Abstract
Purpose
To investigate the utility of intraoperative optical coherence tomography (OCT) for Descemet Membrane Endothelial Keratoplasty (DMEK) surgery.
Design
Prospective consecutive interventional case series.
Methods
DISCOVER (NCT02423213) is a prospective consecutive interventional case series examining the feasibility and utility microscope-integrated intraoperative OCT in ophthalmic surgery. This report focuses on those eyes in the DISCOVER study undergoing DMEK surgery. The eight cases were the first DMEK cases performed by the primary surgeon (J.M.G.) with microscope integrated intraoperative OCT feedback (Rescan 700, Carl Zeiss Meditec). Qualitative OCT analysis was performed at multiple surgeon-defined time points, including host and donor tissue preparation, graft orientation, graft apposition, and tissue interface fluid dynamics.
Results
Correct graft orientation was confirmed by intraoperative OCT prior to unscrolling in 100% of cases. Seven of eight grafts were fully attached at the conclusion of surgery and on postoperative day one. One graft had a linear paracentral fixed area of interface separation corresponding to posterior stromal irregularities which was visible during surgery and unchanged on postoperative day one. Two eyes developed significant peripheral graft dehiscence visible by the first postoperative week. Both grafts were successfully re-attached with repeat gas injection. All eyes demonstrated improvement in best corrected visual acuity and there was a 100% graft survival rate at last follow-up (minimum = 4 months). Surgeon feedback indicated that intraoperative OCT provided valuable information in all eight cases.
Conclusions
Real-time intraoperative OCT can provide useful information, which may directly impact surgical decision-making during DMEK surgery. Intraoperative OCT may facilitate the transition for novice DMEK surgeons by increasing surgeon confidence and reducing the risk of iatrogenic graft failure.
Introduction
Descemet Membrane Endothelial Keratoplasty (DMEK) offers several distinct advantages over Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) for the treatment of corneal endothelial disease. Specifically, previous authors have demonstrated benefit with regard to faster visual rehabilitation, better visual acuity, and reduced rates of endothelial rejection.1,2 However, transitioning to DMEK surgery creates novel challenges for the corneal surgeon already skilled in DSAEK surgery. Difficulties and inconsistencies with donor tissue preparation, insertion, orientation, positioning and unscrolling have all resulted in a steep “learning curve” for surgeons beginning DMEK surgery. Significant advances have been achieved in standardizing the steps and techniques utilized during DMEK surgery to reduce the frequency of intraoperative and postoperative complications. Despite these advances, many surgeons remain reluctant to transition to DMEK surgery due to the technical and unique challenges of this surgical procedure.
One potential avenue for improved surgeon confidence and additional refinement of DMEK is the application of high resolution, real-time intraoperative optical coherence tomography (OCT). This technology has already gained acceptance for its utility in visualizing the interface created during Descemet Stripping Automated Endothelial Keratoplasty (DSAEK). In 2014, the PIONEER study described the feasibility, utility and safety of intraoperative OCT in ophthalmic surgery, including lamellar keratoplasty.3 In fact, the PIONEER study reported that intraoperative OCT enhanced surgeon understanding and potentially impacted surgical decision-making in 40% of cases in lamellar keratoplasties.3 Similar to DSAEK, the implementation of intraoperative OCT for DMEK may provide information to the surgeon that helps improve tissue apposition and orientation while minimizing unnecessary and potentially dangerous manipulation of the donor scroll.
The DISCOVER study was initiated to assess the feasibility and utility of microscope-integrated intraoperative OCT for ophthalmic surgery with heads-up display surgeon feedback.4 The purpose of this report is to report the experiences and outcomes of a novice DMEK surgeon’s first consecutive cases performed with real-time intraoperative OCT from the DISCOVER study.
Methods
The DISCOVER Study is a single-site, prospective multi-surgeon investigational device consecutive interventional case series. The study was approved by the Cleveland Clinic IRB and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants. The purpose of the DISCOVER study is to examine the feasibility and utility of a microscope-integrated intraoperative OCT system in both anterior and posterior segment surgery and identified when the system helps to improve surgical outcomes. The integrated systems used in the DISCOVER study are able to provide real-time information regarding instrument-tissue interactions.4,5 For this report, the primary inclusion criterion was enrollment in DISCOVER for DMEK surgery.
Donor Tissue
All donor tissue was pre-stripped and “S-stamped” for DMEK surgery by a Midwest Eye-Bank (Ann Arbor, MI) as previously described (Veldman PB et al., “Eliminating the possibility of upside-down DMEK grafts: a novel stromal side S-stamp technique for DMEK.” Presentted at: Cornea Society Fall Education Symposium; 2013 Nov 15, New Orleans, LA). In brief, the technician initiated a 9.5 mm partial trephination and stripped Descemet membrane (DM) with tying forceps. After the tissue was 80% stripped and elevated, a 2 mm circular trephination was performed eccentrically in the underlying stromal bed. DM was re-apposed and the corneoscleral rim was placed upright. The exposed anterior surface of DM was dry-marked gentian violet on a micro “S” stamp (Moria SA, France) and the 2mm corneal button was replaced. The tissue was placed in Optisol GS (Bausch & Lomb, Rochester, NY, USA) storage media until the time of surgery.
Surgical Technique
Host DM was stripped with a reverse Terry-Sinskey hook (Bausch and Lomb, St. Louis, MO) with Healon (Abbott Laboratories Inc., Abbott Park, IL, USA) filling the anterior chamber. A minimum descemetorrhexis of 8 mm was performed. Healon was evacuated with an irrigating/aspirating hand piece and intraocular Miochol (Bausch & Lomb, Rochester, NY, USA) was injected to constrict the pupil. To optimize the red reflex during decemetorrhexis, Miochol was utilized intraoperatively rather than pilocarpine preoperatively. An inferior peripheral iridectomy was created at 6 o’clock with capsulorrhexis forceps through a dedicated vertical paracentesis site at the limbus.
In all cases, the donor tissue was punched by the surgeon with a 7.75 mm Single-Use Corneal Vacuum Punch (Moria SA, France). Vacuum suction was applied to the epithelial surface but had to be aborted in two cases due to unexpected retraction of the DMEK tissue into the punch site for the “S” stamp. In both of these cases the suction was released and the tissue was successfully repositioned and subsequently cut without suction. The tissue was stained for three minutes in 0.06% trypan blue solution. The trypan blue was replaced with balanced salt solution and the graft was then drawn into a Modified Jones Tube for DMEK (Gunther Weiss Scientific, Portland, OR) attached to a 3 cc syringe. The tissue was injected into the anterior chamber through a 3.5 to 4 mm wide triplanar corneal tunnel. The tissue was centered and oriented by a combination of external taps and successive fluid infusions through the paracentesis sites.
Once a properly centered and oriented double scroll was achieved, the tissue was unscrolled by anterior chamber collapse followed by external taps (Supplemental Video 1). After the tissue was completely unscrolled, 20% sulfur hexafluoride gas was injected beneath the graft to elevate it against the posterior corneal surface (Supplemental Video 2). The pressure was increased to approximately 40–50 mmHg and the cornea was then swept in a center-to-periphery fashion to evacuate any remaining interface fluid. After the ten minutes with a complete gas fill, a partial gas-fluid exchange was performed until an approximately 75% to 90% gas fill was obtained and the gas was no longer obstructing the inferior peripheral iridectomy.
Use of Intraoperative Optical Coherence Tomography
Intraoperative imaging was performed using a microscope-integrated intraoperative OCT system (RESCAN 700, Carl Zeiss Meditec), as previously described.4 This integrated system includes a “heads-up display” with a visible transparent overlay of the OCT data stream in the surgeon’s right ocular and an external video display panel. Surgeon foot-pedal control or external assistant control is possible. Intraoperative OCT was employed for select surgical steps, including host descemetorrhexis, graft orientation, and graft apposition. Both real-time and static intraoperative OCT feedback were utilized by the surgeon. A surgeon feedback form was utilized to evaluate the impact of intraoperative OCT on surgeon decision-making as part of the DISCOVER study.
Results
Clinical Results
Eight eyes of seven patients (4 females, 3 males; mean age of 74, range 49 – 88) that underwent DMEK in the DISCOVER study were included in this analysis. All surgeries were performed by a single surgeon (J.M.G.). Of note, these were the first DMEK cases performed by this surgeon (with or without intraoperative OCT). The median “un-scrolling time” (onset of intraocular graft manipulation until full apposition with 100% gas fill) was 6 minutes and 15 seconds (range 2:25–27:36). The median duration of postoperative follow-up was 7 months (range 4–9 months).
Seven of eight grafts were fully attached on intraoperative OCT at the conclusion of the initial surgery. One graft had a linear area of non-adherence corresponding to a significant posterior stromal irregularity, which was visible on intraoperative OCT. There was no change in the adherence in any of the grafts on postoperative day one. At week 1, two eyes developed partial graft detachments. Both grafts underwent successful re-bubbling procedures and remained attached. All DMEK eyes experienced an improvement in best-corrected visual acuity and there was a 100% graft survival rate at last follow-up.
Intraoperative Optical Coherence Tomography Utility and Surgeon Feedback
During descemetorrhexis, intraoperative OCT allowed identification of the presence of DM fragments and posterior stromal irregularities. In two of eight cases, significant irregularities were visible after descemetorrhexis (Cases #1 and #2, Figures 1 and 2) and both grafts developed partial postoperative graft detachment requiring rebubbling.
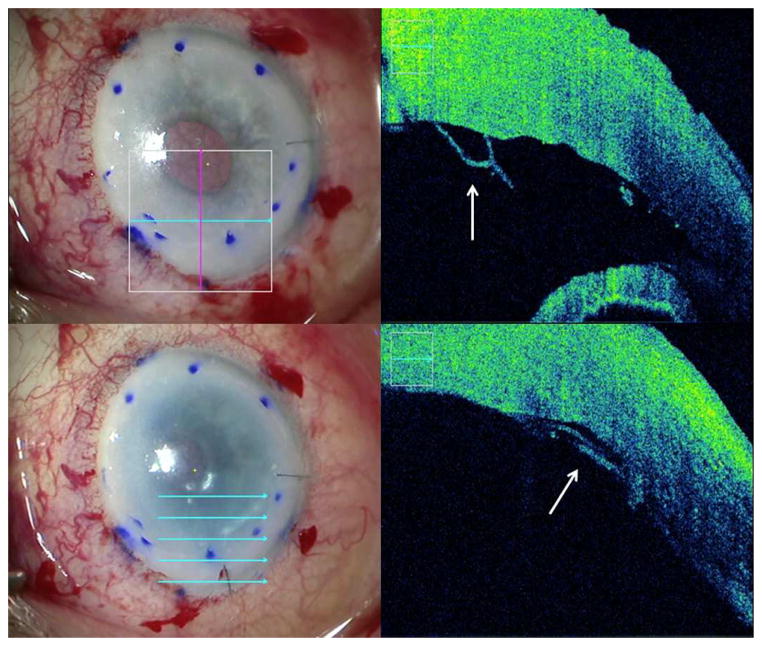
Figure 1. Intraoperative Optical Coherence Tomography Depictions of Peripheral Descemet Membrane Remnants During Descemet Membrane Endothelial Keratoplasty.
The left column shows en face views. In the right column, the accompanying intraoperative optical coherence tomography images from the en face views. Top row: Prior to graft insertion, peripheral remnants of Descemet membrane (white arrow). Bottom row: Attached graft overlapping the peripheral Descemet membrane remnants (white arrow), corresponding to an area of postoperative detachment.
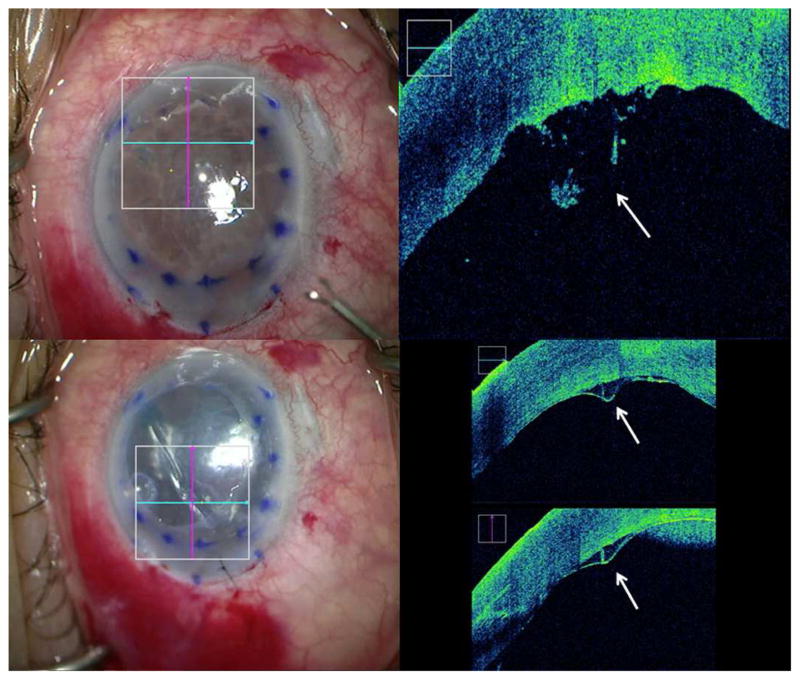
Figure 2. Identification of Posterior Stromal Irregularities with Intraoperative Optical Coherence Tomography During Descemet Membrane Endothelial Keratoplasty.
The left column shows en face views. In the right column, the accompanying intraoperative optical coherence tomography images from the en face views. Top row: Posterior stromal irregularities (white arrow) due to excessive manipulation. Bottom row: Posterior irregularity led to a fixed linear area of incomplete apposition intraoperatively (white arrows) and subsequent partial graft detachment at week one.
The graft orientation was rapidly identifiable with intraoperative OCT in 100% of cases based on the rolling behavior of the graft (Figure 3). Real-time intraoperative OCT feedback contradicted surgeon’s immediate initial “en-face” impression of tissue orientation in 4 of 8 cases. Of note, however, no concerted effort was made to identify the S-stamp prior to intraoperative OCT verification. The properly oriented “S” stamp was clearly visible in six of eight cases at the conclusion of surgery. In two cases, the “S” stamp could not be clearly identified, one case in which the anterior chamber visibility was extremely poor due to severe edema and dense arcus senilis, and one case in which the S-stamp was already barely discernible prior to graft preparation.
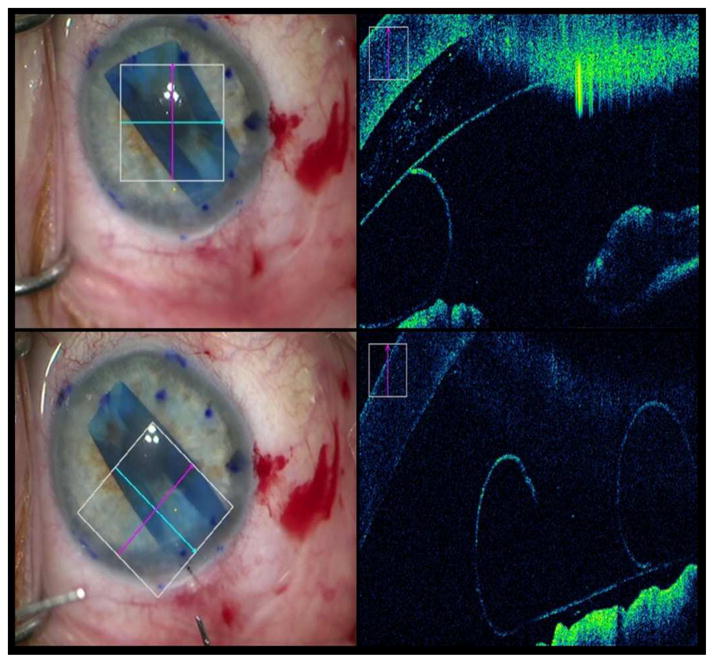
Figure 3. Intraoperative Optical Coherence Tomography Depictions of Graft Orientation During Descemet Membrane Endothelial Keratoplasty.
The left column shows en face views. In the right column, the accompanying intraoperative optical coherence tomography (OCT) images from the en face views. Top row: Inverted graft after insertion that required manipulations to flip it over. Bottom row: Graft orientation corrected after manipulations and verified on intraoperative OCT.
Intraoperative OCT was also utilized to evaluate graft apposition and the impact of external sweeping on interface fluid, when visible (Figures 1 and 4). Six grafts were completely apposed on intraoperative OCT after gas injection alone. In two cases, intraoperative OCT identified areas of incomplete apposition. In one case, additional manipulation had no effect on the area of non-apposition. In the other case, external sweeping resulted in complete elimination of the interface separation.
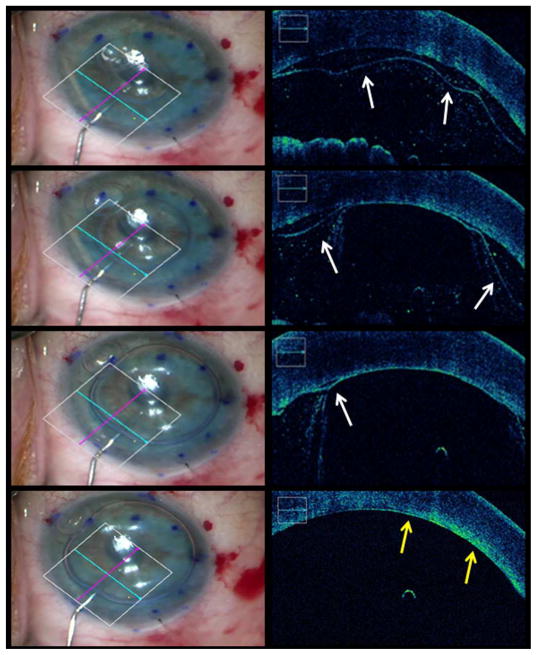
Figure 4. Intraoperative Optical Coherence Tomography Time-lapse Depictions of Graft Apposition Following Gas Infusion During Descemet Membrane Endothelial Keratoplasty.
From top to bottom the rows represent consecutive time-points. The left column shows en face views. In the right column, the accompanying intraoperative optical coherence tomography images from the en face views depict graft localization and the progression of graft apposition during gas infusion (white arrows indicate presence of interface fluid, yellow arrows indicate complete graft attachment to host tissue, see supplemental video 2).
In all eight cases, the surgeon subjectively reported that the intraoperative OCT system provided useful feedback. The OCT did not interfere with the surgical procedure in any of the cases. In all eight cases the external video display panel was used for intraoperative OCT image interpretation. In seven out of eight cases, the surgeon also attempted to use the heads-up display integrated into the microscope oculars, but overall the size and resolution of the image was too small to easily and reliably visualize the donor tissue and the external video display was also used for verification.
Case Examples
Case 1
An 83 year old Caucasian male was referred for pseudophakic bullous keratopathy as a result of a large previous intraoperative DM detachment with loss of the central 40% of DM during cataract surgery. During DMEK, visualization of the descemetorrhexis was rendered difficult due to severe edema and dense arcus senilis. Intraoperative OCT allowed identification of nasal and temporal peripheral irregularities which likely represented peripheral remnants of the host DM (Figure 1). Despite several attempts to excise these fragments, not all could be removed. Owing to the poor view and absence of a clearly visible “S” stamp, intraoperative OCT was critical for rapid visualization of the scroll orientation. The graft was fully attached at the conclusion of surgery, although it appeared the nasal and temporal aspects of the graft overlapped the peripheral DM remnants identified previously (Figure 1). The graft was fully attached on postoperative day one. At postoperative week one partial detachment of the nasal and temporal aspect of the graft was visible (approximately 40%). Repeat gas injection was performed on day 18 with successful reattachment.
Case 2
A 71 year-old Caucasian male underwent DMEK for endothelial decompensation due to possible toxic anterior segment syndrome following cataract surgery three months previously. The view was extremely poor due to severe corneal edema necessitating epithelial debridement at the beginning of the surgery. During descemetorrhexis, intraoperative OCT identified a paracentral area of posterior stromal irregularity similar to Case 1, likely due to excessive manipulation of the pre-Descemet posterior stroma (Figure 2). After the graft was unscrolled and gas instilled into the anterior chamber, a fixed linear area of incomplete apposition was visible originating from the area of posterior irregularity and extending obliquely in both directions (Figure 2). Evacuation of the gas and re-insufflation did not alter the appearance of the interface gap. External sweeping was similarly ineffective. One week postoperative, an inferior-temporal graft detachment of approximately 1/3rd the graft area was visible, extending to the aforementioned area, which did not resolve with observation. The graft was successfully re-attached with gas. The same area of interface separation remained present, but the inferior-temporal aspect of the graft remained attached postoperatively. At month 3, the graft remained attached despite persistence of a fixed fold.
Case 3
A 75 year-old Caucasian female with Fuchs dystrophy underwent DMEK surgery. Following initial insertion, the graft was thought to be properly oriented. Real-time intraoperative OCT revealed that the graft was improperly oriented and inverted contrary to the surgeon’s initial en-face impression, allowing for appropriate reorientation (Figure 3).
Discussion
The field of intraoperative OCT is currently going through substantial growth and increased availability. Until recently, all systems that were commercially available in the United States were portable systems, such as the Bioptigen and Optovue systems.3,6,7 More recently there has been significant interest in microscope integration of this technology.4,5,8–10 There are two commercially available microscope integrated systems: the Haag-Streit integrated system and the Zeiss RESCAN 700.4,8
The specific role for intraoperative OCT in ophthalmic surgery continues to be defined. DMEK is an emerging surgery that has specific technical challenges that may be particularly mitigated with the use of intraoperative OCT. One report described 26 patients who underwent DMEK with an integrated OCT microscope platform that did not include a heads-up display (Haag-Streit iOCT, Koeniz, Switzerland). Intraoperative OCT provided reliable visualization and identification of remnants of Descemet’s membrane during stripping as well as the correct localization of the endothelial side of the graft, both during preparation of graft and after injection into the anterior chamber of the patient’s eye were described.8 Furthermore, these authors noted the added utility of intraoperative OCT in cases in which the anterior chamber visualization was compromised by corneal pathology. The authors in this study were already experienced with DMEK surgery but concluded that intraoperative OCT may improve the learning curve for novice DMEK surgeons wishing to adopt the procedure.
In our study, the surgeon was a novice DMEK surgeon performing his first consecutive DMEK cases. Similar to the other report, intraoperative OCT allowed for rapid, almost immediate, identification of graft orientation upon activation of OCT imaging in this study. In contrast to the device, the integrated system in this study was also equipped with a “heads-up” OCT data feedback system. However, the external monitor was superior to the “heads-up” image for visualization of host and donor DM and consequently relied almost exclusively on the external display due to the size and quality of the image.
When possible in this study, the “S” stamp technique and intraoperative OCT to assess graft orientation were dually employed. An additional technique that has been described is a handheld slit lamp may also be employed to identify the correct orientation of the graft.11 Each of these techniques are viable methods to identify the scroll orientation. The principle advantage of OCT in a microscope-integrated system is that no disruption of flow is required and there are no concerns related to field contamination from the handheld slit lamp. Intraoperative OCT may hold some specific advantages of “S” stamping. For example in this study, the “S” was variably distinct following stamping. Also, methods for “S” stamping may invite additional opportunities for tissue damage both during the actual stamping process as well as the subsequent surgeon donor tissue preparation. As described in this study, the “button punch site” allowed unexpected donor tissue retraction following suction in two cases. Conversely, the relative costs of the methods should also be considered. Currently, a microscope-integrated intraoperative OCT systems would require a major financial investment for purchasing the combined system. That system would have applications beyond DMEK, but the relative value is still being elucidated.
As seen in this study, OCT may reveal posterior stromal irregularities that may be obscured by corneal pathology (especially peripherally in the setting of a dense arcus). The OCT may allow the surgeon to address these areas prior to graft insertion, or if necessary, decrease the size of the graft to avoid the peripheral irregularities. In one case, intraoperative OCT identified small detached remnants of DM, likely interposed between the donor tissue and host surface, again resulting in a potential areas for decreased adherence. These findings are consistent with previous publications, supporting the theory that interface abnormalities including disrupted stromal fibers or host DM fragments may increase the odds of postoperative detachment.12,13 The posterior stromal irregularities were the result of disturbing the pre-Descemet’s stroma which results in a thicker, more fibrous plane of tissue immediately anterior Descemet’s membrane. Disturbing this tissue may create an irregular surface, which impedes normal graft attachment.
Review of side-by-side microscope video and intraoperative OCT imaging may further enhance DMEK surgery techniques and understanding. Of note, the endothelial surface of the graft was noted to rarely make contact with the iris or intraocular lens surface during the tissue unscrolling process (Supplemental Video 1). Even with forceful chamber collapse, the graft was frequently separated from other structures by a measureable amount of fluid. It is possible that this tissue behavior explains the ability to successfully perform DMEK in eyes with anterior chamber lenses without incurring significant endothelial cell loss.14
Beyond the two partial graft dislocations, there were no serious intraoperative or postoperative complications. The incidence of re-bubbling was 25%, which is comparable to previously reported DMEK case series.12 More importantly, there were no incidences of primary graft failure and no postoperative graft inversions. Larger studies are necessary to determine if these early favorable results are generalizable to other novice surgeons adopting DMEK surgery with intraoperative OCT feedback.
The limitations of this study and the technology should be acknowledged. Our sample size was relatively small, non-randomized, unmasked and all cases were performed by a single surgeon. Also, there may be a case selection bias toward more straightforward cases based on the surgeon’s preference and relative inexperience with DMEK surgery. A larger, randomized and masked approach to intraoperative OCT utilization would provide more definitive results regarding the potential benefit in operative efficiency and patient outcomes for DMEK facilitated by intraoperative OCT. With current intraoperative OCT systems, automated tracking to areas of interest is not available. Additionally, software analysis of fluid dynamics is not available. There is significant ongoing work related to the development of automated algorithms for fluid interface quantification in lamellar keratoplasty that may facilitate intraoperative decision-making.15 Additionally, with the intraoperative OCT system used in this study, the current scan range limits how much of the anterior segment can be visualized simultaneously. However, this study demonstrates encouraging results for the novice DMEK surgeon and deserves further research for facilitating the transition for corneal surgeons to DMEK surgery. Increased availability of intraoperative OCT technology may ease the transition to DMEK surgery for novice surgeons wishing to adopt this procedure.
This report provides an intriguing examination of the use of real-time image-guided feedback for DMEK surgery with a microscope-integrated system with a heads-up display from the DISCOVER study. This case series suggests that the data obtained from intraoperative OCT may enhance surgeon understanding of the anatomic configurations of the tissue of interests, facilitating optimal surgical decision-making, and potentially improving surgical outcomes. This may improve the adoption of DMEK surgery through reducing some of the anxiety and learning curves around graft orientation and placement.
Supplementary Material
Donor graft unscrolling following placement in the anterior chamber with intraoperative optical coherence tomography visualization of graft behavior. Note the paucity of actual tissue contact to the surrounding iris and posterior stroma despite the shallow anterior chamber.
Time-lapse slow-motion video of gas infusion for graft apposition. Video intraoperative optical coherence tomography reveals progressive apposition of the graft on the host cornea.
Acknowledgments
Funding/Support: NIH/NEI K23-EY022947-01A1, Bethesda, MD (JPE); Ohio Department of Development TECH-13-059, Columbus, OH (JPE, SKS); Research to Prevent Blindness (Cole Eye Institutional), New York, NY
Other Acknowledgements: N/A
Footnotes
- BC: None
- JG: None
- SKS: Bausch and Lomb Rochester, NY (C, R); Bioptigen Durham, NC (P); Allergan, Irvine, CA (R); Synergetics, O’Fallen, MO (P); Leica, Buffalo Grove, IL (C), Carl Zeiss Meditec, Oberkochen, Germany(C)
- JPE: Bioptigen, Durham, NC (C, P); Thrombogenics, Iselin, NJ (C, R); Synergetics, O’Fallon, MO (P); Genentech, San Francisco, CA (R); Leica, Buffalo Grove, IL (C); Carl Zeiss Meditec, Zeiss, Oberkocken, Germany (C), Alcon, Fort Worth, TX (C)
Contributions to Authors: Design of the study (JPE, SKS, JG); Conduct of the study (All authors); Data collection (JPE, JG, BC); Data management (JPE, BC); Data analysis (All authors); Data Interpretation (All authors); Preparation of the manuscript (JPE, JG, BC); Review and approval of the manuscript (All authors)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melles GR, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty. Cornea. 2006;25(8):987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 2.Tourtas T, Laaser K, Bachmann B. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153(6):1082–1090. doi: 10.1016/j.ajo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014;158(5):999–1007. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98(10):1329–1332. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Srivastava SK, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLOS ONE. 2014;9(8) doi: 10.1371/journal.pone.0105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Benito-Llopis L, Mehta J, Angunawela R, Ang M, Tan D. Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty. Am J Ophthalmol. 2014;157(2):334–341. doi: 10.1016/j.ajo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Sharma A, Arora T, et al. Application of anterior segment optical coherence tomography in glaucoma. Surv Ophthalmol. 2014;59(3):311–327. doi: 10.1016/j.survophthal.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Steven P, Le Blanc C, Velten K, et al. Optimizing Descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmology. 2013;131(9):1135–1142. doi: 10.1001/jamaophthalmol.2013.4672. [DOI] [PubMed] [Google Scholar]
- 9.Ehlers JP, Tao YK, Farsui S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3152–3159. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart Z, Feng M, Price M, Price F. Handheld slit beam techniques to facilitate DMEK and DALK. Cornea. 2013;32(5):722–724. doi: 10.1097/ICO.0b013e31827797e7. [DOI] [PubMed] [Google Scholar]
- 12.Tourtas T, Schlomberg J, Wessel JM, et al. Graft adhesion in Descemet membrane endothelial keratoplasty dependent on size of removal of host’s Descemet membrane. JAMA Ophthalmology. 2014;132(2):155–161. doi: 10.1001/jamaophthalmol.2013.6222. [DOI] [PubMed] [Google Scholar]
- 13.Anshu A, Price M, Price F. Risk of corneal transplant rejection significantly reduced with descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119(3):536–540. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Liarkos VS, Ham L, Dapena I, et al. Endothelial Keratoplasty for bullous keratopathy in eyes with an anterior chamber intraocular lens. J Cataract Refract Surg. 2013;39(12):1835–1845. doi: 10.1016/j.jcrs.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Ehlers JP, Itoh Y, Xu L, et al. Factors associated with persistent subfoveal fluid and complete macular hole closure in the PIONEER study. Invest Ophthalmol Vis Sci. 2015;56(2):1141–1146. doi: 10.1167/iovs.14-15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Donor graft unscrolling following placement in the anterior chamber with intraoperative optical coherence tomography visualization of graft behavior. Note the paucity of actual tissue contact to the surrounding iris and posterior stroma despite the shallow anterior chamber.
Time-lapse slow-motion video of gas infusion for graft apposition. Video intraoperative optical coherence tomography reveals progressive apposition of the graft on the host cornea.