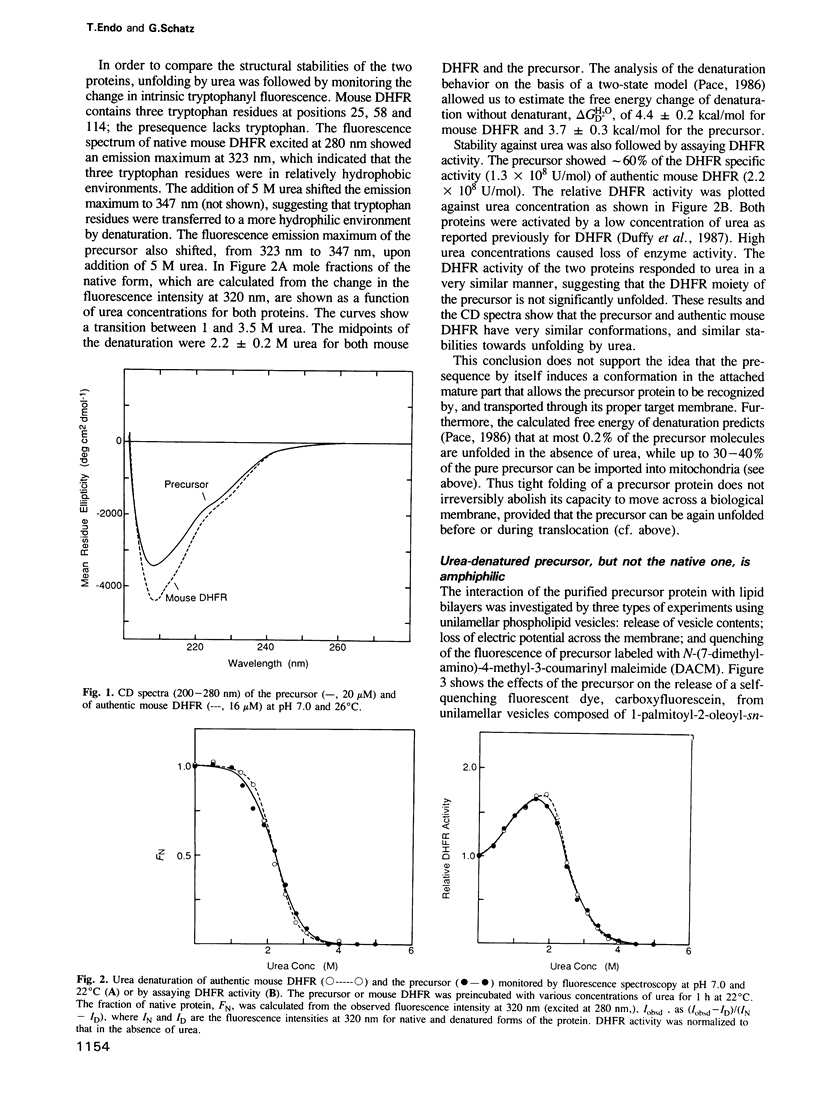
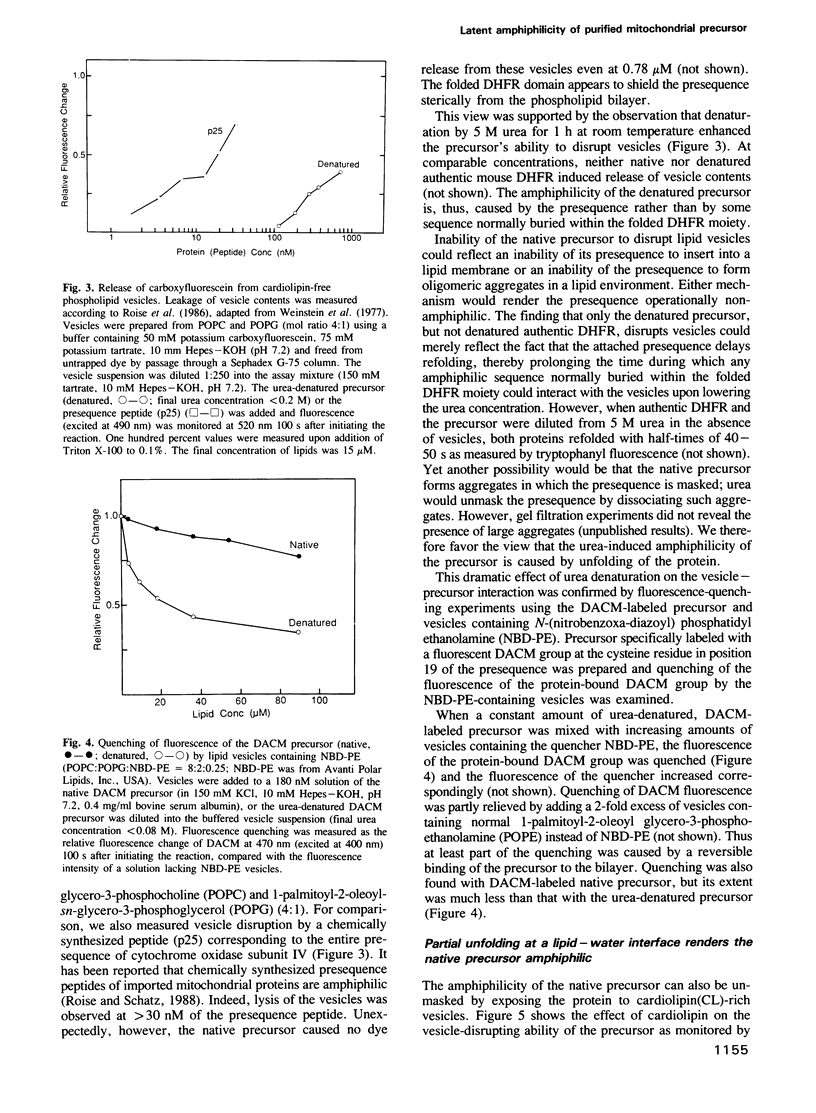
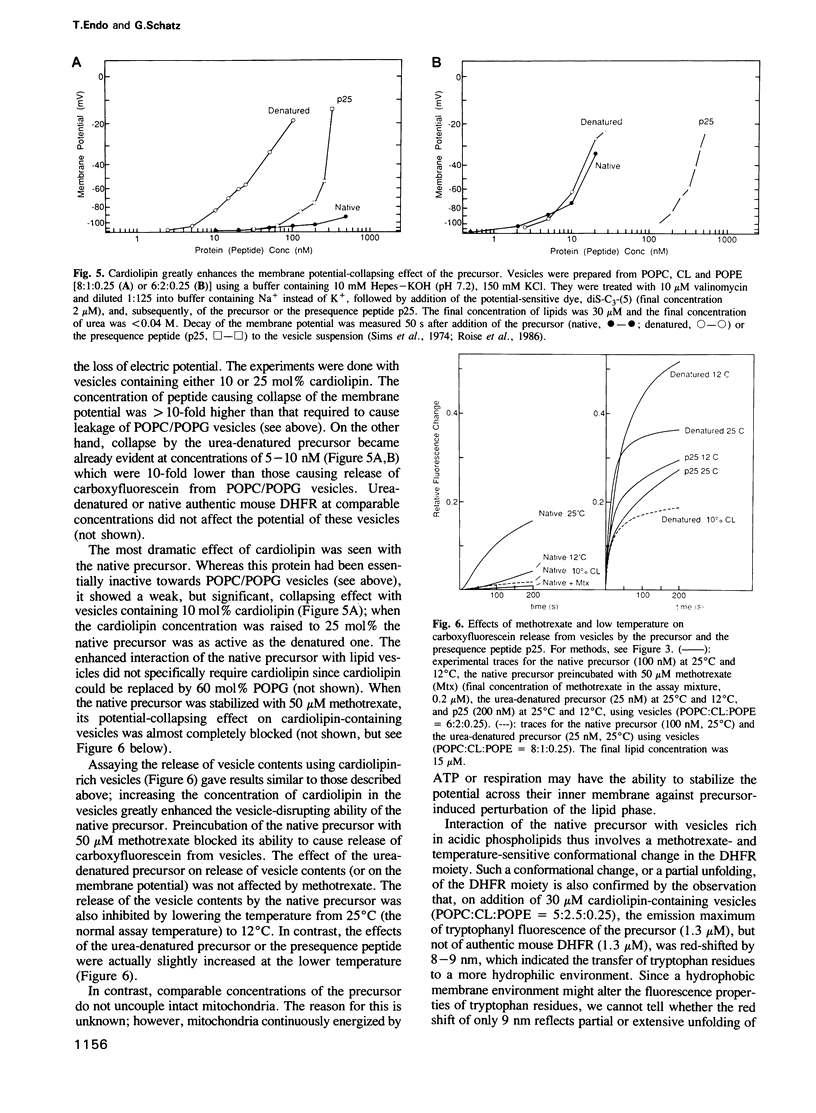
Abstract
We have purified milligram amounts of an importable mitochondrial precursor protein [the presequence of yeast cytochrome oxidase subunit IV fused to mouse dihydrofolate reductase (DHFR)]. This has made it possible, for the first time, to perform detailed studies on the conformation of a precursor protein and its interaction with lipid membranes. The precursor protein closely resembled authentic mouse DHFR with respect to secondary structure (measured by CD spectra) and stability towards urea (measured by tryptophan fluorescence and enzyme activity). With this precursor protein, the presequence thus does not significantly alter the folding of the attached 'passenger protein'. In contrast to the corresponding presequence peptide, the native precursor exhibited only weak ability to disrupt vesicles with a low mol% of negatively charged lipids, suggesting that the passenger protein masks the amphiphilic properties of the presequence. The membrane-perturbing properties of the precursor were greatly enhanced by increasing the vesicles' content of negatively charged lipid or by denaturing the precursor in 5 M urea. Interaction with vesicles rich in acidic phospholipid was accompanied by partial unfolding of the precursor, suggesting that such a conformational change may also be involved in the interaction of the precursor with the mitochondrial membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien S. M., Freeman K. B. Mitochondrial malate dehydrogenase and its precursor have different conformations. Biochem Biophys Res Commun. 1986 Nov 26;141(1):313–318. doi: 10.1016/s0006-291x(86)80370-9. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy T. H., Beckman S. B., Peterson S. M., Vitols K. S., Huennekens F. M. L1210 dihydrofolate reductase. Kinetics and mechanism of activation by various agents. J Biol Chem. 1987 May 25;262(15):7028–7033. [PubMed] [Google Scholar]
- Eilers M., Hwang S., Schatz G. Unfolding and refolding of a purified precursor protein during import into isolated mitochondria. EMBO J. 1988 Apr;7(4):1139–1145. doi: 10.1002/j.1460-2075.1988.tb02923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Taniuchi H., Anfinsen C. B. On the role of heme in the formation of the structure of cytochrome c. J Biol Chem. 1973 May 10;248(9):3188–3195. [PubMed] [Google Scholar]
- Giudice L. C., Weintraub B. D. Evidence for conformational differences between precursor and processed forms of thyroid-stimulating hormone beta subunit. J Biol Chem. 1979 Dec 25;254(24):12679–12683. [PubMed] [Google Scholar]
- Gupta S. V., Greenfield N. J., Poe M., Makulu D. R., Williams M. N., Moroson B. A., Bertino J. R. Dihydrofolate reductase from a resistant subline of the L1210 lymphoma. Purification by affinity chromatography and ultraviolet difference spectrophotometric and circular dichroic studies. Biochemistry. 1977 Jul 12;16(14):3073–3079. doi: 10.1021/bi00633a005. [DOI] [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortt A. A., Liu T. Y. On the mechanism of action of streptococcal proteinase. I. Active-site titration. Biochemistry. 1973 Jan 16;12(2):320–327. doi: 10.1021/bi00726a023. [DOI] [PubMed] [Google Scholar]
- Miyata S., Akazawa T. alpha-Amylase biosynthesis: signal sequence prevents normal conversion of the unprocessed precursor molecule to the biologically active form. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7792–7795. doi: 10.1073/pnas.79.24.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Yanofsky C. Amino-terminal sequence and processing of the precursor of the leucine-specific binding protein, and evidence for conformational differences between the precursor and the mature form. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2005–2009. doi: 10.1073/pnas.77.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C. N. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Neupert W. Transport of proteins into mitochondria. Posttranslational transfer of ADP/ATP carrier into mitochondria in vitro. Eur J Biochem. 1980 Aug;109(1):217–229. doi: 10.1111/j.1432-1033.1980.tb04787.x. [DOI] [PubMed] [Google Scholar]



