Abstract
Movements are the main measurable output of central nervous system function. Developing behavioral paradigms that allow detailed analysis of motor learning and execution is of critical importance in order to understand the principles and processes that underlie motor function. Here we present a paradigm to study movement acquisition within a daily session of training (within-session) representing the fast learning component and primary acquisition as well as skilled motor learning over several training sessions (between-session) representing the slow learning component and consolidation of the learned task. This behavioral paradigm increases the degree of difficulty and complexity of the motor skill task due to two features: First, the animal realigns its body prior to each pellet retrieval forcing renewed orientation and preventing movement execution from the same angle. Second, pellets are grasped from a vertical post that matches the diameter of the pellet and is placed in front of the cage. This requires a precise grasp for successful pellet retrieval and thus prevents simple pulling of the pellet towards the animal. In combination with novel genetics, imaging and electrophysiological technologies, this behavioral method will aid to understand the morphological, anatomical and molecular underpinnings of motor learning and memory.
Keywords: Behavior, Issue 100, Motor Learning, Rat, Skilled Reaching Task, Single Pellet Grasping, Procedural Learning
Introduction
Movement control is a core function of the central nervous system (CNS). Motricity is the main measurable output of CNS function and the main possibility for individuals to interact with the external world. Understanding the principles of motor function and the mechanisms that underlie the learning of a motor task is currently one of the big challenges in neuroscience. Morphological, physiological and molecular changes were found upon acquisition of a new motor task. For example, the shape and number of synapses change in response to skilled motor training1-5, and functional changes of the synaptic machinery were observed after motor learning. Synaptic responses were higher in the connections of the forelimb-representing region of the trained motor cortex compared to the untrained hemisphere of the same animal or to responses from untrained animals6,7. Electrophysiological observations also suggest that long-term potentiation (LTP) and long-term depression (LTD) like mechanisms take place during the learning of a new motor skill, and that the range of synaptic operation, which is defined between the limiting borders of LTP and LTD saturation, is modified8. Furthermore, it has been shown that activity markers and plasticity promoting molecules such as c-fos, GAP-43, or BDNF but also plasticity inhibiting molecules such as Nogo-A display regulatory roles for learning-related neuronal plasticity9-16.
These advances towards a better understanding of the mechanisms underlying motor learning could only be achieved with the use of behavioral paradigms that allow precise control of the acquisition of a new motor skill, e.g., skilled forelimb-reaching. Only a well-structured behavioral task allows to monitor and capture correlative changes that occur upon learning and execution of the respective task. Here we visually demonstrate a modified version of the skilled forelimb single-pellet reaching task in rats adapted from Buitrago et al.17 The presented paradigm allows the analysis of movement acquisition within a daily training session (within-session) representing the fast learning component and primary acquisition as well as skilled motor learning over several sessions (between-session) representing the slow learning component and maintenance of the learned task18. Importantly, this behavioral paradigm increases the degree of difficulty and complexity of the motor skill task due to two features: First, the rats are trained to turn around their axis after each grasp and thus to realign their body prior to the next pellet reach and renew the body orientation, preventing constant movement execution from the same angle. Second, pellets are retrieved from a vertical post placed in front of the cage. Due to the small diameter of the post, pellets can easily be kicked off requiring a precise grasp for successful retrieval and preventing simple pulling of the pellet towards the animal.
Such complex behavioral testing allows deeper insights into the mechanisms underlying motor learning. Compared to mice, rats are superior in their performance of complex behavioral tasks and thus better suited for complex paradigms as presented in this study. Considering the increasing genetic possibilities available for rats19,20, the combination of precise and well controlled behavioral testing methods with genetic manipulations, imaging and physiological techniques represents a powerful toolbox to better understand the neurobiological basis of motor learning and memory.
Protocol
All experiments were performed in accordance with the guidelines of the Veterinary Office of the Canton Zurich, Switzerland.
1. Animal Handling and Habituation
- Animal Handling Note: 5 days prior to the start of the experiment, perform step 1.1.1 daily.
- For behavioral experiments, accustom the animals to the experimenter. Have daily handling sessions lasting 10-15 min per animal. Clean the box after each animal’s session.
- Initially, place the experimenter’s hand into a cage allowing the animal to explore and smell to become familiarized with the experimenter.
- Next, gently lift the animal with the experimenter’s hand in a safe manner by grasping the rat’s body between the front- and hind paws allowing further familiarization.
- Weigh each animal daily to obtain a baseline bodyweight before food deprivation.
- Apparatus Habituation and Food Familiarization
- Weigh each animal daily to obtain a baseline bodyweight before food deprivation.
- Start food depriving rats 3 days prior to the beginning of pre-training on a standard laboratory diet. Give rats 0.05 g of food per 1 g bodyweight per day (e.g., a rat weighing 200 g, start with 10 g of food). Ensure bodyweight to not decrease more than 10% per day by monitoring bodyweight daily.
- If several animals are held in one cage, dominant rats may eat more than less dominant ones. In case the bodyweight of an animal decreases, feed the animal separately instead of group feeding. Give water ad libitum.
- To acquaint the rat with the grasping apparatus, place the animal into the training box (Figure 1). Have sugar pellets placed in the training box in proximity to the slit opening to familiarize the animal with the food pellets. Perform this step 10-15 min daily for 3 days.
2. Pre-training and Motor Skill Learning
- Pre-training
- One day after familiarization, place the animal into the training box and place the pellet closely to the slit opening so it can be reached by the animal’s tongue. Exclude animals that retrieve pellets with their forelimb during pre-training Note: At this stage, pellet retrieval with the tongue is crucial and usually the animal’s method of choice. Pellets should be in no case grasped with the forelimb until the first day of motor skill learning to allow adequate monitoring of the learned reaching task. Pellet retrieval with the forelimb during pre-training is an exclusion criterion and is in most cases not observed.
- Teach the rat to either run to the rear of the cage and return to the slit opening or step back and turn around its own axis in order to receive the next food pellet with the tongue. Allow time for the animal to explore the cage, run to the rear and return to the slit opening. If the animal does not correctly execute the task, use a forceps to gently tap at the end rear of the cage and catch the animal’s attention. Once the animal is at the rear, gently tap at the cage front to guide the animal to the slit opening. Note: Once the animal reaches a defined standard value (e.g., 50 successful pellet retrievals with the tongue in less than 15 min), the animal qualifies for the motor skill learning stage. On day 1 and 2 of pre-training, learners can already be distinguished from non-learners. Non-learners can be excluded from the study at this step. This decreases the probability of having a high number of non-learners during the motor learning step (2.2).
- During pre-training, food deprive rats on a standard laboratory diet. Give water ad libitum. Monitor body weight daily throughout the study. Do not use same room to train male and female rats. Ensure a calm and noise-free environment for the animal.
- Determination of Paw Preference and Motor Skill Learning
- During the first session of motor skill learning, replace the slide in front of the window with a post. Place the sugar pellet about 1.5 cm away from the window on the post so that the animal cannot reach the pellet with its tongue but only retrieve it by a precise forelimb reaching and grasping movement.
- To enforce pellet retrieval by the forelimb, use a forceps to gently bring the pellet close to the animal’s mouth and retract the pellet while the animal attempts consumption with the tongue. Carry out this task repetitively until the animal stretches out the forelimb and grasps a pellet.
- Place the post central to the window opening. To determine paw preference, carefully observe the first 10 trials on training day 1. More than 70% of the reaching attempts (i.e., 7 out of 10) have to be executed with the same forelimb. If this is not achieved, continue with another round of 10 trials until the 70% threshold is reached.
- After paw preference determination, shift the post towards the preferred forelimb and align central to the border of the window opening. Preferred paw alignment means the post is shifted contrary to the respective paw to allow an optimal angle for reaching (Figure 1B, C).
- Classify a trial, defined as a new pellet presented to the animal, as successful (reach, grasp, retrieve and eat the pellet), drop (reach, grasp and lose pellet during retrieval) or fail (knock pellet off the post). Note down every trial in your sheet and analyse the data after the experiment.
- Perform one daily session consisting of a defined number of trials (e.g., 150) or a maximum time (e.g., 1 hr) for each animal.
- Investigate precision and fine-tuning of the movement using first attempt analysis. A first attempt is defined by grasping of the pellet in a single monolithic movement without disruption, hesitation or repetition of individual movement components. Carefully observe each grasp by the rat.
- If the rat hesitates or retracts during a reach or attempts several trials to correctly grasp the pellet, note the respective trial as success but not first attempt. If the animal successfully grasps the pellet in a single monolithic reach, note down the respective trial as successful first attempt in a separate column in your sheet.
- During motor skill learning, food deprive rats on a standard laboratory diet. Give water ad libitum. Monitor body weight daily throughout the study.
Representative Results
Successful motor skill acquisition is only achieved through consistent practice. Despite careful consideration of all aspects, some rats fail to learn the task (Figure 2). These 'non-learners' either lack motivation resulting in few or absent attempts of pellet retrieval from the start of the experiment or generally lose interest in reaching for the pellets leading to continuously failed attempts. By contrast, some animals show aggressive and over-motivated behavior resulting in overhasty and rushed grasping attempts that lead to failures. A third group of unsuccessful learners are those animals that start with high success rates and do not make significant improvement leading to stagnate or even decreased learning curves.
Successful motor skill learning consists of two phases: A fast learning component representing primary acquisition usually observed within the first day of motor training (within-session; Figure 3) and skilled motor learning over several repetitive training sessions (between-session, Figure 4) representing the slow learning component and consolidation of the learned task. Between-session learning curves are characterized by steep learning curves during days 1-3 and a plateau level during the last days of learning.
To analyze the performance during each daily session (within-session analysis), divide the total number of trials into 25 trial bins (e.g., 6 bins for 150 trials). After the experiment, calculate the percentage of successfully grasped pellets for each bin by dividing the number of successes in the respective bin by the total number of trials on the respective day.
It should be considered that motor skill acquisition can vary among individual animals. Different rats need different numbers of days to reach the plateau level. Thus, individual learning curves are usually not as smooth as the average learning curve.
Another expression of successful motor learning is the analysis of fine tuned motor learning. To assess this aspect, we measured the number of pellets grasped on the first attempt during a monolithic movement without hesitation or disruption in comparison to all successfully measured pellets (Figure 5).
The examples in Figure 2-5 show the learning curves of 150 grasped pellets/day over the course of 6 days. Day 1 refers to the first day of motor learning.
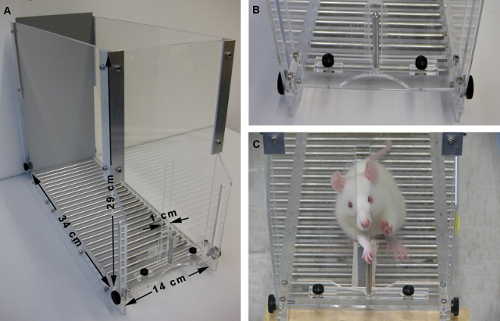 Figure 1: Design of the rat training box. (A) The training box with its respective dimensions. To avoid retrieval of pellets that were lost inside the box, the ground of the training chamber is made of metal rods, through which lost pellets fall. (B) Close view of the slit opening and the post. Note the position of the post aligned central to the slit opening. (C) Example of a rat successfully grasping a pellet through the slit opening. Note the shifted post position and the resulting angle towards the animal’s preferred forelimb (in this case right). Please click here to view a larger version of this figure.
Figure 1: Design of the rat training box. (A) The training box with its respective dimensions. To avoid retrieval of pellets that were lost inside the box, the ground of the training chamber is made of metal rods, through which lost pellets fall. (B) Close view of the slit opening and the post. Note the position of the post aligned central to the slit opening. (C) Example of a rat successfully grasping a pellet through the slit opening. Note the shifted post position and the resulting angle towards the animal’s preferred forelimb (in this case right). Please click here to view a larger version of this figure.
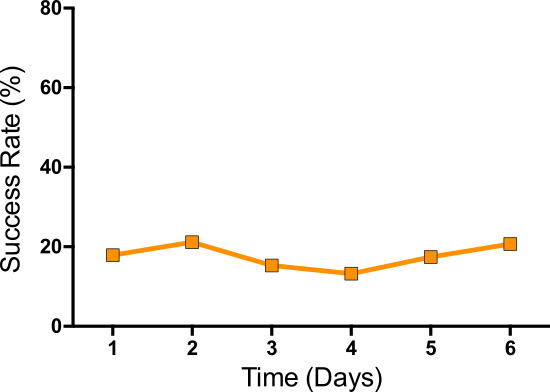 Figure 2: Example of an animal that did not exhibit successful acquisition of the motor skill over the course of 6 days. The success rate stagnates around 20% with no improvement on further training.
Figure 2: Example of an animal that did not exhibit successful acquisition of the motor skill over the course of 6 days. The success rate stagnates around 20% with no improvement on further training.
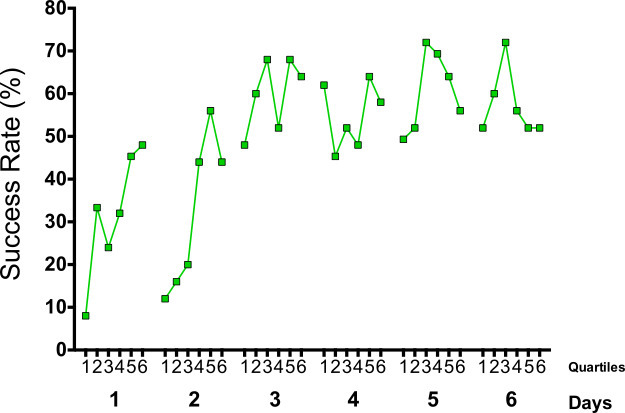 Figure 3: Within-day analysis of the same example as shown in Figure 4. The success rate is divided into 6 bins of 25 pellets illustrating the improvement in motor learning throughout a single daily session. Note the initial success rate during the first 25 trials of the first 2 days in comparison with the average success rate of the respective days in Figure 4 as well as the general improvement within day 1 and 2 in comparison to Figure 4.
Figure 3: Within-day analysis of the same example as shown in Figure 4. The success rate is divided into 6 bins of 25 pellets illustrating the improvement in motor learning throughout a single daily session. Note the initial success rate during the first 25 trials of the first 2 days in comparison with the average success rate of the respective days in Figure 4 as well as the general improvement within day 1 and 2 in comparison to Figure 4.
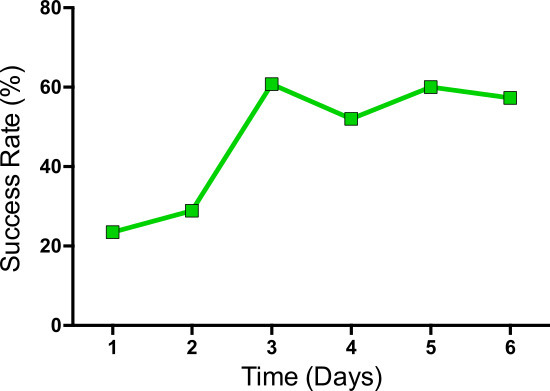 Figure 4: Example of an animal with a typical, successful learning curve over the course of 6 days. The percentage of successfully grasped pellets (success rate) increases during the first 3 days and reaches a plateau during the remaining days.
Figure 4: Example of an animal with a typical, successful learning curve over the course of 6 days. The percentage of successfully grasped pellets (success rate) increases during the first 3 days and reaches a plateau during the remaining days.
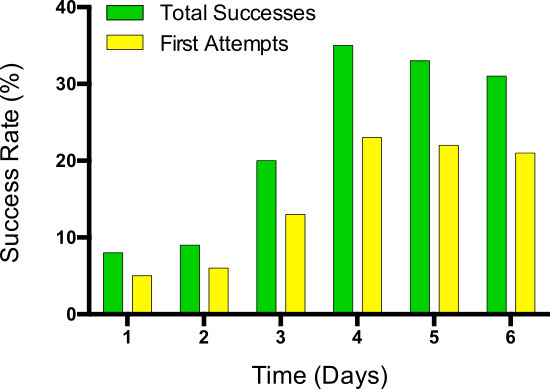 Figure 5: Successful first attempts are shown in comparison to the total number of correctly grasped pellets as a measure for fine tuned movement learning.
Figure 5: Successful first attempts are shown in comparison to the total number of correctly grasped pellets as a measure for fine tuned movement learning.
Discussion
The paradigm shown in this study is adapted from Buitrago et al.18 and differs from the classical single pellet reaching paradigm17 mainly in two aspects:
First, studying within-session improvement allows analysis of the learned task within a single day, which can provide a different level of information such as investigation of the fast learning component compared to the slow learning component represented by the average daily values (see Figures 3 and 4). Second, the behavioral paradigm presented here increases the degree of difficulty and complexity of the motor skill task. The animal is forced to realign its body and reaching orientation prior to each pellet retrieval. This prevents simple repetitive movement execution from the same angle and requires profound spatial orientation. Moreover, pellets are retrieved from a thin vertical post that is placed in front of the cage and matches the diameter of the pellet thus requiring a precise grasp for successful retrieval and preventing simple pulling of the pellet towards the animal.
An important step is teaching the animal to run to the rear of the cage and return to the slit opening during pre-training. Hinting the rat to the desired location by using a forceps and gently tapping at the cage’s rear panel aids the animal to understand the task. The experimenter should use this tool carefully as overtapping produces too much noise and irritates the animal. Once the rat has returned to the front of the cage, place several sugar pellets at the slit opening for the animal to get accustomed with the taste, smell and location of the pellet. Another critical step is pellet retrieval with the forelimb on the first day of motor learning. The animal often continues attempts to retrieve the pellet with the tongue as learned during pre-training. Using a forceps to gently bring the pellet close to the animal’s mouth and retraction of the pellet while the animal attempts consumption with the tongue enforces the rat to stretch out the forelimb for successful pellet retrieval. The goal is to allow the animal to grasp the pellet from the forceps using the forelimb and retrieve the sugar pellet to its mouth. Once this has been achieved, pellets can be placed at the post and paw preference determination can be evaluated. These two steps are the most crucial parts of the entire experiment and require a careful approach to the animal as well as the experimenter’s patience.
It is important to note that differences in learning exist between individual animals and between gender (males learn slower than females18) and strains (according to our observation, Sprague-Dawley rats used in this study and Long-Evans rats show superior learning compared to e.g., Lewis rats). Thus, in order to meet the challenging requirements of the task, success rates may require modifications of the paradigm depending on gender and strain. To keep the variance low, do not mix strains and gender in an experimental series.
Well-structured and controllable behavioral paradigms are of crucial importance for studies investigating cellular correlates underlying mechanisms of pathologic and physiologic behavior. The more complex behavioral tasks that rats are capable to learn represent an important advantage of rats over mice. The ongoing advances in technologies for genetic manipulations in the rat will allow in the very near future to conduct experiments in rats that were so far only possible in mice19-21. In combination with novel imaging technologies and techniques allowing targeted manipulation of neuronal circuits, behavioral paradigms in rats may open new avenues towards the understanding of physiologic and pathologic principles of nervous system functions.
Disclosures
The authors have nothing to disclose
Acknowledgments
This work was funded by grants of the Swiss National Science Foundation (Grant 31003A-149315-1 to M.E.S. and Grant IZK0Z3-150809 to A.Z.), to A.Z. the Heidi Demetriades Foundation, to M.E.S. the European Research Council (‘Nogorise’) and the Christopher and Dana Reeve Foundation (CDRF).
References
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends in neurosciences. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature reviews. Neuroscience. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zuo Y. Spine plasticity in the motor cortex. Current opinion in neurobiology. 2011;21:169–174. doi: 10.1016/j.conb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nature neuroscience. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Donoghue JP, Dunaevsky A. Plasticity of the synaptic modification range. Journal of neurophysiology. 2007;98:3688–3695. doi: 10.1152/jn.00164.2007. [DOI] [PubMed] [Google Scholar]
- Fritsch B, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Ying Z, de Vellis J, Gomez-Pinilla F. Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia. 2007;55:966–975. doi: 10.1002/glia.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson A, et al. Activity-induced and developmental downregulation of the Nogo receptor. Cell and tissue research. 2003;311:333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- Karlen A, et al. Nogo receptor 1 regulates formation of lasting memories. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20476–20481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova YA, Giger RJ. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends in neurosciences. 2013;14:7–23. doi: 10.1016/j.tins.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nature reviews. Neuroscience. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Zemmar A, et al. Neutralization of Nogo-A enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8685–8698. doi: 10.1523/JNEUROSCI.3817-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behavioural brain research. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- Buitrago MM, Ringer T, Schulz JB, Dichgans J, Luft AR. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behavioural brain research. 2004;155:249–256. doi: 10.1016/j.bbr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Geurts AM, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tews B, et al. Synthetic microRNA-mediated downregulation of Nogo-A in transgenic rats reveals its role as regulator of synaptic plasticity and cognitive function. Proceedings of the National Academy of Sciences of the United States of America. 2013. [DOI] [PMC free article] [PubMed]
- Li D, et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nature. 2013;31:681–683. doi: 10.1038/nbt.2661. [DOI] [PubMed] [Google Scholar]


