. This correlates with a measureable decrease in left ventricular ejection fraction (EF), cardiac stroke volume (SV), and cardiac output (CO). Histological analysis of healthy (c) and infarcted (d) hearts shows dilation of the left ventricle and thinning of the left ventricular wall of the infarcted myocardium, both signs of tissue remodeling and scar tissue deposition. Histological images are cross sectional slices capturing the left (larger chamber) and right (smaller chamber) ventricles.
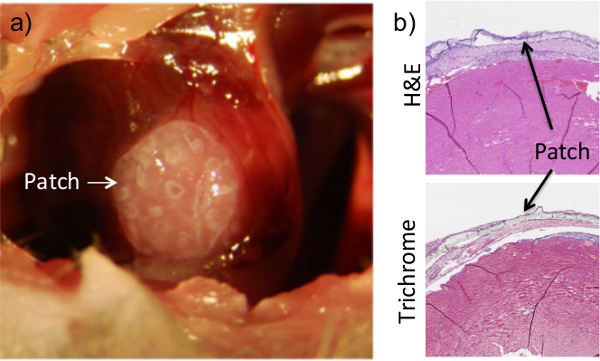 Figure 2: Application of Fibrin Flue Ensures Patch Adhesion to the Heart. The patch is placed on the surface of the heart while the chest cavity is exposed during the procedure (a). Once placed, a fibrin-based glue is added on top of the heart and allowed to dry. The patch can clearly be seen on histological sections of heart tissue that were collected at 1 week post-procedure (b). Dissociation of the patch from the heart surface in histological images is an artifact of the collection and sectioning process. Results are consistent for up to 8 weeks of adhesion.
Figure 2: Application of Fibrin Flue Ensures Patch Adhesion to the Heart. The patch is placed on the surface of the heart while the chest cavity is exposed during the procedure (a). Once placed, a fibrin-based glue is added on top of the heart and allowed to dry. The patch can clearly be seen on histological sections of heart tissue that were collected at 1 week post-procedure (b). Dissociation of the patch from the heart surface in histological images is an artifact of the collection and sectioning process. Results are consistent for up to 8 weeks of adhesion.
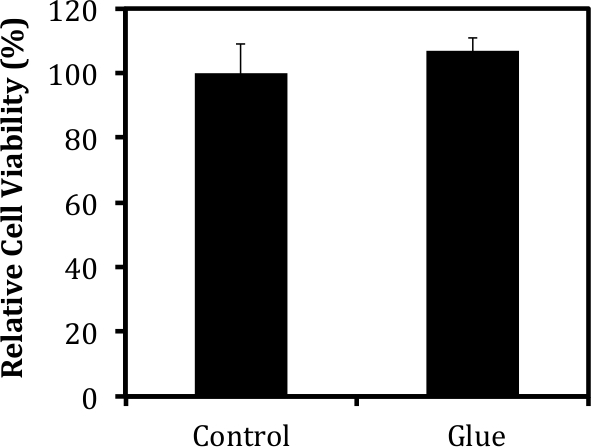 Figure 3: Fibrin-based Glue Does Not Harm Encapsulated Tissues. Cell viability was measured via MTT analysis 1 week after the administration of a fibrin-based glue using protocols described in detail in previous publications8. The viability of cells within the patch was not affected by the addition of the glue to the surface of the construct.
Figure 3: Fibrin-based Glue Does Not Harm Encapsulated Tissues. Cell viability was measured via MTT analysis 1 week after the administration of a fibrin-based glue using protocols described in detail in previous publications8. The viability of cells within the patch was not affected by the addition of the glue to the surface of the construct.
Discussion
With this approach to the murine MI model, we have developed a system that minimizes the damage to non-myocardium areas that are associated with other murine MI techniques. These areas include damage caused by a tracheostomy, the cutting of the muscular layer, and the breakage of ribs to expose the chest cavity. We believe that these changes improve the overall surgical outcome thanks to the care taken to keep as much of the major structures, including the ribs and muscle layers, intact during the surgical procedure.
It should be noted that there are some limitations to this technique, with the largest being its time-consuming nature, resulting in only 8-10 full surgical procedures in one day. To overcome this, a second ventilator can be used, allowing for the procedure to be performed on two animals concurrently. Additionally, major vessels, such as the intercostal vessels and interior thoracic artery, should be avoided when performing the thoracotomy. If near one of these vessels, a cauterizer should be used to prevent bleeding out due to a ruptured artery.
Fibrin glue is known in both the laboratory and clinical settings thanks to its ability to quickly aid in clot formation, as well as its potential to deliver and host MSCs14-17,19. For our purposes, fibrin glue served as a biocompatible approach to adhering a water-based hydrogel construct to the surface of the heart. The use of a fibrin-based glue has proven successful in keeping the hydrogel construct at the damaged site for up to 8 weeks, with the possibility for tissue contact of a longer amount of time. The use of this adhesion system allowed control for placement of the delicate construct without the need for additional sutures that harm the myocardium as well as the construct itself. We have found that the glue itself is non-toxic to the encapsulated cells and does not negatively affect the underlying tissue. The hydrogel and fibrin-based glue system can be applied to a variety of other tissue engineering purposes that desire cell or therapeutic contact for an extended period of time. Furthermore, with the use of the SLA fabrication method, designs and structures may be incorporated within the patch to create a more complex system for cell delivery18.
One setback to the use of the fibrin-based glue is the fact that more than one researcher must be present in the surgical room during patch placement and glue addition. It is the job of the first researcher to make sure the patch remains in place and does not get lost in the chest cavity, while the second researcher mixes the solution and applies it to the patch after proper viscosity is reached. To optimize the timing of the glue delivery, it is suggested that the second researcher also tests and times the gelation of the glue prior to patch placement, since proper gelation of the glue is critical for patch adhesion.
There are a number of critical steps and error prone areas within the protocol, with the first being the induction of the myocardial infarction. It is imperative to check for signs, such as the blanching of the left ventricular wall and the enlargement of the left atrium, to ensure occlusion of the left coronary artery. This can be done prior to the addition of the stitch by simply crossing and tightening the end of the suture strings. If it is determined that the left coronary artery is not enclosed by the suture, the string can be taken out and re-applied. A second critical measure is to continue to monitor the intubation tube throughout the procedure, to ensure that it remains within the trachea and does not get pulled out mid-surgery. Finally, throughout the surgery, all attempts should be made to avoid contact with the lungs, as any contact has the potential to lead to a collapsed lung. If, during the surgery, lung collapsing occurs, simply block the outflow tube of the intubator for up to 3 sec in an attempt to re-inflate troubled regions of the lungs.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was funded by US Army Grant (W81XWH-08-1-0701) and a Fellowship from Carle Foundation Hospital.
References
- Kolk MV, et al. LAD-ligation: a murine model of myocardial infarction. Journal of visualized experiments : JoVE. 2009. [DOI] [PMC free article] [PubMed]
- Li Y, Yao Y, Sheng Z, Yang Y, Ma G. Dual-modal tracking of transplanted mesenchymal stem cells after myocardial infarction. International journal of nanomedicine. 2011;6:815–823. doi: 10.2147/IJN.S17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. American journal of physiology. Heart and circulatory physiology. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- Wang JS, Shum-Tim D, Chedrawy E, Chiu RC. The coronary delivery of marrow stromal cells for myocardial regeneration: pathophysiologic and therapeutic implications. The Journal of thoracic and cardiovascular surgery. 2001;122:699–705. doi: 10.1067/mtc.2001.116317. [DOI] [PubMed] [Google Scholar]
- Cashman TJ, Gouon-Evans V, Costa KD. Mesenchymal stem cells for cardiac therapy: practical challenges and potential mechanisms. Stem cell reviews. 2013;9:254–265. doi: 10.1007/s12015-012-9375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circulation. Cardiovascular quality and outcomes. 2012;5:532–540. doi: 10.1161/CIRCOUTCOMES.111.964700. [DOI] [PubMed] [Google Scholar]
- Chan V, Zorlutuna P, Jeong JH, Kong H, Bashir R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab on a chip. 2010;10:2062–2070. doi: 10.1039/c004285d. [DOI] [PubMed] [Google Scholar]
- Kai D, et al. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta biomaterialia. 2014;10:2727–2738. doi: 10.1016/j.actbio.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Serpooshan V, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34:9048–9055. doi: 10.1016/j.biomaterials.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo LM, Solari D, Somma T, Savic D, Cappabianca P. The Awake Endoscope-Guided Sealant Technique with Fibrin Glue in the Treatment of Postoperative Cerebrospinal Fluid Leak After Extended Transsphenoidal Surgery: Technical Note. World neurosurgery. 2013. [DOI] [PubMed]
- Chung HW, Mehta JS. Fibrin glue for Gundersen flap surgery. Clinical Ophthalmology. 2013;7:479–484. doi: 10.2147/OPTH.S42105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CJ, Goa KL. Fibrin sealant: a review of its use in surgery and endoscopy. Drugs. 1999;58:863–886. doi: 10.2165/00003495-199958050-00010. [DOI] [PubMed] [Google Scholar]
- Chi NH, et al. Cardiac repair achieved by bone marrow mesenchymal stem cells/silk fibroin/hyaluronic acid patches in a rat of myocardial infarction model. Biomaterials. 2012;33:5541–5551. doi: 10.1016/j.biomaterials.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Erb MA, Claus T, Hartrumpf M, Bachmann S, Albes JM. The use of Tachosil surgical patch or fibrin glue in coronary artery surgery does not affect quality of anastomosis or provoke postoperative adhesions in pigs. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009;36:703–707. doi: 10.1016/j.ejcts.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Okada K, et al. Surgical treatment for rupture of left ventricular free wall after acute myocardial infarction. Interactive cardiovascular and thoracic surgery. 2005;4:203–206. doi: 10.1510/icvts.2004.100321. [DOI] [PubMed] [Google Scholar]
- Simpson D, Liu H, Fan TH, Nerem R, Dudley SC. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, et al. Living' microvascular stamp for patterning of functional neovessels; orchestrated control of matrix property and geometry. Advanced Materials. 2012;24:58–63. doi: 10.1002/adma.201103207. [DOI] [PubMed] [Google Scholar]
- Wu X, Ren J, Li J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy. 2012;14:555–562. doi: 10.3109/14653249.2011.638914. [DOI] [PubMed] [Google Scholar]


