Abstract
Glutamate is an important neurotransmitter in regulation of the neural portion of hypothalamus-pituitary-adrenal (HPA) axis activity, and signals through ionotropic and metabotropic receptors. In the current studies we investigated the role of hypothalamic paraventricular group I metabotropic glutamate receptors in regulation of the HPA axis response to restraint stress in rats. Direct injection of the group I metabotropic glutamate receptor agonist 3,5-dihydroxyphenylglycine (DHPG) into the PVN prior to restraint leads to blunting of the HPA axis response in awake animals. Consistent with this result, infusion of the group I receptor antagonist hexyl-homoibotenic acid (HIBO) potentiates the HPA axis response to restraint. The excitatory effect of blocking paraventricular group I metabotropic glutamate signaling is blocked by co-administration of dexamethasone into the PVN. However, the inhibitory effect of DHPG is not affected by co-administration of the cannabinoid CB1 receptor antagonist AM-251 into the PVN. Together, these results suggest that paraventricular group I metabotropic glutamate receptor signaling acts to dampen HPA axis reactivity. This effect appears to be similar to the rapid inhibitory effect of glucocorticoids at the PVN, but is not mediated by endocannabinoid signaling.
Keywords: Glutamate, metabotropic, HPA axis, glucocorticoids, stress, rats
1. Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system [1], and has significant involvement in regulation of the hypothalamus-pituitary-adrenal (HPA) axis [2]. Glutamate signaling in the paraventricular nucleus of the hypothalamus (PVN) is known to play a role in this regulation. For example, direct infusion of glutamate into either the 3rd ventricle [3] or the PVN [4] leads to increased ACTH secretion, suggesting an excitatory role for glutamate in regulating the HPA axis at the level of the PVN. However, the glutamate receptor family has numerous members that may mitigate effects of glutamate at the cellular level. The ionotropic glutamate receptor GluR5, for example, appears to inhibit the HPA axis response to restraint when signaling at the PVN [5], while other ionotropic glutamate receptors typically play an excitatory role in HPA axis regulation [2]. In addition to the ionotropic glutamate receptors, metabotropic glutamate receptors are also involved in regulating the HPA axis.
Metabotropic glutamate receptors (mGluR) are generally categorized into 3 different families, based on homology and pharmacology [6]. Groups II and III, comprising mGluR 2, 3, 4, 6, 7, and 8, are typically expressed presynaptically and negatively regulate synaptic release of neurotransmitters [6]. Group I consists of mGluRl and mGluR5, which have been reported to be both presynaptic [7] and post-synaptic [8] [9]. Post-synaptic receptors cause excitation of the post-synaptic neuron [6], while presynaptic receptors can mediate either an excitatory feed-forward signal [7] or an inhibitory signal. Inhibition is mediated by reducing presynaptic neurotransmitter release, in at least some cases by downstream signaling through endocannabinoid/CB1 receptor signaling [10] [11].
Metabotropic glutamate receptor signaling is involved in regulating HPA axis responses [12]. In general, group III metabotropic glutamate receptor signaling increases ACTH and corticosterone release [13]. Group II receptors appear to exert tonic inhibition on the HPA axis [14]. Studies on group I metabotropic glutamate receptors have suggested both excitatory and inhibitory actions of these receptors on HPA axis activity. Intracerebroventricular treatment of animals with either group I agonists or antagonists leads to increased plasma corticosterone [13]. Likewise, intraperitoneal administration of an mGluR5 antagonist also leads to increased plasma corticosterone and ACTH levels [15], likely due at least in part to effects at the adrenal gland [16]. It has been proposed that this apparent contradiction in group I metabotropic glutamate receptor actions in the HPA axis may due to stimulatory effects on CRH-releasing cells in the PVN by group I receptor signaling, and disinhibition of GABAergic interneurons in the peri-PVN area of the hypothalamus [13]. However, it should also be noted that peripheral or intracerebroventricular administration would be expected to cause effects on glutamate receptors distant from the PVN.
Thus, the case for an involvement of group I metabotropic glutamate receptor signaling at the PVN in regulation of the HPA axis has not yet been convincingly made. Group I metabotropic glutamate receptors are expressed in the PVN [8] [9], but it is currently unknown whether these receptors play a role in regulating the HPA axis response to stress. The purpose of the present studies is to determine the effect of group I metabotropic glutamate receptor signaling in the PVN on HPA axis responses to acute stress. We tested the hypothesis that metabotropic glutamate receptor signaling in the PVN leads to an excitation of the HPA axis response to restraint stress using intra-PVN injections of group I metabotropic agonist or antagonist.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats weighing 300-350g were used in these studies. Animals were kept in dedicated animal holding rooms with controlled temperature and humidity. The animals were kept on a 12:12 hour light:dark cycle, with lights on at 6:00 AM. After arrival at the facility, rats were allowed to accommodate to the new surroundings for at least 1 week before undergoing surgery. All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee, and were consistent with guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Stereotaxic surgery
Rats were anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively), with additional dosing as necessary to maintain a surgical plane of anesthesia. They were also given pre-emptive analgesia with butorphanol. They were implanted with 26-gauge bilateral guide cannulas (1.0 mm center-center distance, Plastics One, Roanoke VA) aimed at the PVN, using the following stereotaxic coordinates: 1.9 mm posterior to bregma, centered over the sagittal sinus, and 6.3 mm ventral from the dura mater at the site of implantation. Cannulas were anchored to the skull using stainless steel screws and cranioplastic cement (Plastics One). Dummy cannulas were placed in the guide cannula to maintain patency, and dust caps were placed over the dummy cannulas (cut to project 0.1 mm beyond the end of the guide cannula) to hold them in place. Animals were treated with gentamicin post-operatively, and observed through recovery before being returned to their home cages. All animals were observed post-surgery for signs of pain or illness, and additional analgesic was given as necessary for pain. After several days of recovery, the dummy cannulas were removed and replaced daily until the day of the experiment, to maintain an open guide cannula.
2.3. Drugs
(S)-3,5-dihydroxyphenylglycine (DHPG), hexyl-homoibotenic acid (hexyl-HIBO), and AM-251 were purchased from Tocris (Minneapolis, MN). Dexamethasone was purchased from Sigma (St. Louis, MO). Stock solutions of DHPG and hexyl-HIBO were made by dissolving the drugs in 0.9% saline alkalinized with NaOH. The pH of the resulting solutions was checked using pH paper, and neutralized with HCl as needed to obtain neutral solutions. Dexamethasone was dissolved in sterile 0.9% saline, and AM-251 was dissolved in DMSO. The drugs were then diluted to working concentrations (2 nmol/μl for DHPG, 1 nmol/μl for hexyl-HIBO, 20ng/μl for dexamethasone, and 40 pmol/μl for AM-251) in 0.9% saline (with 0.4% DMSO for drugs in the AM-251 experiment). When 2 drugs were given to the same animal, the drugs were mixed together and administered as a single injection. The injected doses for dexamethasone (10ng/side) and AM-251 (20pmol/side) were previously described by us as effective to blunt the ACTH response to restraint, and to reverse this blunting, respectively [17]. The dose of DHPG was chosen based on reported dosing causing neurobehavioral effects in rats [18], and that of hexyl-HIBO was chosen based on results of in vitro testing of the compound [19]. All drug stock and working solutions were made on the morning they were used.
2.4. Injection and Restraint Challenge
After cannula implantation, animals were allowed to recover for 6-8 days before restraint challenge. Starting 2 days after surgery, animals were handled daily to habituate them to the experimenter and to the injection protocol. On the day of the experiment, the dummy cannulas were removed from the guide cannulas and internal cannulas (cut to project 1.0 mm beyond the end of the guide cannula) placed. Animals received intraparenchymal injections (500 nl per side) through the cannula, using a PHD 2000 syringe pump (Harvard Apparatus, Holliston, MA). Injections were given over 1 minute (500 nl/min injection rate). Immediately following the injection, animals were restrained in plastic restraining tubes, and blood samples (∼250 μl) were collected by tail nick into microcentrifuge tubes containing EDTA (see Figure 1 for a schematic of the experiments). For the experiments using DHPG or hexyl-HIBO, blood samples were taken immediately upon restraint, then 15 and 30 minutes later. After the 30 min blood sample, animals were released into their home cages, then killed by rapid decapitation at 60 minutes post-injection. Trunk blood was collected, and brains removed after decapitation. For the experiments with dexamethasone and AM-251, blood samples were taken immediately upon restraint, and at 15 and 30 minutes post-injection. Rats were then released into their home cages, and a 60 minute blood sample was taken. Animals were perfused with 4% formaldehyde 120 minutes post-injection. All injections and blood sampling procedures were performed between 8AM and noon on the day of the experiment.
Figure 1.
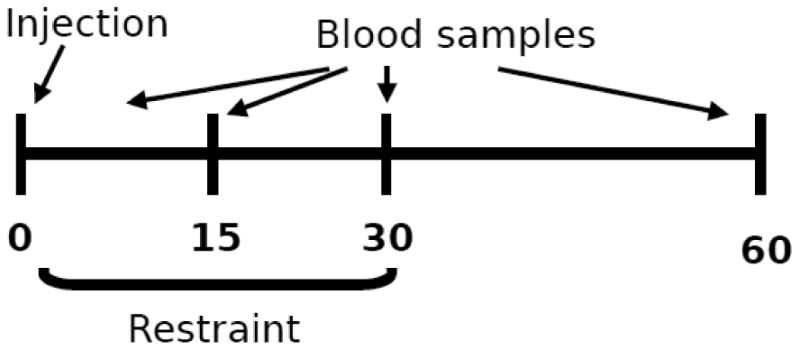
For each experiment, animals were injected into the PVN at time 0, then placed immediately into restrainers. Blood samples were obtained by tail clips immediately upon restraint, and at 15 and 30 minutes after the injections. Animals remained in restraint through the 30 minute time point. For the AM-251 experiment, a fourth blood sample was taken at 60 minutes, and animals were perfused 120 minutes after injection. For the other two experiments, animals were sacrificed by rapid decapitation at the 60 minute time point, and trunk blood obtained for the 60 minute time point.
2.5. Nissl stain for cannula placement
Cannula placement was verified as previously reported [5]. For animals killed by decapitation, brains were immersion fixed in 3.7% formaldehyde in 50 mM potassium phosphate buffer for 5 days. Brains fixed by immersion or by perfusion were subsequently impregnated with 30% sucrose solution, sectioned at 25 μm on a sliding microtome (Leica, Bannockburn, IL). Sections were mounted on glass slides, then Nissl stained with cresyl violet. Cannula placement was verified by observing the cannula tracks in brain sections. Only animals with correctly placed cannulas were included in the analyses of plasma hormones.
2.6. Radioimmunoassay
Plasma was separated from the blood samples by centrifugation. Plasma levels of corticosterone were measured using a commercial 125I-labeled corticosterone radioimmunoassay kit (ICN, Costa Mesa, CA). ACTH levels were assayed with a 125I-labeled ACTH radioimmunoassay as previously described [20], using tracer from DiaSorin (Stillwater, MN). ACTH antiserum was a generous gift of W. Engeland (University of Minnesota).
2.7. Statistical analysis
Plasma hormone levels were analyzed using 2-way or 3-way ANOVA with repeated measures, as appropriate. Area under the curve (AUC) was estimated using the trapezoidal method. Data were square root transformed if necessary to preserve homogeneity of variance, and outliers were removed using Grubb's test [21]. Significant main effects or interactions were analyzed further using the Fisher's LSD post-hoc test. Statistical significance was considered at p < 0.05. Statistical analyses were performed using either GB-Stat (Dynamic Microsystems, Silver Spring, MD) or Statistica (StatSoft, Tulsa OK).
3. Results
3.1. Metabotropic glutamate receptor activation inhibits the HPA axis response to restraint
Results of local PVN mGluRl agonist injections are illustrated in Figure 2. DHPG treatment rapidly attenuated the HPA axis response to restraint stress. There was a significant reduction in the ACTH time course response (Time × DHPG interaction F3,67= 2.942, p < 0.05, Fig. 2A), and in the corticosterone area under the curve (AUC) response (2 tailed t = 2.277, p < 0.05, Fig. 2D).
Figure 2. mGluR group I agonist inhibits the HPA axis response to restraint.
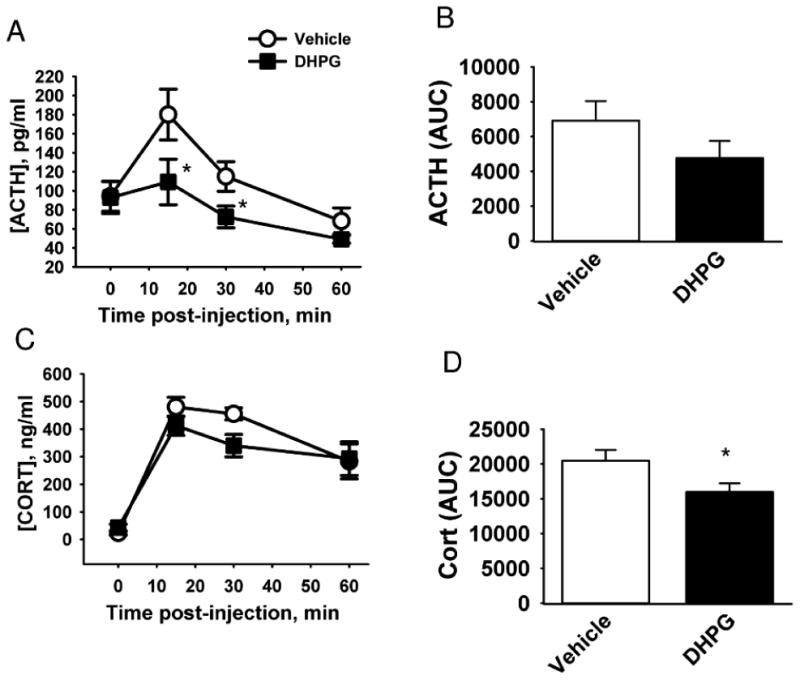
Rats were injected in the PVN with the group I metabotropic glutamate receptor agonist DHPG. A) Time course of ACTH secretion in response to restraint. B) Area under the curve (AUC) of ACTH secretion in response to restraint. C) Time course of corticosterone (CORT) secretion in response to restraint. D) AUC of corticosterone secretion in response to restraint. * indicates p < 0.05 vs vehicle. n = 8 (vehicle) and 9 (DHPG).
3.2 Blocking mGluR signaling potentiates HPA axis response to restraint
Blockade of group I metabotropic receptors resulted in a significant elevation of the ACTH response to restraint (main effect of hexyl-HIBO treatment F1,26 = 6.93, p = 0.014, Fig 3A). Hexyl-HIBO treatment elevated the ACTH AUC measurement as well (main effect of hexyl-HIBO treatment F3,27 = 4.21, p = 0.014 Fig. 3B). There was not a significant change in the corticosterone time course or AUC measurement as a result of hexyl-HIBO treatment (Fig 3C, D). In contrast to the hexyl-HIBO results, dexamethasone treatment led to inhibition of the HPA axis response to restraint, as measured by ACTH secretion (main effect of dexamethasone treatment, F1,26 = 5.32, p = 0.029, Fig 3A, B) and corticosterone secretion (dexamethasone treatment × time interaction F3,93 = 4.81, p = 0.0037, Fig 3C). Post-hoc analysis showed no significant difference in restraint-induced ACTH levels between vehicle treated animals and dexamethasone/hexyl-HIBO co-treated animals at any time point (Fig 3A, B).
Figure 3. Group I mGluR antagonist treatment elevates the HPA axis response to restraint in a manner that is reversed by dexamethasone treatment.
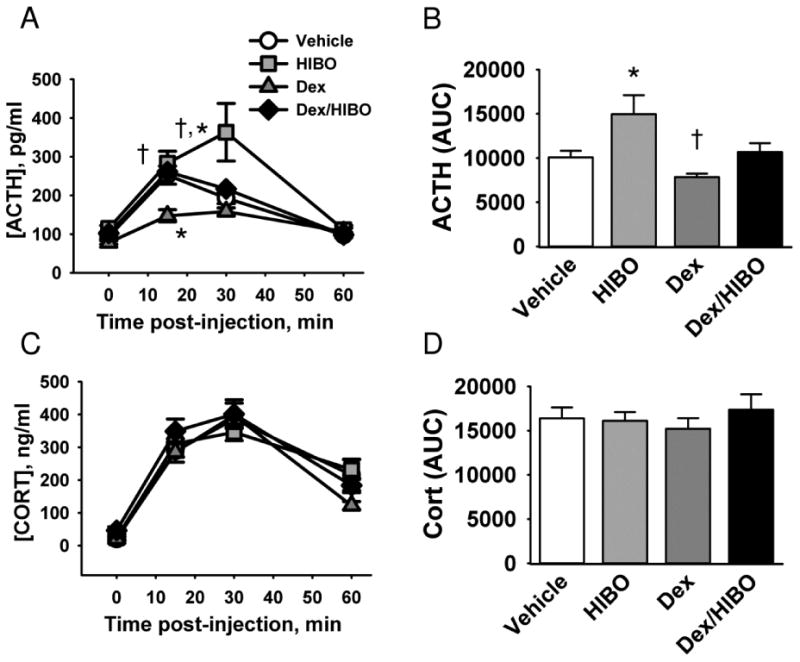
Rats were injected in the PVN with the group I metabotropic glutamate receptor antagonist hexyl-HIBO and/or dexamethasone (Dex), then subjected to 30 minutes of restraint. A) Time course of ACTH secretion in response to restraint. B) Area under the curve (AUC) of ACTH secretion. C) Time course of corticosterone (CORT) secretion in response to restraint. D) AUC of corticosterone secretion in C. * indicates p < 0.05 vs. vehicle, and † indicates p < 0.05 vs dexamethasone in A and vs HIBO in B. n = 8 (vehicle), 10 (hexyl-HIBO), 7 (dexamethasone), and 8 (dexamethasone/hexyl-HIBO).
3.3. Cannabinoid CB1 receptor blockade does not reverse group I metabotropic glutamate receptor-mediated HPA axis inhibition
There was a significant main effect on the ACTH secretion time course of drug treatment (F3,34 = 5.5, p = 0.034), as well as a significant time by drug interaction (F9,102 = 7.09, p < 0.00001). Post-hoc analysis of these results revealed that, as expected, DHPG treatment significantly blunted the ACTH response to restraint stress at 15 minutes and 30 minutes after the onset of restraint stress (Fig. 4A). Administration of the cannabinoid CB1 receptor antagonist AM-251 alone did not affect the ACTH response. AM-251 also did not reverse the blunting of the ACTH response to restraint caused by DHPG treatment. A similar pattern of results was seen with AUC calculations from this experiment (Fig. 4B). There was no statistically significant effect on corticosterone levels by any of these treatments (Fig. 4C, D).
Figure 4. Inhibition of HPA axis response to stress by DHPG is not blocked by AM-251 treatment.
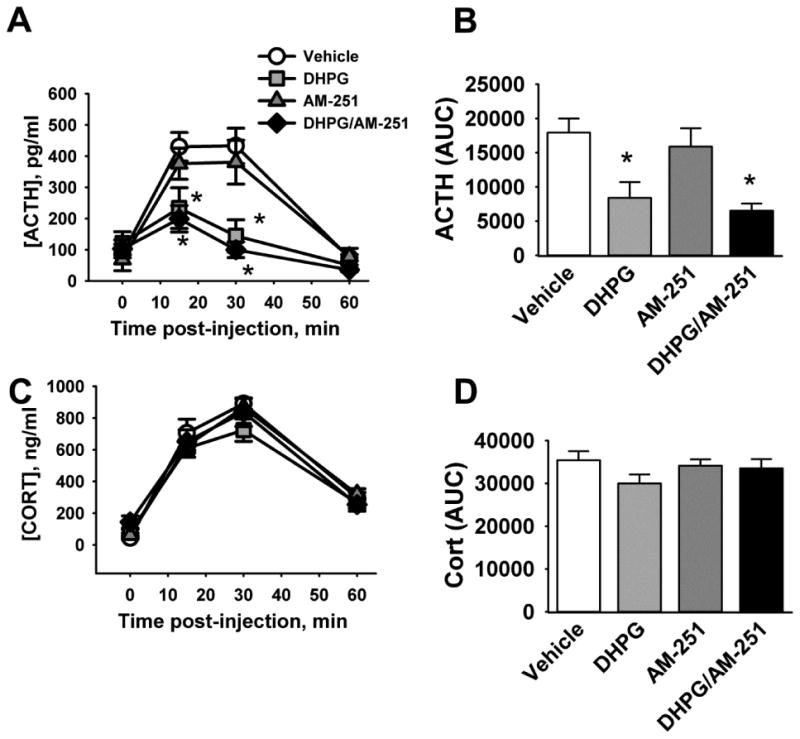
Rats were injected with the cannabinoid CB1 receptor antagonist AM-251 and/or DHPG into the PVN, then subjected to 30 minutes of restraint. A) ACTH time course in response to restraint. B) Area under the curve (AUC) of ACTH secretion. C) Corticosterone (CORT) time course in response to restraint. D) AUC of corticosterone secretion. * indicates p < 0.05 compared to vehicle treated animals. n = 10 (vehicle and DHPG) and 9 (AM-251 and DHPG/AM-251).
4. Discussion
4.1. Overview
In the current studies we demonstrate that paraventricular group I metabotropic glutamate receptor signaling is involved in regulation of the HPA axis response to restraint. Activation of paraventricular group I metabotropic glutamate receptors by DHPG leads to a blunting of the ACTH response to restraint stress, while blocking these receptors using hexyl-HIBO leads to a potentiation of the ACTH response to restraint. Thus, the effect of activating paraventricular group I metabotropic glutamate receptors is inhibitory to the pituitary portion of the HPA axis response to restraint stress. This inhibition, mediated by group I metabotropic glutamate receptors, occurs rapidly, within 15 to 30 minutes of applying the drugs.
4.2. Group I metabotropic glutamate receptor mediated HPA axis inhibition is independent of endocannabinoid receptor signaling in the PVN
The HPA axis response to restraint is rapidly inhibited by glucocorticoids through an endocannabinoid-mediated signaling pathway, on a similar time scale to that reported in the current manuscript [17] [22]. Because of this similarity in time course, and because mGluR I receptors in many cases mediate endocannabinoid signaling [11] [10], we hypothesized that metabotropic glutamate receptors may be part of the signal cascade that mediates the fast inhibitory effects of glucocorticoids on the HPA axis [23]. This hypothesis was initially supported by the finding that while intra-PVN injection of dexamethasone alone inhibits the ACTH response to acute restraint stress, co-treatment with dexamethasone and hexyl-HIBO leads to an ACTH response that is not significantly different from vehicle-treated animals (Fig 3A). We have previously seen this pattern, with dexamethasone treatment at the PVN blunting the ACTH response to restraint, and this blunting being completely reversed by co-treatment with AM-251 [17]. This was done with the same dosing as in the current studies, and with paraventricular administration of both drugs.
However, unlike the case with dexamethasone-induced HPA axis blunting, in the current studies the CB1 receptor antagonist AM-251 fails to reverse DHPG-induced blunting of the HPA axis response to restraint (see Figure 4). In addition, while AM-251 alone does not affect the ACTH response to acute restraint, hexyl-HIBO treatment alone enhances the ACTH response, suggesting different mechanisms for the reversal of dexamethasone-induced ACTH blunting by AM-251 vs. hexyl-HIBO. It is possible that the results illustrated in Fig. 3A represent a balancing of dexamethasone-induced inhibition of ACTH and hexyl-HIBO induced excitation of the ACTH response to acute restraint, although further study will be needed to define this more fully. Taken together, these results appear to disprove the hypothesis that group I metabotropic glutamate receptor mediated signaling at the PVN is part of the signaling cascade that leads to endocannabinoid-dependent rapid negative feedback regulation of the HPA axis.
4.3. Technical considerations
The drugs used in this study are all considered to be selective for the receptors investigated in the current studies. DHPG [24] and HIBO [19] have no effect on Group II or III metabotropic glutamate receptors at doses at least as high as 1mM. Similarly, AM-251 is about 300-fold selective for CB1 vs CB2 [25]. Based on this information, we feel that the results described in these studies represent effects mediated through group I metabotropic glutamate receptors and the endocannabinoid CB1 receptor, respectively. That said, however, we cannot rule out the possibility that there are other receptors that may be affected by these drugs.
Based on previous work in our laboratory, the volume injected in the current studies would be expected to diffuse throughout the entire PVN, including part of the peri-PVN [17]. Group I metabotropic glutamate receptors are found in the parvocellular but not magnocellular portion of the PVN, but are also found in the peri-PVN area of the hypothalamus [9] [8]. Based on this, we cannot rule out the immediate surround of the PVN as the site of action for the metabotropic glutamate receptor modulators used in this study; indeed, disinhibition of peri-PVN GABAergic neurons has been proposed as a possible mechanism for metabotropic glutamate receptor signaling [13]. Further study will be required to determine whether the drugs used in this study exert their effects on parvocellular neurons directly, or on neurons that are one or more synapses away from the parvocellular PVN.
4.4. Complexity of paraventricular glutamate-mediated control of HPA axis
As noted above, glutamate is the main excitatory signal in the central nervous system. Infusion of glutamate into the third ventricle [4] or PVN proper [3] [26] leads to excitation of ACTH secretion, suggesting that the predominant action of glutamate in the PVN is excitatory. However, we have previously reported an inhibitory role for paraventricular glutamate on HPA axis activity, mediated by the GluR5 ionotropic glutamate receptor [5]. In conjunction with the current studies, these data suggest that the glutamatergic regulation of the HPA axis at the PVN is complex, involving actions that depend on synapse-specific inhibitory as well as excitatory signaling mechanisms.
This complexity of regulation is further illustrated by subtle differences in the time course of DHPG-induced inhibition of the ACTH response to restraint (seen at the 15 minute time point) as compared to hexyl-HIBO-induced disinhibition (seen at the 30-minute time point). It is not clear why there is a difference in timing here, although it is possible that the endogenous glutamatergic tone blocked by hexyl-HIBO occurs after the onset of restraint, while DHPG administration occurs prior to the onset of the restraint stress. Future studies will be needed to more fully explore these subtle differences.
4.5. Dissociation of ACTH from corticosterone
It is interesting to note in these experiments that there were consistent effects of our drug treatments on ACTH secretions. The effects on corticosterone were much smaller in magnitude, and not consistently seen in all experiments (for example, a statistically significant decrease in corticosterone AUC was seen with DHPG treatment in Fig. 2 but not in Fig. 4). We have noted this pattern in other studies performed in our laboratory, especially in those focused on the PVN [27] [5]. Dissociation between ACTH and corticosterone production has also been reported by other groups[28-30]. Mechanisms for dissociation of ACTH and corticosterone responses include, for example, sympathetic nervous system-dependent tuning of adrenal sensitivity to ACTH; such dissociation, including mechanisms for the effect, has been reviewed elsewhere [31].
Regardless of the mechanism for this dissociation, however, it is clear that ACTH and corticosterone levels are not always parallel. In the current studies, effects on ACTH secretion provide a direct readout of the output of paraventricular integration of central stress processing, independent of downstream actions in the HPA axis. Thus, we show upstream effects of central blockade and activation of metabotropic glutamate receptors in the PVN.
4.6 Conclusion
In conclusion, we have shown that a group I mGluR-mediated signaling pathway at the PVN is inhibitory to the HPA axis. This inhibitory pathway acts on the same time scale as rapid negative feedback regulation of the HPA axis by glucocorticoids, but appears to represent a distinct signaling pathway from endocannabinoid-mediated fast feedback. These results highlight the complexity of paraventricular glutamate-mediated control of HPA axis reactivity.
Highlights.
-
-
We investigated metabotropic glutamate receptors actions in HPA axis regulation.
-
-
Blocking group I receptors in the PVN potentiates the HPA axis response to stress.
-
-
Group I agonist treatment decreases the HPA axis response to stress.
-
-
This action of glutamate is not mediated by CB1 endocannabinoid receptors.
Acknowledgments
We thank Ben Packard, Anne Christianson, Matia Solomon, Ryan Jankord, Yve Ulrich-Lai, Amanda Jones, Kenny Jones, Rong Zhang, and Mark Dolgas for technical assistance with the animal studies. This work was supported by NIH grants MH069725 and NS007453.
Footnotes
Present Address: 3333 Burnet Ave, ML: 4009, Cincinnati, OH 45229, Phone:+15136367480, Nathan.evanson@cchmc.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brann DW. Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology. 1995;61:213–225. doi: 10.1159/000126843. [DOI] [PubMed] [Google Scholar]
- 2.Jezova D. Control of ACTH secretion by excitatory amino acids: functional significance and clinical implications. Endocrine. 2005;28:287–294. doi: 10.1385/ENDO:28:3:287. [DOI] [PubMed] [Google Scholar]
- 3.Darlington DN, Miyamoto M, Keil LC, Dallman MF. Paraventricular stimulation with glutamate elicits bradycardia and pituitary responses. Am J Physiol. 1989;256:R112–R119. doi: 10.1152/ajpregu.1989.256.1.R112. [DOI] [PubMed] [Google Scholar]
- 4.Makara GB, Stark E. Effect of intraventricular glutamate on ACTH release. Neuroendocrinology. 1975;18:213–6. doi: 10.1159/000122400. [DOI] [PubMed] [Google Scholar]
- 5.Evanson NK, van Hooren DC, Herman JP. GluR5-mediated glutamate signaling regulates hypothalamo-pituitary-adrenocortical stress responses at the paraventricular nucleus and median eminence. Psychoneuroendocrinology. 2009;34:1370–1379. doi: 10.1016/j.psyneuen.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. Metabotropic glutamate receptors: synaptic modulators and therapeutic targets for neurologic disease. Neurology. 2008;70:964–968. doi: 10.1212/01.wnl.0000306315.03021.2a. [DOI] [PubMed] [Google Scholar]
- 7.Musante V, Neri E, Feligioni M, Puliti A, Pedrazzi M, Conti V, Usai C, Diaspro A, Ravazzolo R, Henley JM, Battaglia G, Pittaluga A. Presynaptic mGlu1 and mGlu5 autoreceptors facilitate glutamate exocytosis from mouse cortical nerve endings. Neuropharmacology. 2008;55:474–482. doi: 10.1016/j.neuropharm.2008.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Pol AN. Metabotropic glutamate receptor mGluR1 distribution and ultrastructural localization in hypothalamus. J Comp Neurol. 1994;349:615–632. doi: 10.1002/cne.903490409. [DOI] [PubMed] [Google Scholar]
- 9.van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995;362:134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- 10.Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Curr Opin Pharmacol. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- 11.Kano M, Hashimoto K, Tabata T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and Synapse elimination. Philos Trans R Soc Lond B Biol Sci. 2008;363:2173–2186. doi: 10.1098/rstb.2008.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand D, Pampillo M, Caruso C, Lasaga M. Role of metabotropic glutamate receptors in the control of neuroendocrine function. Neuropharmacology. 2008;55:577–583. doi: 10.1016/j.neuropharm.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MP, Kelly G, Chamberlain M. Changes in rat serum corticosterone after treatment with metabotropic glutamate receptor agonists or antagonists. J Neuroendocrinol. 2001;13:670–677. doi: 10.1046/j.1365-2826.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- 14.Scaccianoce S, Matrisciano F, Bianco PD, Caricasole A, Gerevini VDG, Cappuccio I, Melchiorri D, Battaglia G, Nicoletti F. Endogenous activation of group-II metabotropic glutamate receptors inhibits the hypothalamic-pituitary-adrenocortical axis. Neuropharmacology. 2003;44:555–561. doi: 10.1016/s0028-3908(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Schaffhauser H, Rao SP, Varney MA, Anderson JJ. Metabotropic glutamate receptor 5 antagonist-induced stimulation of hypothalamic-pituitary-adrenal axis activity: interaction with serotonergic systems. Neuropharmacology. 2003;44:562–572. doi: 10.1016/s0028-3908(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 16.Pokusa M, Prokopova B, Hlavacova N, Makatsori A, Jezova D. Effect of blockade of mGluR5 on stress hormone release and its gene expression in the adrenal gland. Can J Physiol Pharmacol. 2014;92:686–692. doi: 10.1139/cjpp-2014-0030. [DOI] [PubMed] [Google Scholar]
- 17.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadlewska A, Car H, Oksztel R, Wiśniewski K. Effect of (S)-3,5-DHPG on learning, exploratory activity and anxiety in rats with experimental hypoxia. Pol J Pharmacol. 2002;54:11–18. [PubMed] [Google Scholar]
- 19.Madsen U, Brauner-Osborne H, Frydenvang K, Hvene L, Johansen TN, Nielsen B, Sanchez C, Stensbol TB, Bischoff F, Krogsgaard-Larsen P. Synthesis and pharmacology of 3-isoxazolol amino acids as selective antagonists at group I metabotropic glutamic acid receptors. J Med Chem. 2001;44:1051–1059. doi: 10.1021/jm000441t. [DOI] [PubMed] [Google Scholar]
- 20.Engeland WC, Miller P, Gann DS. Dissociation between changes in plasma bioactive and immunoreactive adrenocorticotropin after hemorrhage in awake dogs. Endocrinology. 1989;124:2978–2985. doi: 10.1210/endo-124-6-2978. [DOI] [PubMed] [Google Scholar]
- 21.Barnett V, Lewis T. Outliers in statistical data. John Wiley & Sons; New York: 1994. [Google Scholar]
- 22.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–7. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evanson NK, Herman JP, Sakai RR, Krause EG. Nongenomic actions of adrenal steroids in the central nervous system. J Neuroendocrinol. 2010;22:846–861. doi: 10.1111/j.1365-2826.2010.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gereau R, 4th, Conn PJ. Roles of specific metabotropic glutamate receptor subtypes in regulation of hippocampal CA1 pyramidal cell excitability. J Neurophysiol. 1995;74:122–129. doi: 10.1152/jn.1995.74.1.122. [DOI] [PubMed] [Google Scholar]
- 25.Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- 26.Feldman S, Weidenfeld J. Hypothalamic mechanisms mediating glutamate effects on the hypothalamo-pituitary-adrenocortical axis. J Neural Transm. 1997;104:633–42. doi: 10.1007/BF01291881. [DOI] [PubMed] [Google Scholar]
- 27.Kasckow JW, Segar TM, Xiao C, Furay AR, Evanson NK, Ostrander MM, Herman JP. Stability of Neuroendocrine and Behavioral Responsiveness in Aging Fischer 344/Brown-Norway Hybrid Rats. Endocrinology. 2005;146:3105–3112. doi: 10.1210/en.2004-1648. [DOI] [PubMed] [Google Scholar]
- 28.Armario A, Restrepo C, Castellanos JM, Balasch J. Dissociation between adrenocorticotropin and corticosterone responses to restraint after previous chronic exposure to stress. Life Sci. 1985;36:2085–2092. doi: 10.1016/0024-3205(85)90304-2. [DOI] [PubMed] [Google Scholar]
- 29.Dempsher DP, Gann DS. Increased cortisol secretion after small hemorrhage is not attributable to changes in adrenocorticotropin. Endocrinology. 1983;113:86–93. doi: 10.1210/endo-113-1-86. [DOI] [PubMed] [Google Scholar]
- 30.Dobrakovová M, Kvetnanský R, Oprsalová Z, Jezová D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993;18:163–174. doi: 10.1016/0306-4530(93)90001-2. [DOI] [PubMed] [Google Scholar]
- 31.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]


