Abstract
Purpose.
We developed a novel technique for accelerated drug screening and retinotoxin characterization using time-lapse optical coherence tomography (OCT) and a drug microapplication device.
Methods.
Using an ex vivo rabbit eyecup preparation, we studied retinotoxin effects in real-time by microperfusing small retinal areas under a transparent fluoropolymer tube. Known retinotoxic agents were applied to the retina for 5-minute periods, while changes in retinal structure, thickness, and reflectance were monitored with OCT. The OCT images of two agents with dissimilar mechanisms, cyanide and kainic acid, were compared to their structural changes seen histologically.
Results.
We found the actions of retinotoxic agents tested could be classified broadly into two distinct types: (1) agents that induce neuronal depolarization, such as kainic acid, causing increases in OCT reflectivity or thickness of the inner plexiform and nuclear layers, and decreased reflectivity of the outer retina; and (2) agents that disrupt mitochondrial function, such as cyanide, causing outer retinal structural changes as evidenced by a reduction in the OCT reflectivity of the photoreceptor outer segment and pigment epithelium layers.
Conclusions.
Retinotoxin-induced changes in retinal layer reflectivity and thickness under the microperfusion tube in OCT images closely matched the histological evidence of retinal injury. Time-lapse OCT imaging of the microperfused local retina has the potential to accelerate drug retinotoxicological screening and expand the use of OCT as an evaluation tool for preclinical animal testing.
Keywords: microperfusion, optical coherence tomography, retinotoxins
Optical coherence tomography was used with drug microperfusion on ex vivo rabbit retina to characterize the real-time effects of retinotoxins.
Introduction
Retinal neurotoxicity is a major concern for new drug development and evaluation. Retinotoxicity testing is complicated, because drugs can have direct and indirect effects, even affecting specific cell types or layers that might be missed in simple drug screens, such as using cultured retinal cells or fundus imaging. Identifying better methods to predict retinotoxicity in ex vivo animal models would be extremely helpful in the drug evaluation process and could help prevent harmful agents from advancing to human trials.
Many techniques for studying retinal neurotoxicity, such as classical histology and patch clamping, are time-intensive and destructive to tissue. The electroretinogram (ERG) and the multifocal ERG are less intrusive, but have limited lateral spatial resolution and no depth resolution. Optical techniques that can be applied to functional studies, such as retinal angiography and fluorescence imaging, do not collect electrophysiological data, have limited depth sectioning capability, and require intense visible light. Visible light imaging confounds the retina's physiological response by activating its natural detection circuitry. Optical coherence tomography (OCT), however, uses infrared light to nondestructively and noninvasively optically section the living retina in real time with micron level resolution. Optical coherence tomography can also be used as a functional imaging technique to study the physiology of retina, brain, and other nervous tissue.1–8
Optical coherence tomography imaging has shown clinical utility in evaluating ophthalmic drug trial outcomes for glaucoma, macular degeneration, and edema.9–14 The role of OCT as an evaluation tool for retinal function is expanding, including measurement of blood flow,15,16 neuronal activity,2,4,17 and damage induced from either electrical overstimulation18 or neurotoxins19,20 in animal models.
While many retinotoxic compounds are well documented in the clinical literature,21–25 how they affect retinal OCT images in real time is unclear. The OCT evaluation methods of adverse effects following retinal drug application by systemic injection are hampered by the lethal dose limits of the animal. Drug uptake is dependent on the permeability of the blood–retina barrier, while the clearance of the test agent is dependent on the circulation and aqueous outflow pathways. Intravitreal drug injections are better as they act faster, affect a single eye, and allow the use of the contralateral eye to function as a control.26,27 However, intravitreal drug injections act globally on the treated eye and interpretation can be confounded by variability in the control eye thickness, requiring measurements to be matched to the exact same retinal eccentricity between the two eyes. Intravitreal injections also are limited by the slow kinetics of drug diffusion and clearance.28 Many chronically applied drugs result in altered structure in local retinal regions, such as the bulls-eye maculopathy of fluoroquinolones,19,29 tamoxifen retinopathy spots,29,30 and the sector-selective thinning of optic nerve fibers by vigabtrin.29,31 Spatially confined drug perfusion in ex vivo retina could be useful for evaluating local retinotoxicity during drug development as well as providing useful preclinical information for the regulatory evaluation process.
We recently have developed a drug screening method by combining a transparent microperfusion device and OCT in a live rabbit retinal eyecup preparation (Qian H, et al. IOVS 2012;53:ARVO E-Abstract 4979). This method was designed to confine the application of test compounds on small subregions of the retina, which would permit multiple pharmacological tests on a single eye. If successful, drugs that affect specific retinal features, such as the optic nerve, could be probed selectively at only the feature of interest. This method also would greatly increase control over drug application by removing the blood–retina barrier, whole body uptake, and ocular clearance kinetics from drug exposure, while simultaneously reducing the number of test animals and potentially the study duration. This method could improve the use of OCT for detecting changes in retinal morphology, reflectivity, and scattering to screen drug candidates rapidly for retinotoxicity.
Identifying the direct mode of action of drugs from OCT imaging is difficult because the induced changes in optical scattering and refractive index are complex.3 Drugs can act on ion channels, transporters, intracellular and mitochondrial enzymes, blood vessels, and protein synthesis. These, in turn, could cause secondary morphological changes, such as swelling or retinal pigment epithelium detachment, and all of these could potentially have varying effects on the retinal reflectance signatures in OCT images. To help elucidate any possible correlation between known mechanistic and morphological retinotoxin effects with phenomenological changes observed with OCT, we also compared OCT images from live ex vivo tissue treated with retinotoxins with well-known mechanisms (kainic acid [KA] and cyanide) with classical histological sections of the same tissue.
Methods
Eyecup Preparation
Rabbits of either sex were anesthetized with ketamine–xylazine (35–50 mg/kg, 5–10 mg/kg) by a protocol approved by the United States Food and Drug Administration (FDA) Institutional Animal Care and Use Committee, and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eyes were excised, hemisected, and the posterior half of the eyewall containing the retina was everted onto a dome-shaped chamber 13 mm in diameter32 and superfused with oxygenated (95% O2/5% CO2) bicarbonate-buffered Ames Ringer (Sigma-Aldrich Corp., St. Louis, MO, USA or US Biological, Swampscott, MA, USA).33 The Ringer's solution was heated to 34° to 35°C and flowed across the eyecup chamber (volume ≈ 350 μL) at 5 to 6 mL/min. A total of 40 eyes from 26 animals was used for this study.
Test Agents
We tested KA (3–300 μM), sodium cyanide (NaCN, 3 mM), sodium glutamate (10 mM), chloroquine (10 μM; Sigma-Aldrich Corp.), and D-amino adipic acid (10 mM; Alfa-Aesar, Illkirch, UK).
Isolated Drug Perfusion
An optically transparent drug microperfusion device was constructed using a fluorinated ethylene propylene (FEP) tube, 0.8 mm inner diameter, 1.0 mm outer diameter (Zeus, Inc., Orangeburg, SC, USA), which was faced at a 38° angle. Local drug microperfusion was performed by gently apposing the FEP tube lumen against the retina to form a chamber using an MP285 micromanipulator (Sutter Instruments, Novato, CA, USA) and a goniometer stage (Optosigma, Santa Ana, CA, USA). Inside the FEP tube lumen were sealed two fine (AWG 28-30) polyimide microtubes (Amazon.com, Seattle, WA, USA) through which drugs were exchanged (Fig. 1). Test drugs were held in carbogen-gassed wells of Ames Ringer at 37°C and flowed by gravity into the transparent FEP tube chamber (≈1 μL internal volume) at 0.2 mL/min through the polyimide microtube close to the lumen at the retinal surface. Drugs were removed from the transparent FEP tube by the recessed polyimide microtube using a low-flow peristaltic pump (Fisher Scientific, Hampton, NH, USA). Drug inflow and outflow were balanced carefully and achieved a high exchange rate of roughly 200 times per minute (0.2 mL/min flow rate/1 μL volume) while the retina outside the tube was superfused with normal Ames Ringer, which helped limit the effects of drug diffusion. Ames Ringer was applied first for 5 minutes in the tube to establish a baseline retinal OCT image, followed by 5 minutes of the test agent, followed by an Ames Ringer washout period of at least 10 minutes. Controls were performed by applying untreated Ames Ringer for the entire 20-minute period.
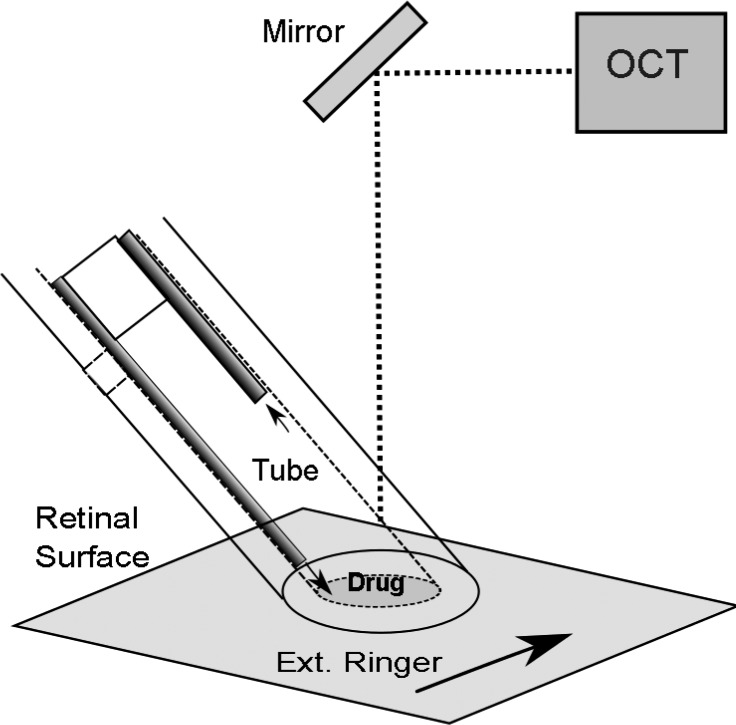
Figure 1.
Schematic diagram of the transparent tube drug microperfusion device experiment. The lumen of an optically matched transparent tube is closely apposed to the retinal surface. The test drug in Ringer's solution enters the transparent microperfusion tube chamber from a microtube (dark tube) close to the retina and is removed by the more recessed microtube. The retinal area external to the microperfusion tube is exchanged rapidly with normal Ames Ringer (black arrow) to limit the effects of drug diffusion outside the tube. An OCT unit continuously scans across the tube lumen to provide cross-sectional B-scans.
Imaging
A custom-made 855-nm Fourier-domain OCT system (Physical Sciences, Inc., Andover, MA, USA) was used to perform the retinal imaging on a mesopic background (70 lux).18,34 The source spectral bandwidth is 56 nm, yielding an axial point spread function, measured with nanoparticles35 of 5.7 μm (1.4 μm/pixel sampling) in retinal tissue assuming a refractive index of 1.38. The system has a signal-to-noise ratio of approximately 94 dB, and the power at the sample was 0.6 mW. A glass coverslip was placed over the eyecup preparation to provide a uniform optical path. The scan width was 2.5 mm. Averaged OCT images were collected from 20, 1024 × 1024 cross-sectional (B-scan) images at 18 Hz and stored as 8-bit grayscale bitmaps. In later runs, OCT image range settings were recorded to reconstruct exact reflectivity values used for segmentation. The OCT system also was equipped with a 764-nm line scanning ophthalmoscope (LSO) that allowed continuous wide-field viewing of the eyecup surface and the microperfuser tube location.
Time-lapse OCT intensity plots (M-scans) were created with the aid of custom LabVIEW software (National Instruments, Austin, TX, USA). Before and after 5-minute drug application, averaged (20 frame) OCT images were taken every 30 seconds. During the drug application, images were taken every 15 seconds. After the images were flattened, a 100 × 300 pixel region of interest (ROI) was selected under the drug applicator. Each ROI was compressed into single median row A-scans to represent individual time points, scaled horizontally by duration between images, registered, and combined into single time lapse M-scan images for analysis (Figs. 2A–C). Example M-scans in Figures are displayed on a logarithmic scale and contrast-enhanced to optimize visibility of drug-induced changes for each run.
Figure 2.
An example of the method used to create an M-scan. An application of glutamate (10 mM) is shown. White scale bars: 100 μm. (A) An OCT B-scan image of retina under the microperfusion tube (upper) is flattened (lower). Median row values in the region of interest (box) under the tube are used to create 1-dimensional A-scans. Vertical lines in (B, C) represent A-scan generated from the region in (A). (B) A-scans for every time point are registered, horizontally scaled proportional to the time interval between image acquisitions, and combined to create one time-lapse M-scan. (C) The M-scan then is colorized for better visualization. Black horizontal bar indicates the 5-minute drug application period. (D) Labeled intensity profile from previous A-scan. Layer thicknesses of the IPL, INL, OPL, IS, OS, and PRT band to RPE band were calculated by the distances between their borders (upper). The IPL, INL, OPL, and ONL reflectivity intensities were calculated as the average intensity between layer borders. Reflectivity intensities for the ELM, ISOS, PRT, and RPE were measured as peak layer values (lower).
Average cross-sectional A-scans from the 0-, 5- (predrug), 10- (postdrug), 15-, and 20-minute time points were segmented at the external limiting membrane (ELM), inner segment-outer segment (IS/OS) junction, photoreceptor tip/apical processes of the retinal pigment epithelium (PRT), RPE layer, and the borders of the ganglion cell layer (GCL, mainly Müller cell endfeet in rabbits), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layer (ONL). These segmentation positions were used to create ImageJ (National Institutes of Health [NIH], Bethesda, MD, USA; available in the public domain at http://imagej.nih.gov/ij) ROIs for layer thickness and intensity measurements.
If acquisition settings were known, bitmaps could be back calculated into their true linearly scaled reflectance values by reversing the proprietary image processing algorithm (supplied by the manufacturer of the OCT machine) with a custom ImageJ macro. Borders of bright layers (IPL and OPL) were defined as the 50% point of peak values nearest the edges of each layer. Nuclear layers (GCL, INL, ONL) were defined as the spaces between bright layers, except for the bottom layer of the ONL, which was defined by the peak of the ELM.36 The ELM, IS/OS, PRT, and RPE were defined as their peak value for intensity measurements, and thickness measurements were calculated from peak to peak distances (Fig. 2D). For structures with weaker reflection, such as the OPL and ELM peak, A-scans before and after were used as reference to maintain consistency and reduce inaccurate segmentations due to noise.
Statistics
Segmentation points were excluded from analysis if they were otherwise indistinguishable from noise. If acquisition settings were unknown, borders were estimated by normalizing image intensities to known values from other runs and defining borders by the corresponding known half point values. A sign test, a variation of the binomial statistical test, was performed because relative intensity changes (positive or negative) were preserved in every experiment regardless of acquisition settings even if the absolute intensities were unknown. Since absolute thicknesses were discernible in every case regardless of absolute intensity, layer thicknesses and swelling statistics were performed using paired 1-tailed t-tests. Both tests were performed with a significance level of P ≤ 0.05.
Histology
To correlate OCT images of drug-treated retina to the retinal anatomy in more detail, some tissues treated with KA and NaCN were imaged live with OCT, and then immediately removed and fixed at the onset of drug washout. To locate the drug-exposed zone post fixation, the positions of the drug microperfusion tube and optic nerve head were recorded in the LSO image. The eyecup was carefully removed from the chamber and immediately fixed in 2% glutaraldehyde/2% paraformaldehyde solution overnight. The microperfused retinal zone then was excised, dehydrated, and embedded in paraffin. Serial sections were taken every 100 μm through the drug-exposed retinal zone, mounted on glass slides, and stained with hematoxylin-eosin. The extent of the drug-affected retinal zone was measured at ×5 to ×20 for comparison with the OCT images.
Results
To better understand how OCT reflectance signals change in the presence of retinotoxic agents, a series of well characterized retinotoxins37–41 were applied to the retina and their effects on reflectance and morphology were tracked over time. We compared OCT images immediately before and after the 5-minute drug application (Tables 1–4). For control Ames Ringer runs (n = 6), the distance between the PRT and RPE lines decreased 4.4 ± 1.6 μm (mean ± SD). No other changes were detected in any retinal layer thicknesses or intensities suggesting all other observed changes induced by retinotoxic agents tested were real and not simply due to our microperfusion method.
Table 1.
Effects of Excitatory Neurotoxins on Retinal Layer Thickness
|
Layer |
Drug |
|||||||
|
Control,n= 6 |
3 μM KA,n= 4 |
10 μM KA,n= 4 |
30 μM KA,n= 5 |
100 μM KA,n= 5 |
300 μM KA,n= 6 |
10 mM Glutamate,n= 6 |
10 mM Adipate,n= 5 |
|
| IPL | +3 ± 10% | +2 ± 12% | −2 ± 3% | +4 ± 9% | +26 ± 14%* | +32 ± 13%* | +5 ± 4%* | +5 ± 4%* |
| INL | −7 ± 43% | −17 ± 9%* | +3 ± 21% | +2 ± 24% | +1 ± 16% | −1 ± 16% | +14 ± 33% | +3 ± 25% |
| OPL | +5 ± 19 % | +6 ± 13% | +3 ± 17% | +29 ± 37% | +32 ± 42%* | +9 ± 13% | +15 ± 22% | −5 ± 23% |
| ONL | +9 ± 15% | +2 ± 14% | −1 ± 7% | 0 ± 8% | −1 ± 10% | −2 ± 12% | +10 ± 12% | −4 ± 6% |
| IS | −2 ± 6% | −7 ± 19% | +2 ± 9% | +7 ± 11% | −15 ± 19% | +4 ± 16% | −6 ± 20% | +4 ± 5% |
| OS | +1 ± 11% | +5 ± 4%* | 0 ± 5% | −7 ± 9% | −9 ± 11%* | −11 ± 20% | +1 ± 6% | +8 ± 12% |
| PRT to RPE | −36 ± 14%* | −10 ± 12% | −4 ± 8% | −12 ± 47% | −35 ± 25%* | −17 ± 10%* | −16 ± 36% | −7 ± 26% |
Comparisons of relative change in average retinal layer thickness before and after the drug application.
Significant changes in layer thickness (1-sided paired t-test, P ≤ 0.05).
Table 2.
Effects of Excitatory Neurotoxins on Retinal Layer Reflectance
|
Layer |
Drug |
|||||||
|
Control,n= 6 |
3 μM KA,n= 4 |
10 μM KA,n= 4 |
30 μM KA,n= 5 |
100 μM KA,n= 5 |
300 μM KA,n= 6 |
10 mM Glutamate,n= 6 |
10 mM Adipate,n= 5 |
|
| IPL | NS | NS | NS | + | + | + | + | NS |
| INL | NS | NS | NS | + | + | + | NS | NS |
| OPL | NS | NS | NS | + | + | NS | NS | NS |
| ONL | NS | NS | NS | NS | − | − | − | NS |
| ELM* | NS | NS | NS | NS | NS | NS | − | NS |
| IS/OS* | NS | NS | NS | − | − | NS | − | NS |
| PRT* | NS | NS | NS | − | NS | NS | − | NS |
| RPE* | NS | NS | NS | NS | NS | NS | NS | NS |
Comparisons of relative change in average retinal layer reflectance before and after the drug application. ±, significant changes in layer reflectance (binomial sign test, P ≤ 0.05); NS, no statistically significant difference.
A measurement of only the peak layer value.
Table 3.
Effects of Chloroquine and Cyanide on Retinal Layer Thickness
|
Layer |
Drug |
||
|
Control,n= 6 |
10 μM Chloroquine,n= 5 |
3 mM NaCN,n= 5 |
|
| IPL | +3 ± 10% | +7 ± 10% | +4 ± 9% |
| INL | −7 ± 43% | −7 ± 35% | +9 ± 18% |
| OPL | +5 ± 19% | +17 ± 17%* | −8 ± 15% |
| ONL | +9 ± 15% | −2 ± 17% | +6 ± 8% |
| IS | −2 ± 6% | −9 ± 9% | 0 ± 10% |
| OS | +1 ± 11% | 0 ± 12% | +9 ± 6%* |
| PRT to RPE | −36 ± 14%* | +7 ± 11% | −27 ± 19%* |
Comparisons of relative change in average retinal layer thickness before and after the drug application.
Significant changes in layer thickness (1-tailed paired t-test, P ≤ 0.05).
Table 4.
Effects of Chloroquine and Cyanide on Retinal Reflectance
|
Layer |
Drug |
||
|
Control,n= 6 |
10 μM Chloroquine,n= 5 |
3 mM NaCN,n= 5 |
|
| IPL | NS | NS | NS |
| INL | NS | NS | NS |
| OPL | NS | NS | NS |
| ONL | NS | NS | NS |
| ELM* | NS | NS | NS |
| IS/OS* | NS | NS | − |
| PRT* | NS | − | − |
| RPE* | NS | NS | − |
Comparisons of relative change (±) in average retinal layer reflectance before and after the drug application.
A measurement of only the peak layer value.
Although our list of retinotoxic agents tested is not exhaustive, we found many retinotoxic agents tested could be classed broadly into two different types. Excitatory agents that induced neuronal depolarization, such as KA and glutamate, caused an increase in reflectivity and thickness of the inner retinal layers due to edematous swelling of fine processes. Agents that disrupt mitochondrial function often caused a disorganization of the photoreceptor bands that correlated with disruption of the pigment epithelium.
Excitatory Neurotoxic Agents Affected the Inner and Outer Retina
Kainic Acid.
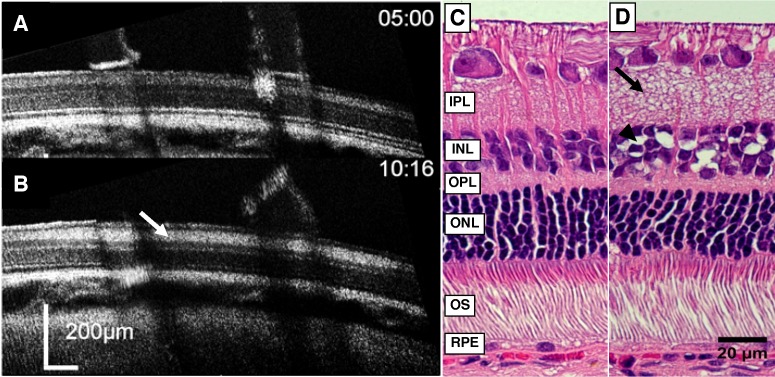
We chose first to examine the retinal sensitivity of the OCT image to application of the retinotoxin KA (3–300 μM, n = 24 total).37,42–44 We compared how application of 100 μM KA affected the retina in OCT images to histology of the same region (n = 3). In the OCT image, 100 μM KA caused an increase in reflectance (scattering) in the inner retinal layers; particularly in the IPL, INL, and OPL (Figs. 3A, 3B). Histological examination of the same region showed a corresponding strong swelling of processes in the IPL and a weaker swelling in the OPL similar to the OCT image (Figs. 3C, 3D). The INL showed large vacuoles between the nuclei, particularly in the innermost part, which coincided with the increased reflectivity observed in the OCT image, but significant swelling was not observed (Tables 1, 2). The size of the retinal zone affected by microapplication of KA measured histologically averaged 2.0 ± 0.8 mm (mean ± SD) in diameter (n = 3).
Figure 3.
Effects of 100 μM KA microperfusion as viewed by OCT and corresponding histology. (A) The OCT B-scan image of retina immediately before application of 100 μM KA. Elapsed time indicated at the top right. The OCT images shown were rotated slightly for clarity. (B) The OCT B-scan image of the same retina immediately after 5 minutes of KA exposure. The tube has been removed in preparation for retinal removal for histological processing. Increases in reflectivity and swelling are observed in the IPL (white arrow) and the OPL. Increases in reflectivity alone were observed in the INL. (C, D) Histology of the same KA-treated retina. (C) Adjacent unaffected retinal control region. (D) Retinal region treated with KA. Large vacuoles are observed in the INL (black arrowhead). The IPL shows extensive swelling of small processes and an increase in thickness (black arrow), while the OPL shows a smaller increase in fine swellings.
Examples of the IPL reflectance time course responses to 3 doses of KA of varying magnitude are shown in Figure 4. At lower doses of KA, we observed a delay in IPL swelling and reflectance increases when compared to higher doses. At higher doses (30–300 μm), KA induced increased reflectivity of the IPL. Also, KA significantly reduced reflectivity in the IS/OS band for 30 to 100 μM and 5/6 cases for 300 μM. Figure 5 shows dose-response curves of IPL swelling for KA (3–300 μM) with 100 to 300 μM producing significant increases in thickness. Tables 1 and 2 summarize the sensitivity of OCT to detecting changes in retinal thickness (swelling) and reflectivity by different doses of KA. Examples of M-scans for the control and 100 μM KA are shown in Figures 6A and 6B.
Figure 4.
Examples of reflectance and swelling increases in the IPL with KA. Plots of individual applications shown. (A) The magnitude and delay of reflectance increases observed are dependent on dose, with higher doses having a shorter delay and higher reflectance increase. (B) The time course of swelling parallels the time course of reflectance increases during the drug application period (300 μM shown).
Figure 5.
Dose response curve for KA-induced IPL swelling as measured with OCT. Ratio indicates mean relative IPL thickness change measured immediately before and after drug application. Error bars represent ±1 SD. The KA was tested at 3, 10, 30, 100, and 300 μM (n = 4, 4, 5, 5, and 6, respectively). Horizontal lines indicate control Ringer mean (solid) ± SD (dotted). *Indicates significant difference (1-tailed paired t-test, P ≤ 0.05).
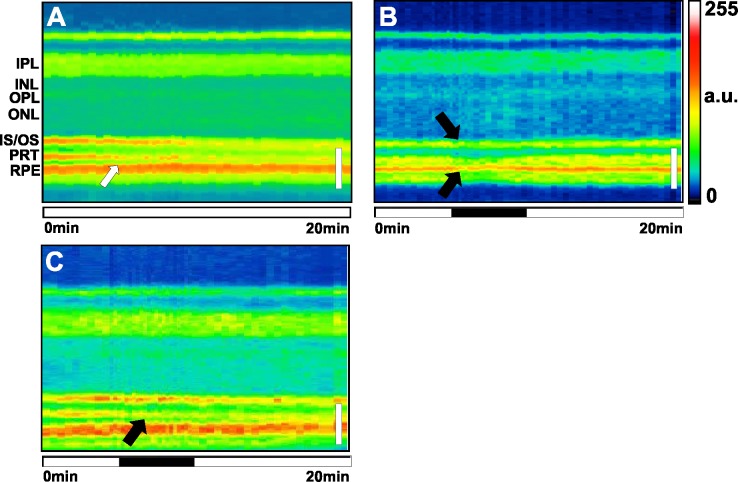
Figure 6.
Retinal M-scan examples of the effects of excitatory neurotoxins on the inner retina. Many excitatory agents caused reflectivity increases and swelling in the IPL (black arrows) and OPL. Black horizontal bar indicates the 5-minute drug application period. a.u., arbitrary units. (A) Control Ames Ringer showed a decreased distance between the PRT and RPE (white arrow). (B) The 100 μM KA elicited prolonged inner retinal swelling and reflectivity increases. (C) The 10 mM glutamate also showed prolonged IPL thickness and reflectivity increases. (D) The 10 mM adipate showed an increased IPL thickness. White vertical scale bar: 50 μm.
Glutamate.
Glutamate was tested at 10 mM (n = 6). The pattern of glutamate-induced retinal damage imaged with OCT was very similar to the effects of KA (Tables 1, 2). Application of glutamate caused a rapid intensity increases in the OCT image; particularly in the IPL and GCL (Müller endfeet) followed by a prolonged swelling, which continued after drug washout. Glutamate-induced increases seen in IPL reflectivity were statistically significant, and were observed in 5/6 cases for the INL and OPL (Fig. 6C). Similar to high doses of KA, the outer retina saw significant decreases in reflectivity (Table 2).
D-Amino Adipic Acid.
Another excitatory retinotoxic agent, D-amino adipic acid was tested at 10 mM (n = 5; Fig. 6D; Tables 1, 2). The pattern of amino adipic acid-induced retinal damage imaged with OCT was similar to the effects of kainate and glutamate, with statistically significant swelling in the IPL, but the reflectance changes generally were weak. The PRT and IS/OS junction decreased in reflectivity in 4/5 cases.
Inhibitory Neurotoxic Agents Affected the Outer Retina and Pigment Epithelium
Sodium Cyanide.
We also tested NaCN, a known respiratory chain inhibitor, to examine if this agent produced a different OCT signature compared to the excitatory neurotoxins. We examined 5 regions using pH balanced 3 mM NaCN (≈75 μM active state, see Discussion) with OCT, which showed reductions in the reflectivity of the photoreceptor IS/OS, PRT, and RPE lines (Tables 3, 4). Surprisingly, cyanide caused only a minor reduction in reflectivity of the IPL in 4/5 retinas and an apparent increase in thickness in the OS.
Histological analysis of the cyanide microprofused retina (n = 3) showed a different pattern than that of KA (Fig. 7). The major effect of acute retinal exposure to cyanide appeared to be on the RPE cells. In these cyanide zones, the RPE cells were swollen with many small vacuoles seen in their cytoplasm and detached from the photoreceptors. The photoreceptor tips in the exposed zones also had severe distortions, becoming highly curved, presumably due to their detachments from the apical processes of the RPE. Because the applicator tube was continuously held to the retinal surface, we could not observe retinal detachments during live OCT imaging, but retinal detachment was seen in the drug application zones of all three fixed tissue histological specimens examined after removal of the applicator tube for tissue processing. The size of the retinal zone affected by microapplication of 3 mM cyanide measured histologically averaged 1.2 ± 0.6 mm (mean ± SD) in diameter. An example M-scan of 3 mM NaCN is shown in Figure 8B.
Figure 7.
Effects of 3 mM sodium cyanide microperfusion as viewed by OCT and corresponding histology. (A) The OCT B-scan image of retina immediately before application of 3 mM NaCN. Elapsed time indicated at the top right. (B) The OCT B-scan image of the same retina immediately after 5 minutes of NaCN exposure. Note that the hyperreflective IS/OS and PRT bands were reduced (white arrows), as well as the RPE. (C, D) Histology of the same NaCN-treated retina. Inset shows enlarged region for comparison. (C) Adjacent unaffected retinal control region. (D) Retinal region treated with NaCN, showing disorganization of outer segments (OS) tips (black arrows) and detachment from the RPE. The pigment epithelium was swollen with numerous vacuoles (black arrowhead). The region shown was near the edge of the drug zone to demonstrate the effects on the RPE were not an artifact due to detachment from histological processing.
Figure 8.
Retinal M-scan examples of the effects of chloroquine and the metabolic inhibitor cyanide on the outer retina and RPE. White vertical scale bar: 50 μm. Black horizontal bar indicates the 5-minute drug application period. (A) Control Ames Ringer (duplicate to Fig. 6A). (B) The 3 mM NaCN induced loss of reflectance in both photoreceptor bands and RPE (black arrows). (C) The 10 μM chloroquine induced loss of reflectance in the PR tips after application, and continued reflectance loss in the PRT and IS/OS junction 10 minutes after the drug removal.
Chloroquine.
Chloroquine, an antiarthritic/malarial drug, was tested at 10 μM (n = 5). We observed a reduction in reflectivity in the IPL and IS/OS in 4/5 eyes examined, and significantly in the PRT after drug application, as well as swelling in the OPL (Tables 3, 4). By 10 minutes after drug washout, there were significant reductions in reflectivity of the IS/OS and PRT lines. An example of a chloroquine retina M-scan can be found in Figure 8C.
Discussion
Pathological changes in the retina have long been known to alter reflectivity in response to chemical injury. Injurious traveling waves of increased retinal reflectance termed “spreading depression” have been reported as early as the 1950's.45 Spreading depression in the chick retina can be triggered by kainate, N-methyl-D-aspartate (NMDA), and a variety of toxins.46,47 More recently, these retinal scattering changes in chick retina have been studied by laser reflectance methods.48 Using OCT in the isolated adult rabbit retina, changes in the reflectance of the inner retinal layers can be seen in response to light stimulation.4 Here, we have shown that when certain retinotoxins are applied to confined areas of ex vivo retina, real time effects can be observed in the OCT signal. However, unlike the chick retina, traveling waves of increase reflectance in response to depolarizing agents have not been reported in the adult rabbit retina, and we did not observe them with microperfusion in the rabbit eyecup preparation. Our microperfusion technique has permitted us to study multiple drug effects on multiple small subregions per eye. These subregions, however, must be spaced carefully, as histological examination of NaCN and 100 μM KA revealed that the drug affected zones were larger than that of the tube lumen, indicating as expected, some drug diffusion did occur radially through the tissue before escaping into the rapidly superfused bath. Additionally, virtually all retinotoxins tested exhibited a reduction in distance between the PRT and RPE lines. Because this existed in our control experiments, we do not attribute this affect to any particular drug.
Two Classes of Retinotoxic Effects
The retinotoxic agents we tested could be classed broadly into two categories with distinct effects on the retinal structure as observed with OCT. Agents that are known to induce neuronal depolarization, such as KA, glutamate, and D-amino-adipic acid, caused increases in the thickness of the IPL and to a lesser extent the OPL due to edematous swelling. Swelling and reflectivity increases in the inner retina typically were associated with decreases in reflectivity in the outer retina. At high doses, KA has been shown to cause severe swelling and cell death in many brain regions, including the retina by opening AMPA/KA type glutamate receptors.49–51 Excessive levels of glutamate also have long been known to injure the retina when chronically applied.38,49 Amino adipic acid and its isomers have been reported to be particularly toxic to the retinal Müller cells and neurons.39,52,53 One of adipate's toxic effects is blocking sodium-dependent glutamate transport into Müller cells, which increases glutamate levels.54 The swelling of the IPL reported in our results is consistent with these glutaminergic excitatory mechanisms. In addition, some retinal layers showed changes in retinal reflectance with KA with little observable swelling (Tables 1, 2). The loss of reflectivity in the outer retina to glutamate and high doses of KA may possibly be due to the presence of glutamate-activated chloride exchangers in photoreceptors.55,56 We found OCT could detect reflectance changes with as little as 30 μM KA (Table 2), somewhat above the level reported to physiologically saturate ganglion cell firing (10 μM).57
In contrast, the aerobic respiration blocker NaCN caused a disorganization and decrease of reflectivity in the photoreceptor (IS/OS and PRT) and RPE lines without an increase of reflectivity or swelling in the IPL in the OCT images (Tables 3, 4). Histological analysis of NaCN-treated zones also revealed retinal detachment and RPE vacuolization. The RPE is normally metabolically active; removing water from the subretinal space, exchanging metabolites, phagocytosis of outer segments, and the regeneration of cis-trans retinal.58 Many RPE cotransport mechanisms are dependent on sodium gradients and are ATP energy-dependent processes.59
The OCT reflectance changes for the photoreceptors and RPE seen in the presence of cyanide are likely due to cyanide's blockade of oxidative phosphorylation and the high metabolic demand of the photoreceptors and RPE, normally supplied by blood-oxygen exchange from adjacent choriocapillaris vessels.40,59–62 The cyanide ion strongly disrupts the mitochondrial electron-transport chain, which leads to a concomitant loss of oxidative metabolism and aerobic cellular energy generation. Cyanide also is a powerful base, with the toxic CN− ion being predominately active at alkaline pH, which can additionally block anaerobic metabolism.40 Thus, cyanide's alkalinity, if left unbuffered, also can confound physiological interpretation of its action. Because cyanide has a pKa of 8.99,63 pH balancing to a biologically relevant pH around 7.4 reduces the effective concentration of active CN− ion to roughly 2.5% of our reported molarity, which limits its effect to blocking only oxidative metabolism. Overall, changes in OCT retinal reflectivity under the drug perfusion tube largely matched histology. However, we could not observe retinal detachment in the OCT images due to the apposition of the applicator tube on the retinal surface. Although NaCN is a known metabolic inhibitor in vitro and ex vivo, in vivo administration causes rapid death by binding to hemoglobin forming HbCN, reducing oxygen delivery to the entire body. We focused solely on its metabolic inhibitory action because our ex vivo preparation does not have blood flow and, thus, disrupting hemoglobin function was not a critical factor for the tissue physiology in these experiments.
Chloroquine is an antiarthritic/malarial drug that has long been known to cause retinopathy to a small subset (~0.5%) of individuals in chronic use,21,41,64 and macular changes in approximately 25% of the population.41 Among its many effects, chloroquine, like cyanide, also is a known inhibitor of the mitochondrial electron transport chain.65–67 Here, we observed a reduction of the PRT reflectance with our accelerated screening procedure. In addition to metabolic effects, prolonged administration of chloroquine corresponded to disappearance of rod and cone outer segments in fish68 and in rats and cats,69 which may be related to our findings. In humans, OCT images of the macula in chloroquine retinopathy often show a fuzzy, “moth-eaten” appearance due to loss of the PRT line.64,70 Swelling of the OPL may be related to damage, as chloroquine is known to cause the complete loss of the OPL with prolonged high-dose administration.71
Our functional imaging method combined OCT, which provides real time morphological and physiological data, with a novel drug microperfusion system. This has allowed us to screen known neurotoxins at a rate much faster than through systemic or intraocular injections, applying drugs multiple times per eye, while continuously collecting morphological and physiological data in time, thereby avoiding the need to euthanize animals at each time point for histopathological analysis. This is especially important as the pathological effects of neurotoxins in tissue can vary with the time after toxin exposure.72 Our limited examination of retinotoxins and their actions is likely to be an oversimplification of all possible drug-induced OCT changes observed in the retina. Future studies will determine if more selective neurotransmitter-specific neurotoxins, such as strychnine, picrotoxin, or MPTP, cellular uptake inhibitors, structural protein disruptors, or voltage-gated channel modulators could produce more layer-selective changes in the OCT reflectance signal. Additionally, our results showed that the same morphological features (vacuoles) could occur with completely different changes in OCT reflectivity. Vacuole formation in the INL from 100 μM KA application showed an increase in reflectivity, whereas vacuole formation in the RPE from 3 mM NaCN showed a decrease in reflectivity. This indicates reflectivity changes alone are insufficient to determine specific morphological changes and that the mechanism behind the retinotoxin, indeed, has an important role in reflectivity, which merits further investigation.
Perhaps the most useful aspect of our microperfusion imaging technique is increased control over drug accumulation and clearance in the retina. In clinical trials, the accumulation of drugs into the retina and brain can be retarded by the permeability of the blood–retina barrier. With this technique, drugs with chronic accumulation or clearance rates, such as fluoroquinolones, can be accelerated, while OCT data can be collected continuously. As drug neurotoxicity is a complex temporal and spatial process,51,71–73 providing useful physiological and morphological data on an acute time scale would clearly provide clinical utility for retinotoxicological analysis.
Conclusions
Using a novel transparent microperfusion device and OCT, we have developed a technique to screen the effects of neurotoxins on the localized retina in real-time. Neurotoxin-induced changes in retinal layer reflectivity under the microperfusion tube closely matched the retinal histology. Our microperfusion technique also has demonstrated the potential to facilitate drug retinotoxicological screening by reducing the number of animals, and accelerate drug accumulation and clearance rates well beyond the pharmacokinetics that would be expected with systemic or intraocular injections. Our results suggested rapid microperfusion coupled with OCT is a potentially useful retinotoxicity screening tool in preclinical animal tests.
Acknowledgments
The authors thank Bruce Fleharty and Randolph Bidinger for drug tank fabrication.
Supported by a grant from the FDA Critical Path Initiative. The content of this publication represents solely the authors' views and may not reflect any position of the United States Government or the Food and Drug Administration. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Disclosure: J.A. Majdi, None; H. Qian, None; Y. Li, None; R.J. Langsner, None; K.I. Shea, None; A. Agrawal, None; D.X. Hammer, None; J.P. Hanig, None; E.D. Cohen, None
References
- 1. Maheswari RU, Takaoka H, Kadono H, Homma R, Tanifuji M. Novel functional imaging technique from brain surface with optical coherence tomography enabling visualization of depth resolved functional structure in vivo. J Neurosci Methods. 2003; 124: 83–92. [DOI] [PubMed] [Google Scholar]
- 2. Srinivasan VJ, Chen Y, Duker JS, Fujimoto JG. In vivo functional imaging of intrinsic scattering changes in the human retina with high-speed ultrahigh resolution OCT. Opt Express. 2009; 17: 3861–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazebnik M, Marks D, Potgieter K, Gillette R, Boppart S. Functional optical coherence tomography for detecting neural activity through scattering changes. Opt Lett. 2003; 28: 1218–1220. [DOI] [PubMed] [Google Scholar]
- 4. Bizheva K, Pflug R, Hermann B, et al. Optophysiology: depth-resolved probing of retinal physiology with functional ultrahigh-resolution optical coherence tomography. Proc Natl Acad Sci U S A. 2006; 103: 5066–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moayed AA, Hariri S, Choh V, Bizheva K. Correlation of visually evoked intrinsic optical signals and electroretinograms recorded from chicken retina with a combined functional optical coherence tomography and electroretinography system. J Biomed Opt. 2012; 17: 016011. [DOI] [PubMed] [Google Scholar]
- 6. Rajagopalan UM, Tanifuji M. Functional optical coherence tomography reveals localized layer-specific activations in cat primary visual cortex in vivo. Opt Lett. 2007; 32: 2614–2616. [DOI] [PubMed] [Google Scholar]
- 7. Yao XC, Yamauchi A, Perry B, George JS. Rapid optical coherence tomography and recording functional scattering changes from activated frog retina. Appl Opt. 2005; 44: 2019–2023. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Rao B, Chen Z. Swept source based Fourier domain functional optical coherence tomography. Conf Proc IEEE Eng Med Biol Soc. 2005; 7: 7230–7233. [DOI] [PubMed] [Google Scholar]
- 9. Loftus JV, Sultan MB, Pleil AM, et al. Changes in vision-and health-related quality of life in patients with diabetic macular edema treated with pegaptanib sodium or sham. Invest Ophthalmol Vis Sci. 2011; 52: 7498–7505. [DOI] [PubMed] [Google Scholar]
- 10. Keane PA, Heussen FM, Ouyang Y, et al. Assessment of differential pharmacodynamic effects using optical coherencetomography in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Csaky KG, Richman EA, Ferris FL III. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008; 49: 479–489. [DOI] [PubMed] [Google Scholar]
- 12. Mansouri K, Leite MT, Medeiros FA, Leung CK, Weinreb RN. Assessment of rates of structural change in glaucoma using imaging technologies. Eye. 2011; 25: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zysk A, Nguyen F, Oldenburg A, Marks D, Boppart S. Optical coherence tomography: a review of clinical development from bench to bedside. J. Biomed. Opt. 2007; 12: 051403. [DOI] [PubMed] [Google Scholar]
- 14. Greenberg BM, Frohman E. Optical coherence tomography as a potential readout in clinical trials. Ther Adv Neurol Disord. 2010; 3: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yazdanfar S, Rollins AM, Izatt JA. In vivo imaging of human retinal flow dynamics by color Doppler optical coherence tomography. Arch Ophthalmol. 2003; 121: 235–239. [DOI] [PubMed] [Google Scholar]
- 16. Wu FI, Glucksberg MR. Choroidal perfusion measurements made with optical coherence tomography. Appl Opt. 2005; 44: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 17. Yamauchi Y, Yagi H, Usui Y, et al. Biological activity is the likely origin of the intersection between the photoreceptor inner and outer segments of the rat retina as determined by optical coherence tomography. Clin Ophthalmol. 2011; 5: 1649–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen ED, Agrawal A, Connors M, Hansen B, Charkhkar H, Pfefer J. Optical coherence tomography imaging of retinal damage in real time under a stimulus electrode. J Neural Eng. 2011; 8: 056017. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez-Padilla JA, Hedges TR III, Monson B, et al. High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007; 125: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hariri S, Moayed AA, Choh V, Bizheva K. In vivo assessment of thickness and reflectivity in a rat outer retinal degeneration model with ultrahigh resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 1982–1989. [DOI] [PubMed] [Google Scholar]
- 21. Penha FM, Rodrigues EB, Maia M, et al. Retinal and ocular toxicity in ocular application of drugs and chemicals-part I: animal models and toxicity assays. Ophthalmic Res. 2010; 44: 82–104. [DOI] [PubMed] [Google Scholar]
- 22. Penha FM, Rodrigues EB, Maia M, et al. Retinal and ocular toxicity in ocular application of drugs and chemicals-part II: retinal toxicity of current and new drugs. Ophthalmic Res. 2010; 44: 205–224. [DOI] [PubMed] [Google Scholar]
- 23. Penha FM, Rodrigues EB, Furlani BA, Dib E, Melo GB, Farah ME. Toxicological considerations for intravitreal drugs. Expert Opin Drug Metab Toxicol. 2011; 7: 1021–1034. [DOI] [PubMed] [Google Scholar]
- 24. Audo I, Warchol ME. Retinal and cochlear toxicity of drugs: new insights into mechanisms and detection. Curr Opin Neurol. 2012; 25: 76–85. [DOI] [PubMed] [Google Scholar]
- 25. Nencini C, Barber L, Runci FM, Micheli L. Retinopathy induced by drugs and herbal medicines. Eur Rev Med Pharmacol Sci. 2008; 12: 293–298. [PubMed] [Google Scholar]
- 26. Meyer CH, Hotta K, Peterson WM, Toth CA, Jaffe GJ. Effect of INS37217, a P2Y2 receptor agonist, on experimental retinal detachment and electroretinogram in adult rabbits. Invest Ophthalmol Vis Sci. 2002; 43: 3567–3574. [PubMed] [Google Scholar]
- 27. Brock WJ, Somps CJ, Torti V, Render JA, Jamison J, Rivera MI. Ocular toxicity assessment from systemically administered xenobiotics: considerations in drug development. Int J Toxicol. 2013; 32: 171–188. [DOI] [PubMed] [Google Scholar]
- 28. Frambach DA, Marmor MF. The rate and route of fluid resorption from the subretinal space of the rabbit. Invest Ophthalmol Vis Sci. 1982; 22: 292–302. [PubMed] [Google Scholar]
- 29. Agarwal A. Toxic diseases affecting the pigment epithelium and the retina. In: Gass' Atlas of Macular Diseases Volume 2. 5th ed. Nashville, TN: Elsevier Saunders; 2012: 756–761. [Google Scholar]
- 30. Gualino V, Cohen SY, Delyfer MN, Sahel JA, Gaudric A. Optical coherence tomography findings in tamoxifen retinopathy. Am J Ophthalmol. 2005; 140: 757–758. [DOI] [PubMed] [Google Scholar]
- 31. Kjellström U, Andréasson S, Ponjavic V. Attenuation of the retinal nerve fibre layer and reduced retinal function assessed by optical coherence tomography and full-field electroretinography in patients exposed to vigabatrin medication. Acta Ophthalmol. 2014; 92: 149–157. [DOI] [PubMed] [Google Scholar]
- 32. Miller RF, Zalutsky R, Massey S. A perfused rabbit retina preparation suitable for pharmacological studies. J Neurosci Methods. 1986; 16: 309–322. [DOI] [PubMed] [Google Scholar]
- 33. Ames A, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981; 37: 867–877. [DOI] [PubMed] [Google Scholar]
- 34. Hammer DX, Iftimia NV, Ferguson RD, et al. Foveal fine structure in retinopathy of prematurity: an adaptive optics fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2008; 49: 2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agrawal A, Chen CW, Baxi J, Chen Y, Pfefer TJ. Multilayer thin-film phantoms for axial contrast transfer function measurement in optical coherence tomography. Biomed Opt Express. 2013; 4: 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baxi J, Calhoun W, Sepah YJ, et al. Retina-simulating phantom for optical coherence tomography. J Biomed Optics. 2014; 19: 1–8. [DOI] [PubMed] [Google Scholar]
- 37. Slaughter MM, Miller RF. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983; 3: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957; 58: 193–201. [DOI] [PubMed] [Google Scholar]
- 39. Pedersen O, Karlsen R. Destruction of Müller cells in the adult rat by intravitreal injection of D,L-alpha-aminoadipic acid. An electron microscopic study. Exp Eye Res. 1979; 28: 569–575. [DOI] [PubMed] [Google Scholar]
- 40. Winkler BS, Dang L, Malinoski C, Easter SS Jr. An assessment of rat photoreceptor sensitivity to mitochondrial blockade. Invest Ophthalmol Vis Sci. 1997; 38: 1569–1577. [PubMed] [Google Scholar]
- 41. Elman A, Gullberg R, Nilsson E, Rendahl I, Wachtmeister L. Chloroquine retinopathy in patients with rheumatoid arthritis. Scand J Rheumatology. 1976; 5: 161–166. [DOI] [PubMed] [Google Scholar]
- 42. Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994; 11: 317–332. [DOI] [PubMed] [Google Scholar]
- 43. Romano C, Price MT, Olney JW. Delayed excitotoxic neurodegeneration induced by excitatory amino acid agonists in isolated retina. J Neurochem. 1995; 65: 59–67. [DOI] [PubMed] [Google Scholar]
- 44. Higa T, Kuniyoshi M. Toxins associated with medicinal and edible seaweeds. J. Toxicol—Toxin Reviews. 2000; 19: 119–137. [Google Scholar]
- 45. Gouras P. Spreading depression of activity in amphibian retina. Am J Physiol. 1958; 195: 28–32. [DOI] [PubMed] [Google Scholar]
- 46. Sheardown MJ. The triggering of spreading depression in the chicken retina: a pharmacological study. Brain Res. 1993; 607: 189–194. [DOI] [PubMed] [Google Scholar]
- 47. Martins-Ferreira H, Ribeiro LJ. do-Carmo RJ. Threshold determination of spreading depression evoking substances in the retina in vitro. Braz J Med Biol Res. 1993; 26: 875–877. [PubMed] [Google Scholar]
- 48. Duarte MA, Almeida ACG, Infantosi AFC, Bassani JWM. Functional imaging of the retinal layers by laser scattering: an approach for the study of Leão's spreading depression in intact tissue. J Neurosci Methods. 2003; 123: 139–151. [DOI] [PubMed] [Google Scholar]
- 49. Olney JW. The toxic effects of glutamate and related compounds in the retina and the brain. Retina. 1982; 2: 341–359. [PubMed] [Google Scholar]
- 50. Bolon B, Garman R, Jensen K, et al. A ‘best practices' approach to neuropathologic assessment in developmental neurotoxicity testing—for today. Toxicol Pathol. 2006; 34: 296–313. [DOI] [PubMed] [Google Scholar]
- 51. Switzer RC III, Lowry-Franssen C, Benkovic SA. Recommended neuroanatomical sampling practices for comprehensive brain evaluation in nonclinical safety studies. Toxicol Pathol. 2011; 39: 73–84. [DOI] [PubMed] [Google Scholar]
- 52. Casper DS, Reif-Lehrer L. Effects of alpha-aminoadipate isomers on the morphology of the isolated chick embryo retina. Invest Ophthalmol Vis Sci. 1983; 24: 1480–1488. [PubMed] [Google Scholar]
- 53. Bonaventure N, Wioland N, Roussel G. Stereospecific effects of the α-aminoadipic acid on the retina: a morphological and electrophysiological study. Doc Ophthalmol. 1985; 61: 71–77. [DOI] [PubMed] [Google Scholar]
- 54. Pannicke T, Stabel J, Heinemann U, Reichelt W. Alpha-aminoadipic acid blocks the Na+-dependent glutamate transport into acutely isolated Müller glial cells from guinea pig retina. Pflügers Archiv. 1994; 429: 140–142. [DOI] [PubMed] [Google Scholar]
- 55. Picaud SA, Larsson HP, Grant GB, Lecar H, Werblin FS. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophys. 1995; 74: 1760–1771. [DOI] [PubMed] [Google Scholar]
- 56. Larsson HP, Picaud SA, Werblin FS, Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophys J. 1996; 70: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen ED, Miller RF. The network-selective actions of quinoxalines on the neurocircuitry operations of the rabbit retina. Brain Res. 1999; 831: 206–228. [DOI] [PubMed] [Google Scholar]
- 58. Mecklenburg L, Schraermeyer U. An overview on the toxic morphological changes in the retinal pigment apithelium after systemic compound administration. Toxicol Pathol. 2007; 35: 252–267. [DOI] [PubMed] [Google Scholar]
- 59. Miller SS, Edelman JL. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol. 1990; 424: 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wood JP, Chidlow G, Graham M, Osborne NN. Energy substrate requirements of rat retinal pigmented epithelial cells in culture: relative importance of glucose, amino acids, and monocarboxylates. Invest Ophthalmol Vis Sci. 2004; 45: 1272–1280. [DOI] [PubMed] [Google Scholar]
- 61. Marmor MF, Yao XY. The metabolic dependency of retinal adhesion in rabbit and primate. Arch Ophthalmol. 1995; 113: 232–238. [DOI] [PubMed] [Google Scholar]
- 62. Yanoff M, Duker J. Retinal pigment epithelium. In: Wiggs J, Miller D, Azar D, et al. , eds. Ophthalmology. 2nd ed. St. Louis, MO: Mosby; 2004: 775–778. [Google Scholar]
- 63. Beck MT. Critical survey of stability constants of cyano complexes. Pure & Appl Chem. 1987; 59: 1703–1720. [Google Scholar]
- 64. Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011; 118: 415–422. [DOI] [PubMed] [Google Scholar]
- 65. Peräsalo R, Rechardt L, Palkama A. Chloroquine-induced ultrastructural changes in the pigment epithelium of the albino rat. Acta Ophthalmol Suppl. 1973; 123: 94–98. [PubMed] [Google Scholar]
- 66. Phelps DC, Crane FL. Inhibition of mitochondrial electron transport by hydroxy-substituted 1,4-quinones. Biochemistry. 1975; 14: 116–122. [DOI] [PubMed] [Google Scholar]
- 67. Katewa SD, Katyare SS. Treatment with antimalarials adversely affects the oxidative energy metabolism in rat liver mitochondria. Drug Chem Toxicol. 2004; 27: 41–53. [DOI] [PubMed] [Google Scholar]
- 68. Matsumura M, Ohkuma M, Tsukahara I. Experimental chloroquine retinopathy. Ophthalmic Res. 1986; 18: 172–179. [DOI] [PubMed] [Google Scholar]
- 69. Ivanina TA, Zueva MV, Lebdeva MN, Bogoslovsky AI, Bunin AJ. Ultrastructural alterations in rat and cat retina and pigment epithelium induced by chloroquine. Clin Exp Ophthalmol. 1983; 220: 32–38. [DOI] [PubMed] [Google Scholar]
- 70. Stepien KE, Han DP, Schell J, Godara P, Rha J, Carroll J. Spectral-domain optical coherence tomography and adaptive optics may detect hydroxychloroquine retinal toxicity before symptomatic vision loss. Trans Am Ophthalmol Soc. 2009; 107: 28–34. [PMC free article] [PubMed] [Google Scholar]
- 71. Gaynes BI, Torczynski E, Varro Z, Grostern R, Perlman J. Retinal toxicity of chloroquine hydrochloride administered by intraperitoneal injection. J Appl Toxicol. 2008; 28: 895–900. [DOI] [PubMed] [Google Scholar]
- 72. Fix AS, Ross JF, Stitzel SR, Switzer RC III. Integrated evaluation of central nervous system lesions: stains for neurons, astrocytes, and microglia reveal the spatial and temporal features of MK-801-induced neuronal necrosis in the rat cerebral cortex. Toxicol Pathol. 1996; 24: 291–304. [DOI] [PubMed] [Google Scholar]
- 73. Franssen CA, Switzer RC IV, Switzer RC III, et al. Neurotoxicity study design: the essential element of sacrifice times (E-Abstract, 25.14). Soc Neurosci. 2007; 37. [Google Scholar]