Summary
Xe-Derma® is a new biological acellular temporary wound cover derived from pig dermis in the form of a mesh of collagen and elastic fibers. It is recommended for use in similar indications as classical pig xenografts. A data collection of 2 burns centres in the treatment of burns with Xe-Derma® was obtained from the medical records of 101 patients admitted from January 1, 2010 to December 31, 2011. The primary objectives of the study were to assess efficacy and safety when using Xe-Derma® in burn treatment, and to analyse the course of healing. The secondary objectives were to define the suitable spectrum of indications of Xe-Derma® in terms of burn depth, and to evaluate subsequent scarring using the Vancouver Scar Scale. No undesirable systemic effects or adverse device events were observed. The use of Xe-Derma® was not associated with a higher risk of burn wound infection. On the other hand, the infection was the most common cause of Xe-Derma® dissolution. The majority of patients (81.4%) had no signs of Xe-Derma® dissolution. The mean healing time in the group of patiens under review was close to 12 days and mean hospitalization time was almost 14 days. Using Xe-Derma® proved to be effective as a temporary covering for partial-thickness burns with the capacity of spontaneous healing. It proved to be a well-tolerated wound coverage with minimal complications and low level of pain during dressing changes. Xe-Derma® firmly adhered to the wound bed. There was a lower frequency of wound dressing changes and only a minimal rate of wound infection.
Keywords: burns, biological temporary wound cover, acellular pig skin, Xe-Derma®
Abstract
Le Xe-Derma® est un nouveau pansement acellulaire biologique provisoire à base de derme porcin, se présentant sous la forme d’un filet de fibres de collagène et d’élastine. Son utilisation est recommandée dans les indications similaires aux xenogreffes porcines classiques. Nous avons recueilli et analysé les données de 101 patients, dont les brûlures ont été traitées par Xe- Derma® dans 2 centres de grands brûlés entre le 1er janvier 2010 et le 31 décembre 2011. L’objectif principal de cette étude consistait à évaluer l’efficacité et la sécurité de l’utilisation du Xe-Derma® pour le traitement des brûlures et d’analyser le processus de guérison. Les objectifs secondaires étaient de déterminer un spectre d’indications pour l’utilisation du Xe-Derma® selon la gravité de la brûlure et d’évaluer le processus de cicatrisation consécutif à l’aide de la VSS (Vancouver Scar Scale, échelle cicatricielle de Vancouver). Aucun effet systémique défavorable ou effet local indésirable n’a été observé. L’utilisation du Xe-Derma® n’a pas été associée à un risque plus élevé d’apparition d’infections de la plaie. En revanche, les infections ont représenté la cause la plus fréquente de dissolution du Xe-Derma®. La majorité des patients (81,4%) n’ont toutefois manifesté aucun signe de dissolution du Xe-Derma®. Quelle que soit la gravité de la brûlure, le durée moyenne de guérison était proche de 12 jours et la durée moyenne d’hospitalisation était de presque 14 jours. L’utilisation du Xe-Derma® s’est avérée efficace en tant que pansement provisoire sur les brûlures de second degré, présentant un potentiel de guérison spontanée. Le Xe-Derma® est un pansement bien toléré, causant peu de complications et réduisant la douleur lors des changements de pansement. Il adhère très bien au fond de la plaie. La faible fréquence de changement de pansement a permis de limiter les infections des brûlures.
Introduction
Pig skin xenografts are viable, cryopreserved or lyophylised skin grafts taken from pig donors. In the Czech Republic there is extensive positive experience from many years of using pig xenografts in burns treatment. The main indications for applying porcine xenografts are partialthickness burns, temporary covering after a necrectomy, and the covering of widely meshed split-thickness skin grafts during intermingled transplantation in extensive burn injuries. During intermingled transplantation, widely meshed autografts are overlapped with non-meshed or meshed and non-expanded pig xenografts to minimize the need for limited donor sites. Since 2004, due to the legislative consequences related to our membership of the European Union, conditions for preparing and storing viable xenografts have changed and become more complicated. This has also meant a significant increase in the associated costs, causing a substantial reduction in the use of viable xenografts. Under these circumstances, the need to find new types of temporary covering that could replace porcine xenografts increased substantially. A new biological temporary cover called Xe-Derma® was introduced into clinical practice in the Czech Republic in 2007. Since then, hundreds of burn patients, both children and adults, have been treated successfully. This biological cover also showed its quality and efficacy in the topical treatment of burn-like ailments (e.g. Lyell´s syndrome), and some types of chronic soft-tissue defects.1 Xe-Derma® is derived from pig dermis in the form of a mesh of collagen and elastic fibers after the enzymatic dissolution of the epidermis and all the cells. The remaining matrix (sterile cell xenodermis) is immunologically inert, bio-compatible, and suitable for the treatment of acute and chronic wounds, such as for temporary cover and accelerating wound healing.1 Xe-Derma®´s natural 3D structure of collagen and elastic fibers stimulates the migration and proliferation of keratinocytes, and contributes to adequate wound granulation.2,3 Xe-Derma® enables easy handling, storage at room temperature, and has an expiration period of 3 years.
Material and methods
Using clinical data from January 1, 2010 to December 31, 2011, we perfomed a retrospective analysis of 101 patients (55 males and 46 females) hospitalized for burn injuries in two burn centres (Ostrava and Prague) and treated with Xe-Derma®. Patients were divided into two agegroups: paediatric patients under 15 years old, and adults over 15 years old. Ninety-one patients were children, with an average age of 2.5 years (range = 0.75 to 14 years old). Ten patients were adults, with an average age of 42 years (range = 21 to 64 years old). The injuries were mostly caused by hot liquids (86%). Xe-Derma® was used mainly for the covering of superficial dermal burns (86% of the covered area) ((Fig. 1A). Deep dermal burns (11%) were the second most frequent category (Fig. 2A). The mean extent of wounds covered by Xe-Derma® was 6%, ranging from 1-20% TBSA. Xe-Derma® was most frequently used during the first two days after an injury.
Fig.1 - A. 12-month-old scalded child with partial-thickness burns on 16% TBSA.
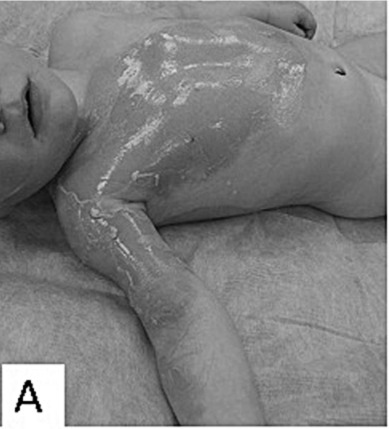
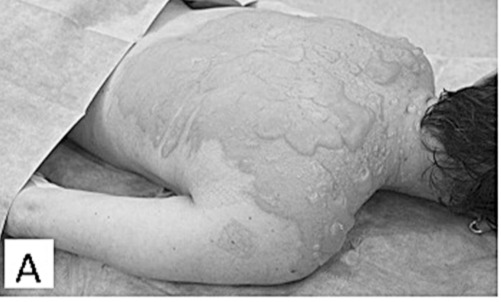
Fig.2 - A. Adult woman with partial-thickness hot water scald burns on 15% TBSA.
The primary objectives of the study were as follows: to assess the efficacy and safety of xenotransplantation of Xe-Derma® in burn treatment; and to analyse the course of healing in terms of possible complications and length of healing. The secondary objectives were: to define the suitable spectrum of indications for Xe-Derma® use in terms of burn depth; and to evaluate the subsequent scarring process according to the Vancouver Scar Scale (VSS).
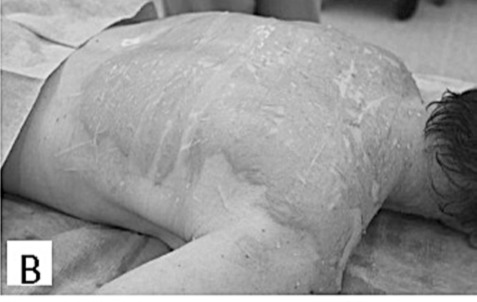
Inclusion criteria for the application of Xe-Derma® were partial-thickness burns, burn unit admission, and informed consent signed by adult patients or by the parents of child patients. Allergy to pig products was the only exclusion criterion. Initial burns treatment was carried out in the operating room under sterile conditions. Children were placed under general anesthesia, while adults, depending on the extent of the burns and the patient’s overall state, were either placed under general anesthesia or analgosedation. The first step - bacteriological surveillance of burn wounds - was performed by the semiquantitative print method. Digital photographs were taken. Initial burn wound care included gentle debridement by blisters perforation in order to release blister fluid and clean any impurities. Simultaneously, Xe-Derma® was prepared for application. After moisturization with water for injection, isotonic saline or Ringer solution, Xe-Derma® was applied directly on the wound (Figs. 1B, 2B).
Fig.1 - B. After primary debridement XeDerma® is applied.
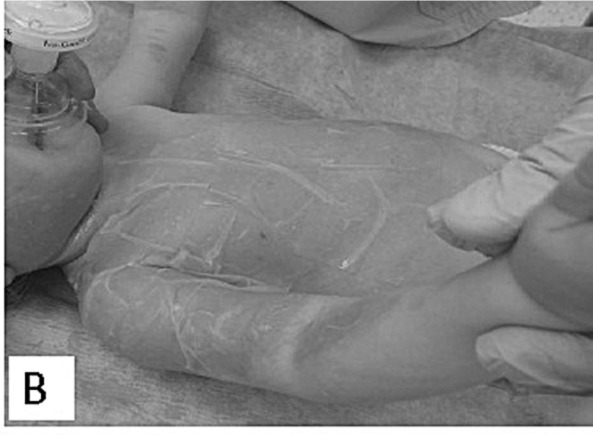
Fig.2 - B. After primary debridement XeDerma® is applied.
The following CE certified sizes of Xe-Derma® are currently available: 5x5cm, 6x8cm, 6x25cm, 10x10cm, and 10x25cm. It is distributed in sterile dry form, and must be stored in a dry dust-free place at room temperature (from +15° to +25° C) away from direct sunlight and the fumes of volatile chemicals. Xe-Derma® was applied on burn wounds very carefully for optimal adhesion to the wound bed and the prevention of fluid collections between the wound bed and Xe-Derma®. On the limbs, hands, fingers and neck, Xe-Derma® was applied longitudinally, leaving about 3 mm space between the sheets to maintain the free flow of blood. Thanks to the excellent ability of Xe-Derma ® to adhere to the wound bed, no additional fixation with staplers or tissue sealants was necessary. In order to lubricate Xe-Derma®, the next layer - an open-weave cotton dressing impregnated with Xeroform balm - was attached. The most superficial layer was formed by a gauze cover fixed with a crepe bandage (Peha-Crepp®, Hartmann Rico Company, Veverská Bitýška, Czech Republic). Evaluation of the healing process was carried out during dressing changes and assessed the following: quantity and quality of Xe-Derma® adhesion, presence or absence of its dissolution, secretion and its kind, and any local signs of infection.
If dissolution occured it was determined whether this was complete or partial, and clinically significant or insignificant. The extent of any dissolution, as a percentage of the total area of the applied Xe-Derma®, was established through clinical assessment. Bacteriological examination of the burn wounds was performed by semiquantitative print method. The local treatment was changed in the areas of dissolution, replacing Xe-Derma® most frequently with Betadine ointment (EGIS PHARMACEUTICALS PLC, Budapest, Hungary) and tulle-grass with boric vaseline. Re-dressings were initially performed every other day. When complication-free healing was evident, dressing changes were performed every third or fourth day, with only the secondary covering being changed. Uncomplicated healing ran from twelve to fourteen days. Once the burns have healed under the Xe-Derma® cover, the edges of this temporary skin begin to lift, indicating that it is also time for its gentle removal (Figs. 1C, 1D, 2C).
Fig.1 - C. Day 8 post-burn, XeDerma® is dry and spontaneous peeling-off begins.
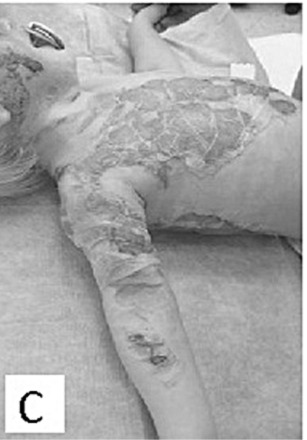
Fig.1 - D. XeDerma® was removed, the burnt surface is healed.
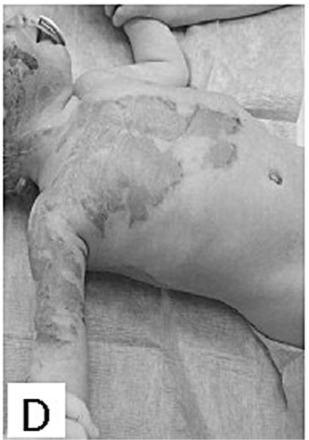
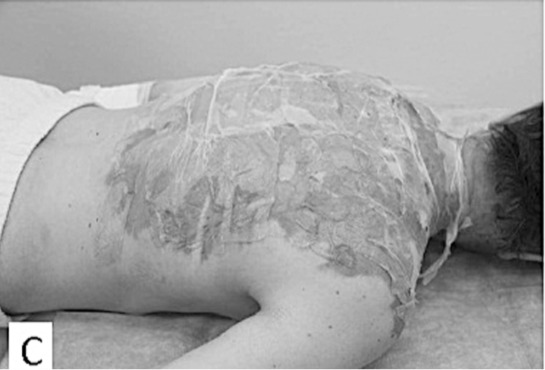
Fig.2 - C. Day 2 post-burn, XeDerma® is dry, clean, and spontaneous epithelisation is under way.
The evaluations of subsequent scarring were performed 1, 3, 6 and 12 months after healing using the Vancouver Scar Scale method, which is the most generally accepted and frequently used scale in postburn scar treatment.
Statistical analysis
The statistical analysis was performed by Leading Clinical Research a.s., Kamenická 655/54, 170 00 Prague 7, Czech Republic. Descriptive epidemiological methods were used. Calculations were performed by the software program R 2.15.1, Free Software Foundation’s GNU General Public License, R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Results
No undesirable systemic effects or adverse device events associated with xenotransplantation of Xe-Derma® were observed in any of the patients. The use of Xe-Derma ® was not associated with a higher risk of burn wound infection. On the other hand, infection was the most common cause of Xe-Derma® dissolution.
The most common complications of treatment were:
Infection - occurring in 5% of patients
Dissolution - occurring in less than 10% of patients
Non-adherence - occurring in 11% of patients
Clinically significant signs of infection were reported in 5 patients (5%). Infection under Xe-Derma® was confirmed by microbiological cultivation in 3 paediatric subjects. Infection under Xe-Derma® was usually associated with its dissolution and/or non-adhesion. With regard to the incidence and extent of dissolution, we can conclude that the majority of patients, 81%, were without any signs of Xe-Derma® dissolution. Partial dissolution, less than 10% of Xe-Derma®, were clinically evident in 9% of patients. It was clinically insignificant in the form of irregularly-spaced small areas of dissolution. Partial dissolution ranging from 10% to 50% of Xe-Derma® was evident in 9% of patients. Dissolution of more than 50% of Xe-Derma® was evident in 1 patient (1% of patients). Only in this 1 case was dissolution the reason for the complete removal of Xe-Derma ®. The most common causes leading to the dissolution of Xe-Derma® were infection, 3rd degree burns, and poorly perfused non-healing deep dermal burns associated with the risk of deterioration into 3rd degree burns. Burn wound infections under the Xe-Derma® caused partial dissolution in 5 patients, and 3rd degree burns accounted for dissolution in 1 patient. Complete non-adhesion was evident in only 1 patient. Partial non-adhesion occurred in 10 cases.
All available data on dressing changes was collected from which the anesthesia and analgosedation requirements were analyzed. 43% of re-dressings were performed without anesthesia or analgosedation. The average number of re-dressings was 7 per patient. During re-dressings, Xe- Derma® was completely replaced in only three cases (3%). In 90% of cases, the change of Xe-Derma® was limited to a smaller area of up to 10% of TBSA. This was due to partial non-adhesion in 16% of patients and partial dissolution in 12% of patients. The mean healing time in the group of patients under review was close to 12 days and mean hospitalization time was almost 14 days.
The mean hospitalization time of all subjects was 16 days, ranging from 2 to 62 days. The mean hospitalization time of paediatric patients was 14 days, ranging from 2 to 62 days. The mean hospitalization time of adults was 34 days, ranging from 12 to 54 days. Three patients were treated as out-patients prior to hospitalization, with a mean duration of 12 days, ranging from 2 to 25 days.
Use of antibiotics during treatment was reported in 21% of paediatric patients and 50% of adult patients. The mean duration of ATB treatment was 11 days (10 days in children, 12 in adults), ranging from 4 to 21 days. Analgesics during burn wound healing were used in 90% of children and 100% of adults. The mean duration of analgesic treatment was 7 days in total, ranging from 1 to 40 days. The mean duration of analgesic treatment in children was 6 days (from 1 to 27 days). The mean duration of analgesic treatment in adults was 14 days (from 3 to 40 days).
The majority of children healed in a conservative way without the necessity of surgical treatment. Partial-thickness burns were present in all children treated with Xe- Derma® and were the most frequent category of burn depth in children. The third and/or deep-second degree burns were present in 37% of paediatric patients. Surgical treatment in the form of necrectomy followed by split-thickness skin grafting was undertaken by 21% of paediatric patients and 90% of adult patients. The average extent of necrectomy and skin grafting was 4% TBSA in paediatric patients and 7% TBSA in adult patients.
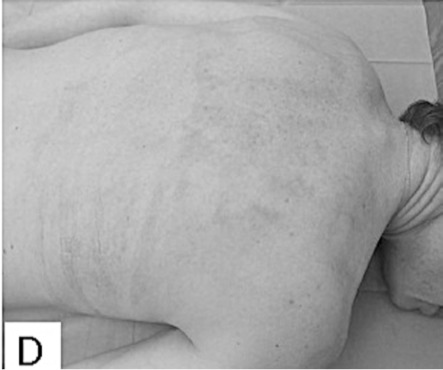
Evaluations of the subsequent scarring process were performed 1, 3, 6 and 12 months after healing. The clinical follow-up evaluations of scars were carried 1 month post healing in 97% of patients, 3 months post healing in 60% of patients, 6 months post healing in 41% of patients, and 12 months post healing in 31% of patients. The median VSS was 2 points in children one month post healing ((Fig. 1E), and increased to 3 points three months post healing before decreasing again to 2 points, six and twelve months post healing. There were no hypertrophic scars on the areas healed with Xe-Derma® in children. The median VSS was 3 points in adults at one month post healing, then it rose to 5 points three months post healing ((Fig. 2D), and to a maximum of 6 points six months post healing. After a six-month-period, the VSS was decreasing to reach the final 5 points by twelve months post healing. The scarring had a more favourable course in children, which correlates to the extent of superficial second-degree and spontaneously healed deep-second degree burns. Comprehensive scar treatment was performed in all patients using various treatment modalities in the form of pressure garments, physiotherapy and rehabilitation, splinting, taping and laser-therapy. A subsequent mild functional limitation occurred in 1% of children and 23% of adults. Moderate or severe functional limitation did not occur in any children. Moderate and/or severe functional limitation occurred in 23% of adult patients. The most frequent areas with functional limitation were axillary regions (35% of patients) and fingers (29% of patients). The subsequent scarring on areas healed under Xe-Derma® had no scar hypertrophy in the majority of patients.
Fig.1 - E. Very good result one month after injury.
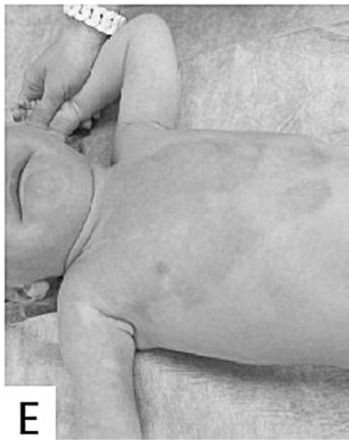
Fig.2 - D. Excellent result 3 months after injury.
Discussion
There has been very good experience with pig xenografts in the field of burn medicine in the Czech Republic since 1973.4 Pig skin xenografts were used as the standard dressing for superficial burns and as temporary cover preparing the wound bed for definitive closure after necrectomy.5 Pig xenografts also confirmed its efficacy in intermingled transplantation.4 Porcine skin is very similar to human skin in terms of epidermal thickness, epithelial turnover and migration, hair distribution, dermal elastin structure, dermal collagen macrostructure, low content of proteolytic enzymes, absence of capillary ingrowth, and absence of vessel-to-vessel connections.3,6,7 On the one hand, pig xenografts, as a temporary biological cover, stimulate epithelisation,8 and minimise losses of liquids, electrolytes, protein and heat from burned areas,8,9 and cause less pain.4 On the other hand, the risk of chronic porcine endogenous retrovirus infection has been widely studied and discussed.10,11 However, the results of multiple studies indicate only potential risk without objective evidence for PERV transmission.12-14 Xe-Derma® very closely meets the properties of xenografts without the risk of zoonosis transition thanks to its acellularity (during production, cells are enzymatically removed) followed by the radiation sterilization of residual dried matrix. The main advantages are that Xe-Derma® proved a painless application with excellent adhesion to burn wounds. The strong adhesion, which is due to a thin layer of fibrin between the wound bed and the scum cover, significantly reduces the traumatization of the wound bed during dressing changes and allows rapid and undisturbed epithelisation.15 The firm adhesion is also associated with reduced risk of hematoma or seroma collection, with a reduced risk of infection as a secondary outcome. Thanks to the transparency of this cover, we can assess the course and advancement of healing during redressings and timely diagnose potential complications. The Xe-Derma® temporary cover seems to also have a beneficial effect on reducing the loss of body fluids from open wound areas. In the case of uncomplicated healing, only a secondary (superficial) cover is changed, Xe-Derma® is left in place until complete healing is reached. If for any reason a partial and clinically insignificant lysis of Xe- Derma® occurs, we combine the use of Xe-Derma® with conventional local treatment, such as hydrogels or alginates for example. In our experience, significant lysis is rare but, when it occurs, infection is the most common cause. In such a case, it is necessary to change the strategy of local treatment and replace the primary coverage. Xe-Derma® is not suitable to cover 3rd degree burns, the healing of which cannot be expected without surgical treatment. We also would not recommend Xe-Derma® for covering escharotomies. Our experience is that Xe-Derma® dissolves itself rapidly when used for escharotomies in surrounding deep burns. The lysis of Xe-Derma® occurs within less than 24 hours after application. Consequently, the cover of the wound during the period between two dressing changes tends to be incomplete and carries the risk of wound conversion. When the complete healing of the areas under Xe-Derma® is achieved, spontaneous separation of the cover edges occurs. It is a clinical sign of complete epithelization and Xe-Derma® can be gently removed.
Xe-Derma® provides a well-tolerated temporary wound cover for the spontaneous healing of partial-thickness burns. It is possible to reduce the frequency of dressing-changes significantly, which correlates with a lower risk of wound infection. In cases of complication-free healing, there is no need to change the cover. Xe-Derma® firmly adheres, which enables painless or low-level pain dressing changes with reduced consumption of analgesics or even general anesthesia procedures. All these factors are associated with reduced costs, which out-weigh the slightly higher initial full-cost of treatment.
However, this study also had its limitations. Viewing this study as a starting point for further research, we focused on a single temporary wound cover, outlined its properties and implementation, and evaluated its use. In the consequent pre-planned work, we would like to perform a comparative evaluation of some more similarly effective products simultaneously.
Conclusion
The analysis of Xe-Derma® use in routine burn management in two burn centres showed its advantageous application in paediatric patients in the treatment of partialthickness burns, mostly caused by scalds. Xe-Derma® temporary biological skin cover has proven to be a robust wound dressing that provides well-tolerated wound coverage with minimal complications during the healing process. Xe-Derma® firmly adheres to the wound bed, and minimizes mechanical micro-traumatization without the need to change this primary cover. It allows a lower frequency of wound dressing changes, which in itself is a factor associated with lower risk of wound infection. Additionally, the lower level of pain involved and subsequent reduction in consumption of analgesics or even general anesthesia, are of great importance too. All these factors are associated with the overall reduced costs of the treatment, which must also be taken into consideration. Based on our experience, Xe-Derma® is a suitable and effective temporary cover for second-degree burns, with a capacity to support spontaneous healing.
Acknowledgments
Conflict of interest. All authors declare that there is no conflict of interest.
References
- 1.Pospisilova A. Treatment of leg ulcers with XeDerma. Dermatologie pro praxi. 2009;3:191–3. [Google Scholar]
- 2.Zajicek R, Broz L, Klein L, et al. Xe-Derma: Nový biologický kryt pro lécbu akutních a chronických ran. Hojení ran. 2008;2:18–27. [Google Scholar]
- 3.Zajicek R, Matoušková E, Broz L, et al. New biological temporary skin cover Xe-Derma® in the treatment of superficial scald burns in children. Burns. 2011;37:333–7. doi: 10.1016/j.burns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Königová R, Bláha J. Komplexní lécba popáleninového traumatu. Karolinum. 2010 [Google Scholar]
- 5.Masellis M, Gunn SWA, editors. Dordrecht. Kluwer Academic Publishers; 1995. Clinical experience with skin xenografts in burned patients; pp. 337–45. [Google Scholar]
- 6.Vardaxis NJ, Brans TA, Boon ME, et al. Confocal laser scanning microscopy of porcine skin: Implications for human wound healing studies. Journal of Anatomy. 1997;190:601–11. doi: 10.1046/j.1469-7580.1997.19040601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armour AD, Fish JS, Woodhouse KA, et al. A comparison of human and porcine acellularized dermis: Interactions with human fibroblasts in vitro. Plastic and reconstructive surgery. 2006;117:845–56. doi: 10.1097/01.prs.0000204567.28952.9d. [DOI] [PubMed] [Google Scholar]
- 8.Chiu T, Burd A. Xenograft dressing in the treatment of burns. Clinics in dermatology. 2005;23:419–23. doi: 10.1016/j.clindermatol.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Pruitt BA, Levine NS. Characteristics and uses of biologic dressings and skin substitutes. Archives of surgery. 1984;119:312–22. doi: 10.1001/archsurg.1984.01390150050013. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi Y, Fishman J. Long life with or without PERV. Xenotransplantation. 2010;17:429–30. doi: 10.1111/j.1399-3089.2010.00614.x. [DOI] [PubMed] [Google Scholar]
- 11.Fishman JA, Scobie L, Tajeuchi Y. Xenotransplantation-associated infectious risk: A WHO consultation. Xenotransplantation. 2012;19:72–81. doi: 10.1111/j.1399-3089.2012.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Nicuolo G, D´Alessandro A, Andria B, et al. Long-term absence of porcine endogenous retrovirus infection in chronically immunosuppressed patients after treatment with the porcine cellbased Academic Medical Center bioartificial liver. Xenotransplantation. 2010;17:431–9. doi: 10.1111/j.1399-3089.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- 13.Irgang M, Sauer I M, Karlas A, et al. Porcine endogenous retroviruses: No infection in patients treated with a bioreactor based on porcine liver calls. Journal of clinical virology. 2003;28:141–54. doi: 10.1016/s1386-6532(02)00275-5. [DOI] [PubMed] [Google Scholar]
- 14.Valdez-Gonzalez R, Dorantes L M, Bracho-Blanchet E, et al. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long-term porcine islet xenotransplantation. Journal of medical virology. 2010;82:331–4. doi: 10.1002/jmv.21655. [DOI] [PubMed] [Google Scholar]
- 15.Klosova H, Nemeckova Crkvenjas Z, Petras L, et al. The use of acellular biological xenografts in local treatment of Lyell’s syndrome. Rozhledy v chirurgii. 2014;93:76–81. [PubMed] [Google Scholar]


