Abstract
Here we present a case of refractory hypoglycaemia associated with use of the antibiotic trimethoprim-sulfamethoxazole (TMP-SMX). This was used to treat Pneumocystis jirovecii pneumonia (PCP) infection. The patient had significant pre-existing renal impairment with a kidney transplant in situ. Refractory hypoglycaemia occurred 5 days after starting the antibiotic and persisted for 36 h after its cessation. SMX contains the same sulphanilamide structural group as the oral hypoglycaemic agents called sulphonureas. SMX could therefore act as an insulin secretagogue. The inappropriately raised insulin and c-peptide levels seen in our patient support this theory. The 5-day asymptomatic period would allow sufficient time for the drug to accumulate and the extended period seen after its cessation would be seen in a dose-dependent side effect. Following 3 days of observation and continuous glycaemic support on the High Dependency Unit she was discharged back to the ward, with no further occurrence of hypoglycaemia.
Background
Co-trimoxazole is a very commonly used antibiotic. A case series by Strevel et al1 describes how hypoglycaemia can be associated with its use. Hypoglycaemia is a medical emergency. Prompt recognition and treatment of less commonly encountered aetiologies is important to avoid morbidity. Renal impairment is a risk factor particularly associated with development of hypoglycaemia in patients treated with co-trimoxazole.2–5
Case presentation
Ms M is a 56-year-old Caucasian lady weighing 68 kg with chronic kidney disease (CKD) stage 5. She received a renal transplant in 1990 and is on long-term mycophenolate mofetil 250 mg once daily and prednisolone 5 mg once daily with no known drug allergies. She complains of significant dyspnoea on exertion and a dry non-productive cough for the previous 6 months. She had received three oral antibiotic courses that were penicillin based to little benefit, the most recent course being 1 month before this episode. Examination was unremarkable and a chest radiograph yielded no positive findings. Because of her immunosuppression, an induced sputum analysis was performed to look for Pneumocystis jirovecii infection. The PCR was positive but immunofluorescence negative for P jirovecii pneumonia (PCP). She was subsequently started on dose-adjusted co-trimoxazole. Five days later she was found to have a decreased Glasgow Coma Scale of 12. Her blood glucose was found to be 1.7 mmol/l. The severe hypoglycaemia was resistant to general ward measures involving intramuscular glucagon and intravenous boluses of 50% dextrose. Accordingly, she necessitated transfer to the High Dependency Unit for continuous dextrose replacement and close monitoring. Electrolytes and renal function were unchanged. No other medications were administered. Specifically, she received no known diabetic hypoglycaemic agent. Her morning cortisol was greater than 450 nmol/l excluding an Addisonian component, and the serum insulin and c-peptide levels were double the upper limit of normal. Her maintainence oral steroid was switched to intravenous hydrocortisone 50 mg four times a day. Co-trimoxazole was switched to second-line PCP therapy, which consisted of clindamycin and primaquine. After 36 h her blood glucose had stabilised and she was transferred back to the ward. She was sucessfully discharged and completed her course of antibiotics. No long-term sequalae were noted.
Outcome and follow-up
Our patient reported much improved respiratory function in follow-up clinic appointments confirming a good clinical response to PCP therapy.
Discussion
It is postulated that the sulfamethoxazole (SMX) component of her co-trimoxazole antibiotic caused her adverse reaction. SMX contains the same sulphanilamide structural group as a class of oral hypoglycaemic agents called sulfonylureas (figure 1). SMX could mimic the action of sulphonylureas on the pancreatic islet cells by acting as an insulin secretagogue, increasing endogenous insulin secretion. This hypothesis is supported by the inappropriately raised insulin and c-peptide levels seen in our patient. Co-trimoxazole is metabolised in the liver but a large portion can be excreted unchanged in the urine.2 We believe her renal impairement put her at an increased risk by increasing the half-life of co-trimoxazole2,5 which can be up to 20–50 h in patients with renal impairment. The 5 day asymptomatic period would allow sufficient time for the drug to accumulate and the extended hypoglycaemia seen after its cessation would be consistent with a dose-dependent side effect.3 Hypogylcaemia is not a well-known adverse effect of co-trimoxazole. Strevel et al1 undertook a review of the literature and found that concomitant renal impairement was the most commonly associated risk factor. Due to the common usage of this antibiotic in a variety of clinical settings, clinicians should be aware of the possibility of refactory hypoglycaemia when prescribing co-trimoxazole, paying particular care to patients with renal impairment. In these patients perhaps an alternative should be chosen if possible.
Figure 1.
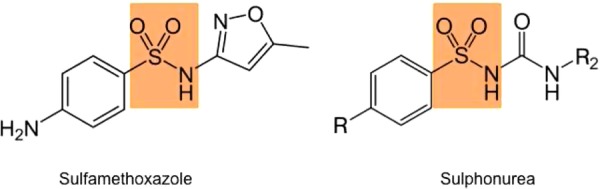
Same sulphanilamide structural group in sulfamethoxazole and the sulfonylureas.
Learning points.
Clinicians should be aware of the rare possibility of refactory hypoglycaemia when prescribing trimethoprim-sulfamethoxazole, particularly to patients with renal impairment.2,5
Careful consideration should be given before commencing co-trimoxazole in non HIV infected patients based on positive PCR results alone which could suggest mere colonisation as opposed to disease.6
Primum non-nocere First do no harm.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Strevel LS, Kuper A, Gold WL. Severe and protracted hypoglycaemia associated with co-trimoxazole use. Lancet Infect Dis 2006;6:178–82. [DOI] [PubMed] [Google Scholar]
- 2.Paap CM, Nahata MC. Clinical use of trimethoprim/sulfamethoxazole during renal dysfunction. Drug Intell Clin Pharm 1989;23:646–54. [Google Scholar]
- 3.Poretsky L, d Moses AC. Hypoglycemia associated with trimethoprim/sulfamethoxazole therapy. Diabetes Care 1984;7:508–9. [DOI] [PubMed] [Google Scholar]
- 4.Dobmeier ME. Symptomatic hypoglycemia secondary to a glipizide-trimethoprim/sulfamethoxazole drug interaction. Ann Pharmacother 1990;24:250–1. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JA, Kappel JE, Sharif MN. Hypoglycemia secondary to trimethoprim/sulfamethoxazole administration in a renal transplant patient. Ann Pharmacother 1993;27:304–6. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1111/j.1469-0691.2010.03400.x. Alanio A, Desoubeaux G, Sarfati C, et al. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect 2011; 17(10):1531–7. [DOI] [PubMed] [Google Scholar]


