Abstract
We evaluated a magnetic resonance venography (MRV) approach with gadofosveset to quantify total thrombus volume changes as the principal criterion for treatment efficacy in a multicenter randomized study comparing edoxaban monotherapy with a heparin/warfarin regimen for acute, symptomatic lower extremities deep vein thrombosis (DVT) treatment. We also used a direct thrombus imaging approach (DTHI, without the use of a contrast agent) to quantify fresh thrombus. We then sought to evaluate the reproducibility of the analysis methodology and applicability of using 3D magnetic resonance venography and direct thrombus imaging for the quantification of DVT in a multicenter trial setting. From 10 randomly selected subjects participating in the edoxaban Thrombus Reduction Imaging Study (eTRIS), total thrombus volume in the entire lower extremity deep venous system was quantified bilaterally. Subjects were imaged using 3D-T1W gradient echo sequences before (direct thrombus imaging, DTHI) and 5 min after injection of 0.03 mmol/kg of gadofosveset trisodium (magnetic resonance venography, MRV). The margins of the DVT on corresponding axial, curved multi-planar reformatted images were manually delineated by two observers to obtain volumetric measurements of the venous thrombi. MRV was used to compute total DVT volume, whereas DTHI was used to compute volume of fresh thrombus. Intra-class correlation (ICC) and Bland Altman analysis were performed to compare inter and intra-observer variability of the analysis. The ICC for inter and intra-observer variability was excellent (0.99 and 0.98, p <0.001, respectively) with no bias on Bland-Altman analysis for MRV images. For DTHI images, the results were slightly lower (ICC = 0.88 and 0.95 respectively, p <0.001), with bias for inter-observer results on Bland-Altman plots. This study showed feasibility of thrombus volume estimation in DVT using MRV with gadofosveset trisodium, with good intra- and inter-observer reproducibility in a multicenter setting.
Keywords: Medicine, Issue 100, venous thrombosis, magnetic resonance imaging, magnetic resonance contrast enhanced venography, factor Xa inhibitor, gadofosveset, image analysis
Introduction
Venous thromboembolism (VTE) affects 300,000-600,000 individuals in the United States every year1. Deep vein thrombosis (DVT) is the most common presentation of VTE, and most commonly affects the calf, thigh or pelvic veins. The diagnosis, management, and follow-up of subjects with DVT cannot be based solely on clinical examinations, since the signs and symptoms of this disease are non-specific2,3. While blood tests (such as D-dimer) can help rule out the diagnosis of DVT, imaging is required to establish the presence of DVT4. Compression ultrasound (CUS) is currently the most commonly used imaging test in the diagnosis of suspected acute DVT. CUS is inexpensive and has high sensitivity and specificity to detect acute DVT5. However, CUS cannot reliably assess the deep veins in the pelvis6. Additionally, CUS cannot directly quantify thrombus volume and composition, which are important when distinguishing between acute DVT (a potential source of pulmonary embolism (PE)) and chronic DVT (less likely to embolize) and for evaluation of therapeutic efficacy7.
Unlike computed tomography (CT), magnetic resonance imaging (MRI) does not deliver ionizing radiation, and is therefore suitable for serial examinations to evaluate thrombus evolution or regression. Compared with CUS, MRI can detect pelvic DVT and can more precisely define proximal (popliteal vein and above) and distal leg (below popliteal vein) DVT8, to better assess the risk of PE. MRI can characterize thrombus age and organization, and may help differentiate acute from chronic DVT9-11 (refs updated). Quantification of thrombus volume, an important metric to assess the disease evolution and response to treatment, is feasible with MR imaging. Current magnetic resonance venography protocols are performed after injection of gadolinium (Gd) based contrast agents12. These are small molecular weight molecules that extravasate quickly after injection, and require careful timing to capture the venous enhancement phase needed to correctly visualize the thrombus13,14.
A proof-of-concept study, edoxaban Thrombus Reduction Imaging Study (eTRIS), utilizing an open label design, investigated the efficacy and safety of edoxaban 90 mg once a day for 10 days, followed by edoxaban 60 mg once a day in the treatment of acute, symptomatic DVT (ClinicalTrials.gov Identifier: NCT01662908). eTRIS addresses whether edoxaban monotherapy, without concomitant low molecular weight heparin (LMW heparin) at the time of treatment initiation, is more effective than standard treatment with LMW heparin/warfarin therapy in subjects with DVT, as assessed by the percent (%) change from baseline in thrombus volume/size (measured by MRI) at Day 14-21.
Another goal of eTRIS was to develop and validate a straightforward MR venography (MRV) image acquisition and analysis protocol for the quantification of thrombus volume in DVT. To overcome some challenges faced by current MRV protocols in multicenter settings, we utilized a recently FDA-approved, long-circulating, gadolinium-based blood pool contrast agent (gadofosveset trisodium). Compared to the use of extracellular Gd-based chelates (e.g., Gd-DTPA) for MRV, gadofosveset has a significantly longer circulation time, which allows use of a simpler MR acquisition scheme, without any timing of acquisitions. Gadofosveset trisodium is a blood pool MRI contrast agent that circulates for 2-3 hr after intravenous injection15,16. Its safety profile is similar to those of traditional extravascular extracellular MRI contrast agents17. It allows steady-state imaging of the vasculature over a period of 1 hr. Therefore, no operator dependent timing of image acquisition is required after contrast agent injection. The additional advantage of using this contrast agent is that it is a small molecule (molecular weight 857 Da)18 and can permeate the sides of even a fully occluded thrombus, thereby providing excellent contrast of the DVT from surrounding areas on the MRV and enabling quantitative computation of DVT volumes. Previous studies have established the inter-rater reliability of visualizing veins using the MR Volume Interpolated Breath-hold Examination (VIBE) venography using gadofosveset trisodium19. Here, we use a similar approach in a multicenter clinical trial setting to evaluate deep vein thrombosis and use the volume of DVT measured by MRI as an endpoint. eTRIS provides an ideal platform to evaluate the feasibility and reproducibility of analysis of the MRV imaging approach proposed here, using a long-circulating Gd-based blood pool contrast agent for evaluating DVT volumes. We also evaluate the use of a direct thrombus imaging (DTHI) approach to quantify the extent of fresh DVT prior to the injection of contrast agents.
Two MRI examinations were performed during the course of the study: the first within 36 hr after randomization into the edoxaban monotherapy group or heparin/warfarin group, and the second between 14 to 21 days after randomization. The analyses of all the images were performed by a centralized core laboratory. Volume of fresh thrombus is calculated from a Direct Thrombus Imaging (DTHI) in the legs and lower pelvis before the injection of any contrast agent. The total thrombus volume (fresh and old) is computed from a post contrast magnetic resonance venography (MRV) images of the veins in the legs and lower pelvis.
Protocol
This study was approved by the local institutional review boards at all participating centers. All subjects in the multicenter trial provided written informed consent to participate in eTRIS at their respective institutions.
1. Image Acquisition
- Perform the MR imaging on a 1.5 T or 3 T whole body scanner using specialized phased-array coils for MRV such as a peripheral vascular coil, body matrix coils or run-off coils. Use these coils in conjunction with other body matrix coils or spine coils. If no suitable specialized coils are available, use the body coil instead. NOTE: Use commercially available scanners such as Siemens Symphony, Sonata, etc.
- Screen subject, and review MRI safety questionnaire prior to scan. Have subject change into a gown.
Place an intravenous line in the antecubital vein of subject for the injection of the contrast agent. Follow standard safety procedures for injecting a gadolinium-based contrast agent.
- Place subject in supine, feet first position in the MRI machine and position appropriate coils on regions to be scanned. Secure coils using Velcro straps as needed.
- Fasten subject’s legs/feet to prevent motion artifacts during the MRI scan.
- Turn on the centering laser and move the table until the laser crossbeams are located just below the subject’s knees (patella). Accept this position for the iso-center of the scan and move the patient table to the center position of the scanner bore.
Measure the creatinine clearance (CrCL) and determine the dose of the contrast agent to be used for the subject based on body weight. If CrCL <30 ml/min, subject is excluded from the study. For individuals with CrCL >30 ml/min but less than 45 ml/min, 0.01 mmol/kg of contrast agent is used. For individuals with CrCL >45 ml/min but <60 ml/min, 0.02 mmol/kg of gadofosveset is injected. For individuals with normal renal function (creatinine clearance >60 ml/min), a dose of 0.03 mmol/kg (0.12 ml/kg) gadofosveset trisodium is used. An MRI compatible power injector is used to inject the contrast agent.
Perform bilateral imaging of both legs and lower pelvis in a single examination session lasting approximately 60 min as shown in Table 1 and described in MRI protocol section. NOTE: Anonymize patient data prior to transfer of images to a central core laboratory for analysis.
2. MRI Protocol
Execute the imaging protocol from the scanner console by selecting each protocol step from the protocol window and dragging it to the execution list. Once ready, run the sequence by pressing Scan/Execute or equivalent button.
Acquire 2D gradient echo based sequences using sequence parameters in Table 1, in the three orthogonal axes to image from mid-calf to above the iliac crest and use as localizers/scouts.
Perform time of flight (TOF) angiography scans in each segment as specified in Table 1 NOTE: These scans are also used as localizers to help differentiate arteries from veins as a long circulating contrast agent is used in steady state in this protocol. This view of the arterial tree is quite limited and only serves to help the image analyst differentiate the arteries from the venous vasculature and does not serve as a full-fledged angiogram.
To avoid acquiring poor quality images at the inferior and superior extent of the imaging volume, acquire three coronal 40 cm segments in the foot to head direction overlapping by 10 cm during the 3D T1-weighted Gradient Echo scans. NOTE: These three segments encompass: a) mid-calf to above knee b) above knee to thigh/lower pelvis, and c) thigh/pelvis to abdomen. Figure 1 shows sample images with the three acquisition locations illustrated.
To distinguish between acute and chronic venous thrombosis, acquire T1W direct thrombus imaging (DTHI) and 3D gradient echo (GRE) sequences using parameters in Table 1. NOTE: These 3 scans also serve as the pre-contrast scan for the MRV acquisition. The extent of the coverage of these acquisitions is the same as the initial localizers acquired in the three locations illustrated in Figure 1. GRE sequence is also easily implementable on various scanning platforms and across imaging field strengths.
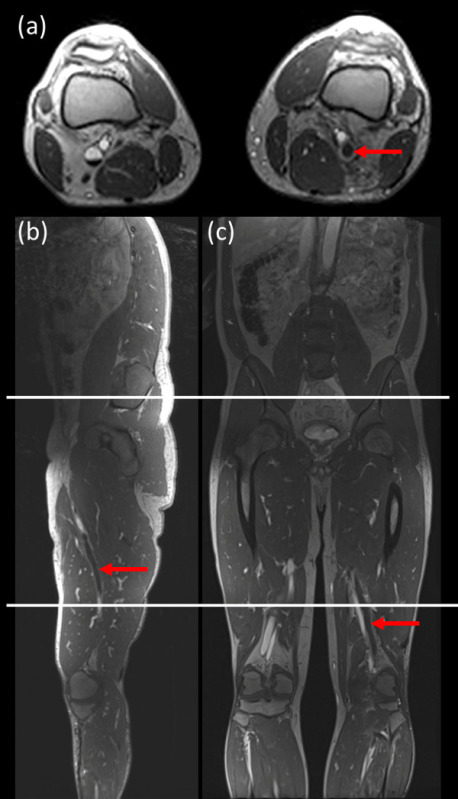 Figure 1: Sample images showing acquisition locations for the 3 stations used for imaging. The field of view in the coronal direction and axial directions was 40 cm for each bed position [acquisition: (mid-calf to above knee), (above knees to thigh/pelvis), (thigh/pelvis to abdomen)] with 10 cm overlap between each bed position. (a) axial image; (b) sagittal image and (c) coronal image. Red arrow indicates DVT. Please click here to view a larger version of this figure.
Figure 1: Sample images showing acquisition locations for the 3 stations used for imaging. The field of view in the coronal direction and axial directions was 40 cm for each bed position [acquisition: (mid-calf to above knee), (above knees to thigh/pelvis), (thigh/pelvis to abdomen)] with 10 cm overlap between each bed position. (a) axial image; (b) sagittal image and (c) coronal image. Red arrow indicates DVT. Please click here to view a larger version of this figure.
Administer the contrast agent (gadofosveset trisodium) at a dose of 0.03 mmol/kg (0.12 ml/kg) intravenously into the subject at a rate of 2 ml/sec and flush with 20 ml of saline. Let the contrast agent circulate for 5 min to ensure a steady state in the blood pool.
Acquire MRV images; post contrast 3D gradient echo sequences at 3 locations described previously. See the sequence parameters in Table 1 and Table 2. Use these images to determine the locations and size of the thrombi.
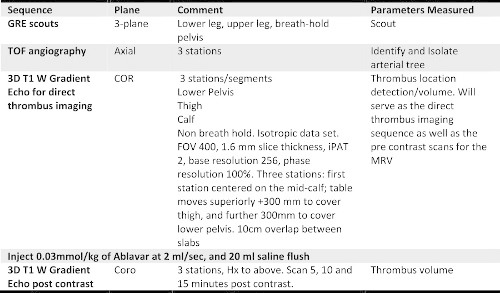 Table 1: Lower extremity MR venography protocol with the contrast agent, gadofosveset trisodium, including direct thrombus imaging.
Table 1: Lower extremity MR venography protocol with the contrast agent, gadofosveset trisodium, including direct thrombus imaging.
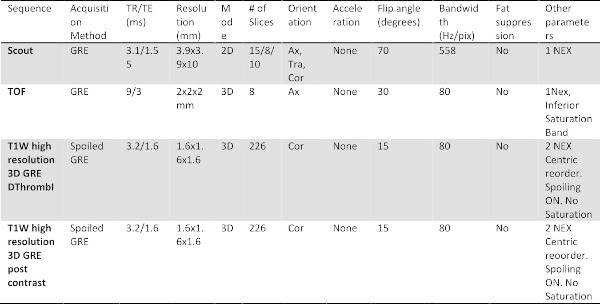 Table 2: The specific imaging parameter for each sequence acquired in the protocol.
Table 2: The specific imaging parameter for each sequence acquired in the protocol.
After scan is completed, remove subject from the MRI scanner and take out the intravenous line. Ask the subject to change out of gown and exit the facility.
3. Image Analysis
Perform image analysis on a dedicated image analysis workstation running FDA-approved open source image processing software such as OsiriX MD20, by 2 trained image analysts. Ensure the analysts have a minimum of 3 years experience each.
Temporarily blind all images before transfer to the image analyst workstation for analysis.
Ensure that a radiologist assesses each MRI scan for the presence and location of thrombi. Provide the radiologist’s assessment to the image analyst to be used as a guideline throughout the analysis.
- Load all DICOM images from both MRI visits of a subject into the image processing software by selecting “import”, and compare the two imaging time-points of the MRV series for each subject to ensure adequate spatial coverage and registration across time points.
- Select the “3D MPR” tool in viewer to provide simultaneous browsing of image data in 3 orthogonal views (axial, coronal, and sagittal). NOTE: The image processing software’s 3D MPR mode enables the viewing of the vessels of interest.
Analyze the following vessels if DVT is present: external iliac, common femoral, superficial femoral, deep femoral, popliteal, anterior tibial, posterior tibial I & II, gastrocnemius I & II, and peroneal I & II veins. All analysis is restricted to regions of the vessels that are present in images from both time-points.
Assess the quality of the image for each vein with known DVT as indicated by the radiologist’s assessment on a 0-5 scale, with 0-2 being unanalyzable and 3-5 being analyzable for both the DTHI and MRV sequences. For the breakdown of scoring system see Figure 2.
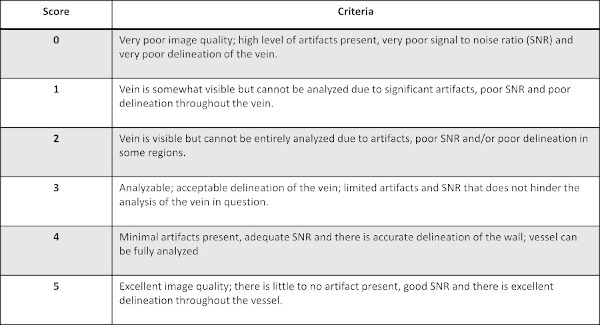 Figure 2: The breakdown of the scoring system used for assessing the quality of images for each vein of interest with known DVT.
Figure 2: The breakdown of the scoring system used for assessing the quality of images for each vein of interest with known DVT.
- For MRV analysis, analyze each vessel individually. After 3D curved multiplanar reconstruction, use contours following the centerline of each vein, traced by the analyst, to generate curved path for each vein and save to file.
- Establish the position of the thrombus in the three dimensional space. From the Curved MPR plane, a 3D Bezier path will be displayed.
- Delineate the centerline of the vein by selecting of the “Contour Path” tool in “Creation Mode”.
- Place points repeatedly on any of the orthogonal MPR view to straighten the entire vessel of interest.
- Make adjustments when necessary to ensure that the vessel is entirely straightened in “Editing Mode”
- When the contour path is accurately delineated at centerline of vessel, save by selecting the “curved path” icon to export the file
- Generate 1 mm axial slices perpendicular to the curved path and save as DICOM files (Figure 3). Observe the the curved path, straightened vessel, and corresponding axial images for quantifying DVT from MRV images. NOTE: The images rendered in the 3D Curved MPR viewer must then be exported in DICOM format and added to the database as a new series of images.
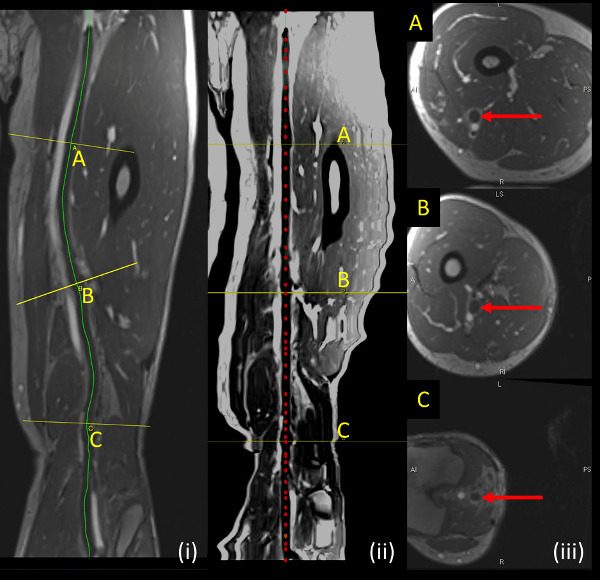 Figure 3: Sample DVT shown on MRV sequence. (i, left panel) Curved path (yellow line) illustrates the contour followed by the vein being analyzed. (ii, middle panel) Straightened vessel along centerline of vessel being analyzed (red dotted line) (iii, right panel) shows axial slices perpendicular to vein being analyzed at locations indicated by yellow lines (A, B, C) on longitudinal sections. Please click here to view a larger version of this figure.
Figure 3: Sample DVT shown on MRV sequence. (i, left panel) Curved path (yellow line) illustrates the contour followed by the vein being analyzed. (ii, middle panel) Straightened vessel along centerline of vessel being analyzed (red dotted line) (iii, right panel) shows axial slices perpendicular to vein being analyzed at locations indicated by yellow lines (A, B, C) on longitudinal sections. Please click here to view a larger version of this figure.
- On these axial DICOM images, manually segment regions of interest encompassing the thrombus (see Figure 4) by using the “Closed Polygon ROI Tool”. Save regions of interests to file by selecting the “Save all ROIs of this Series” located in the “ROI” drop down menu and save ROI metrics by navigating to the the “Plugins” menu and select “ROI Tools” followed by “Export ROIs”. This should be saved in a CSV format.
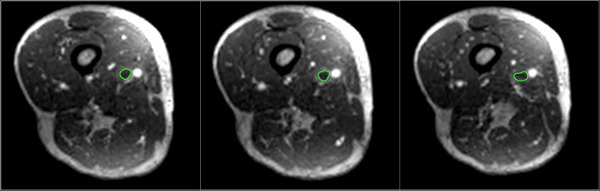 Figure 4: Manually segmented regions of interest (green) are shown encompassing the thrombus on axial reformatted DICOM images.
Please click here to view a larger version of this figure.
Figure 4: Manually segmented regions of interest (green) are shown encompassing the thrombus on axial reformatted DICOM images.
Please click here to view a larger version of this figure.
- Calculate thrombus volume by multiplying the area on each analyzed slice by the slice thickness (1 mm) using a custom built Matlab script. Calculate total thrombus volume in each subject by adding the thrombus volumes in each vessel.
- For DTHI analysis, identify fresh thrombus as bright regions on pre-contrast T1W 3D gradient echo scans13 within the DVT regions segmented by the MRV.
- On axial pre-contrast images, compute the volume of fresh thrombus by drawing regions of interest (ROI) manually as shown in Figure 5. In Figure 6, sample images indicating DVT measured by DTHI in conjunction with the MRV images are depicted.
- Open the pre-contrast and post-contrast sequences alongside each other. Select “axial” view and delineate bright regions along the vessel of interest using the “closed polygon ROI Tool”.
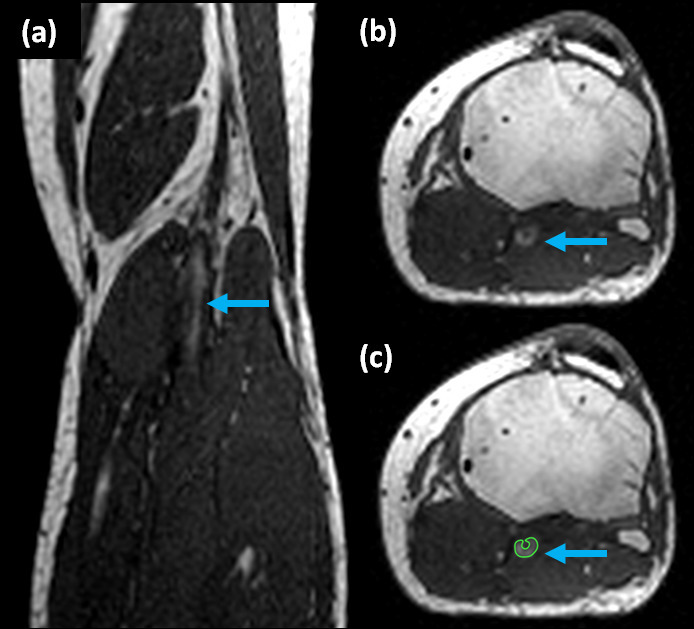 Figure 5: Sample DVT shown on DTHI imaging sequence. (a) coronal image, (b) axial image, and (c) axial image showing region of interest (green) traced around fresh thrombus (blue arrows) on pre-contrast images. The DTHI images are acquired prior to the injection of contrast agent and rely on met-hemoglobin content of thrombus to produce bright signal.
Figure 5: Sample DVT shown on DTHI imaging sequence. (a) coronal image, (b) axial image, and (c) axial image showing region of interest (green) traced around fresh thrombus (blue arrows) on pre-contrast images. The DTHI images are acquired prior to the injection of contrast agent and rely on met-hemoglobin content of thrombus to produce bright signal.
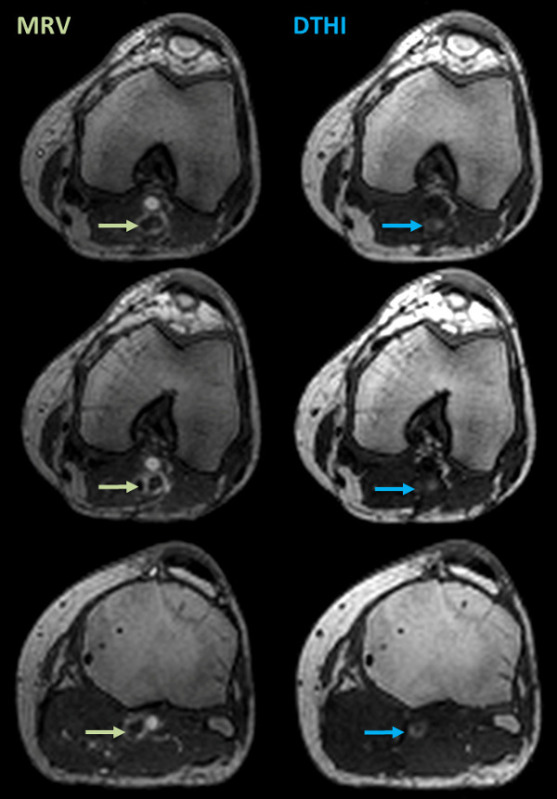 Figure 6: Sample DVT shown on DTHI imaging sequence. The left panels show MRV images with signal voids indicating total DVT (green arrows). The right panel show corresponding DTHI images with bright signal indicating presence of fresh thrombus (blue arrows). Please click here to view a larger version of this figure.
Figure 6: Sample DVT shown on DTHI imaging sequence. The left panels show MRV images with signal voids indicating total DVT (green arrows). The right panel show corresponding DTHI images with bright signal indicating presence of fresh thrombus (blue arrows). Please click here to view a larger version of this figure.
4. Assessment of Reproducibility
Evaluate a subset of ten subjects for the reproducibility of the analysis.
Perform analysis, as described above, by two separate image analysts (primary and secondary readers) to assess inter-observer variability
Ensure that primary reader also performs a second image analysis three months after the first analysis to assess intra-observer variability of the results.
Compute intra-class-correlation coefficients (ICC) and perform a Bland-Altman analysis to assess inter and intra-observer reproducibility. Carry out a one sample t-test to look for bias on Bland-Altman analysis. ICC >0.9 and no bias on Bland-Altman analysis are considered acceptable.
Representative Results
For the purpose of the reproducibility assessments, the baseline and follow up scans were pooled and analyzed as separate cases. From the 10 randomly selected subjects (2 visits each), there were 59 vessels with DVT identified using the MRV approach and 29 vessels with fresh thrombus identified by the DTHI. In the subset of these 10 randomly selected subjects analyzed for the reproducibility metrics, no vessels with DVT were deemed to be of un-analyzable quality (defined as subjective scoring 0-2 for both MRV and DTHI images). The average total thrombus volume (sum of volumes of each individual vessel with thrombus identified) measured by MRV with contrast was 3.13 ± 6.23 cm3 for the first analysis of the primary reader.
Assessment of reproducibility:
MRV thrombus volume (total thrombus): The intra- and inter-reader variability by intra-class correlation coefficients was 0.98 and 0.96, respectively. Bland-Altman analysis also showed no bias for both intra- and inter-observer assessments as well (one sample t-test with 0, p = 0.537 and 0.834 respectively).
DTHI thrombus volume (fresh thrombus): The intra- and inter-reader variability by intra-class correlation coefficients was 0.88 and 0.95 respectively. Bland-Altman analysis showed no bias for intra-observer assessments (one sample t-test with 0, p = 0.598). However, there was a significant bias observed for inter-observer variability on Bland-Altman analysis (one sample t-test with 0, p = 0.002). This indicates poorer reproducibility for the volume measured by DTHI compared to the MRV measured volumes. Figure 7 shows the ICC and Bland Altman plots for the intra-reader assessments of variability and Figure 8 shows the same plots for the inter-reader assessments.
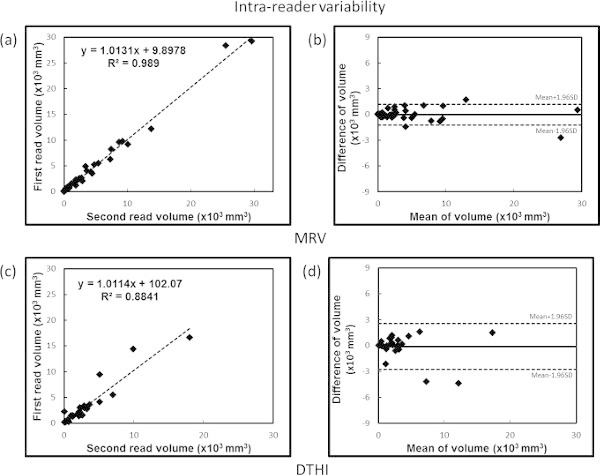 Figure 7: Intra-observer variability analysis. Top panels show (a) ICC and (b) Bland Altman plots for MRV data and bottom panels show (c) ICC and (d) Bland-Altman plots for DTHI data. Please click here to view a larger version of this figure.
Figure 7: Intra-observer variability analysis. Top panels show (a) ICC and (b) Bland Altman plots for MRV data and bottom panels show (c) ICC and (d) Bland-Altman plots for DTHI data. Please click here to view a larger version of this figure.
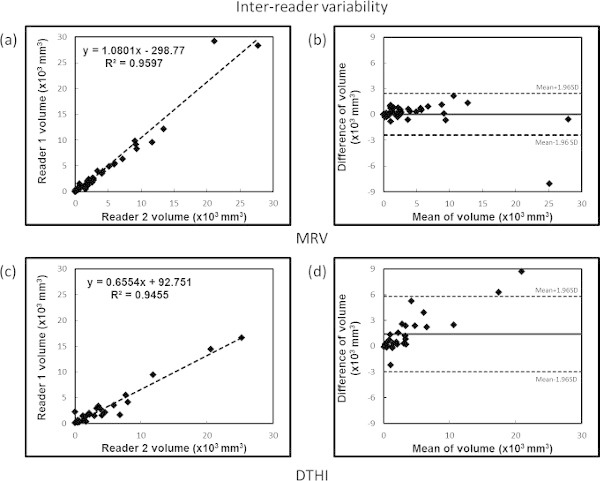 Figure 8: Inter-observer variability analysis. Top panels show (a) ICC and (b) Bland Altman plots for MRV data and bottom panels show (c) ICC and (d) Bland-Altman plots for DTHI data. Please click here to view a larger version of this figure.
Figure 8: Inter-observer variability analysis. Top panels show (a) ICC and (b) Bland Altman plots for MRV data and bottom panels show (c) ICC and (d) Bland-Altman plots for DTHI data. Please click here to view a larger version of this figure.
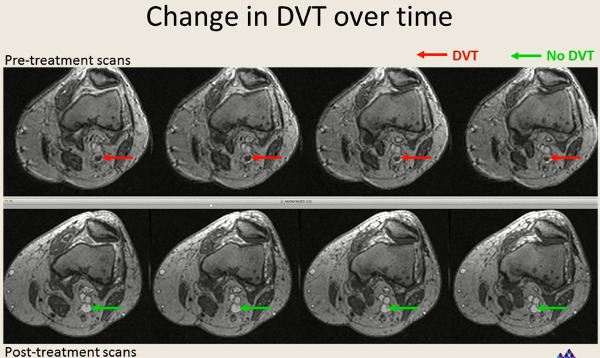 Figure 9: Sample images showing reduction in thrombus size 14-21 days after treatment.
Please click here to view a larger version of this figure.
Figure 9: Sample images showing reduction in thrombus size 14-21 days after treatment.
Please click here to view a larger version of this figure.
Discussion
This study demonstrated the feasibility of quantification of deep vein thrombosis on MR venography using gadofosveset trisodium as a contrast agent, with excellent reproducibility of analysis for quantifying thrombus volume in a multicenter setting. To compute total thrombus volume, the primary method utilized the post-contrast MRV scan to measure thrombus volume. The secondary method used was the direct thrombus imaging approach (DTHI), which leverages the presence of met-hemoglobin within a fresh thrombus to produce a bright signal on a T1W MR image13,14. The MRV volume measures were highly reproducible. The DTHI approach had less reproducibility. The DTHI images were acquired without the use of any contrast agent injection, and intrinsically had poorer contrast to noise ratios for delineating thrombus as they rely only on met-hemoglobin content within thrombus to produce an MR signal. The thrombus volumes observed were comparable to those obtained in previous studies with ultrasound and other imaging modalities21.
We used a 3D gradient echo acquisition for imaging. 3D imaging with isotropic voxels allows for multi-planar image reformatting. These images have fewer partial volume artifacts as compared to 2D acquisitions and may therefore result in more accurate DVT quantification. The use of MR venography addresses some of the drawbacks inherent to the current imaging methods used for diagnosing and monitoring DVT. CUS is ideal to be used as a screening tool, since it is inexpensive and has high sensitivity and specificity to detect acute DVT5. However, CUS cannot reliably assess the proximal deep veins in the thigh and lower pelvis, which are the most common sources of PE22. Additionally, CUS cannot directly quantify thrombus volume and composition, which are important when distinguishing between acute DVT (a potential source of PE) and chronic DVT (less likely to embolize) and for evaluation of therapeutic efficacy23. Alternatives to CUS include venography techniques (X-ray or computed tomography, CT) to detect thrombi as constant intra-luminal filling defects after contrast material infusion. X-ray venography is invasive, costly, and rarely used2,4. CT venography has gained recent interest for the evaluation of DVT and PE during the same imaging session. However, CT scans involve ionizing radiation and carry the risk of contrast nephropathy. Furthermore, while the sensitivity/specificity of CT is similar to CUS, its diagnostic value and potential to quantify and characterize the extent of thrombosis have not been well established24.
Our MRV and DTHI methods have limitations. No modifications can be made to the protocol as this can compromise the quality of the images obtained and distort the positioning of the scanning regions. MR imaging is expensive and unlike ultrasound, is not portable. Individuals with hip/knee replacements and other metal implants such as screws cannot be imaged if the material is made of ferromagnetic substances. The use of Gd-based contrast agents is also contraindicated in individuals with impaired renal function due to nephrogenic systemic fibrosis (NSF)25. The contrast agent we used, gadofosveset trisodium, appears to have the lowest rates of NSF of all the Gd-based agent in the market16,26. This protocol is also acquired in a multicenter setting on scanners of different imaging vendors and field strengths. This makes the acquisition parameters not identical at all sites and may contribute to reduced robustness of results. It is also possible that thrombus size as measured by volume may not be linearly related to disease evolution but rather only provide clues about the evolution of the thrombus.
Disclosures
Venkatesh Mani has received research grants from Novartis. Nadia Alie, Sarayu Ramachandran, Cecilia Besa, Philip Robson and Bachir Taouli have nothing to disclose. Michele Mercuri and Michael Grosso are employees of Daiichi Sankyo. Greg Piazza has received research grants from Daiichi Sankyo, BMS, EKOS, and the Thrombosis Research Institute. Samuel Goldhaber has received grants from BMS, Daiichi Sankyo, EKOS, NHLBI and the Thrombosis Research Institute. He is a consultant for Ariad, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Janssen, Merck, Pfizer and Portola. Zahi Fayad has received research grants from Daiichi Sankyo, Roche, GlaxoSmithKline, Merck, VBL Therapeutics, Novartis, Bristol-Myers Squibb, and Via Pharmaceuticals.
Acknowledgments
The authors have no acknowledgements.
References
- Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235–242. doi: 10.1016/j.beha.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost. 2013;11:412–422. doi: 10.1111/jth.12124. [DOI] [PubMed] [Google Scholar]
- Ramzi DW, Leeper KV. DVT and pulmonary embolism. Part I. Diagnosis. Am Fam Physician. 2004;69:2829–2836. [PubMed] [Google Scholar]
- Wilbur J, Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am Fam Physician. 2012;86:913–919. [PubMed] [Google Scholar]
- Goodacre S, Sampson F, Thomas S, van Beek E, Sutton A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6. doi: 10.1186/1471-2342-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias A, et al. A single complete ultrasound investigation of the venous network for the diagnostic management of patients with a clinically suspected first episode of deep venous thrombosis of the lower limbs. Thromb Haemost. 2003;89:221–227. [PubMed] [Google Scholar]
- Farahmand S, Farnia M, Shahriaran S, Khashayar P. The accuracy of limited B-mode compression technique in diagnosing deep venous thrombosis in lower extremities. Am J Emerg Med. 2011;29:687–690. doi: 10.1016/j.ajem.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Sampson FC, Goodacre SW, Thomas SM, van Beek EJ. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17:175–181. doi: 10.1007/s00330-006-0178-5. [DOI] [PubMed] [Google Scholar]
- Moody AR. Direct imaging of deep-vein thrombosis with magnetic resonance imaging. Lancet. 1997;350:1073. doi: 10.1016/s0140-6736(97)24041-9. [DOI] [PubMed] [Google Scholar]
- Phinikaridou A, et al. In vivo magnetization transfer and diffusion-weighted magnetic resonance imaging detects thrombus composition in a mouse model of deep vein thrombosis. Circ Cardiovasc Imaging. 2013;6:433–440. doi: 10.1161/CIRCIMAGING.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinikaridou A, Qiao Y, Giordano N, Hamilton JA. Detection of thrombus size and protein content by ex vivo magnetization transfer and diffusion weighted MRI. J Cardiovasc Magn Reson. 2012;14:45. doi: 10.1186/1532-429X-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JP, et al. Magnetic resonance venography for the detection of deep venous thrombosis: comparison with contrast venography and duplex Doppler ultrasonography. J Vasc Surg. 1993;18:734–741. doi: 10.1067/mva.1993.49364. [DOI] [PubMed] [Google Scholar]
- Westerbeek RE, et al. Magnetic resonance direct thrombus imaging of the evolution of acute deep vein thrombosis of the leg. J Thromb Haemost. 2008;6:1087–1092. doi: 10.1111/j.1538-7836.2008.02986.x. [DOI] [PubMed] [Google Scholar]
- Koizumi J, et al. Magnetic resonance venography of the lower limb. Int Angiol. 2007;26:171–182. [PubMed] [Google Scholar]
- Goyen M. Gadofosveset: the first intravascular contrast agent EU-approved for use with magnetic resonance angiography. Future Cardiol. 2007;3:19–26. doi: 10.2217/14796678.3.1.19. [DOI] [PubMed] [Google Scholar]
- Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi K, Yucel EK, Chamberlin P. A summary of safety of gadofosveset (MS-325) at 0.03 mmol/kg body weight dose: Phase II and Phase III clinical trials data. Invest Radiol. 2006;41:822–830. doi: 10.1097/01.rli.0000242836.25299.8f. [DOI] [PubMed] [Google Scholar]
- Zhang H. Trisodium-[(2-(R)-[(4,4-diphenylcyclohexyl)phosphono-oxymethyl]-diethylenetriamin epentaacetato)(aquo)gadolinium(III) Gadofosveset. 2004. [PubMed]
- Pfeil A, et al. Magnetic resonance VIBE venography using the blood pool contrast agent gadofosveset trisodium--an interrater reliability study. Eur J Radiol. 2012;81:547–552. doi: 10.1016/j.ejrad.2011.01.102. [DOI] [PubMed] [Google Scholar]
- Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouriel K, Greenberg RK, Green RM, Massullo JM, Goines DR. A volumetric index for the quantification of deep venous thrombosis. J Vasc Surg. 1999;30:1060–1066. doi: 10.1016/s0741-5214(99)70044-3. [DOI] [PubMed] [Google Scholar]
- Elias A, et al. A single complete ultrasound investigation of the venous network for the diagnostic management of patients with a clinically suspected first episode of deep venous thrombosis of the lower limbs. Thromb Haemost. 2003;89:221–227. [PubMed] [Google Scholar]
- Farahmand S, Farnia M, Shahriaran S, Khashayar P. The accuracy of limited B-mode compression technique in diagnosing deep venous thrombosis in lower extremities. Am J Emerg Med. 2011;29:687–690. doi: 10.1016/j.ajem.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Goodacre SW, Sampson FC, van Beek EJ. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol. 2008;63:299–304. doi: 10.1016/j.crad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Heverhagen JT, Krombach GA, Gizewski E. Application of Extracellular Gadolinium-based MRI Contrast Agents and the Risk of Nephrogenic Systemic Fibrosis. Rofo. 2014. [DOI] [PubMed]
- Alhadad A, et al. Safety aspects of gadofosveset in clinical practice - analysis of acute and long-term complications. Magn Reson Imaging. 2014. [DOI] [PubMed]


