Abstract
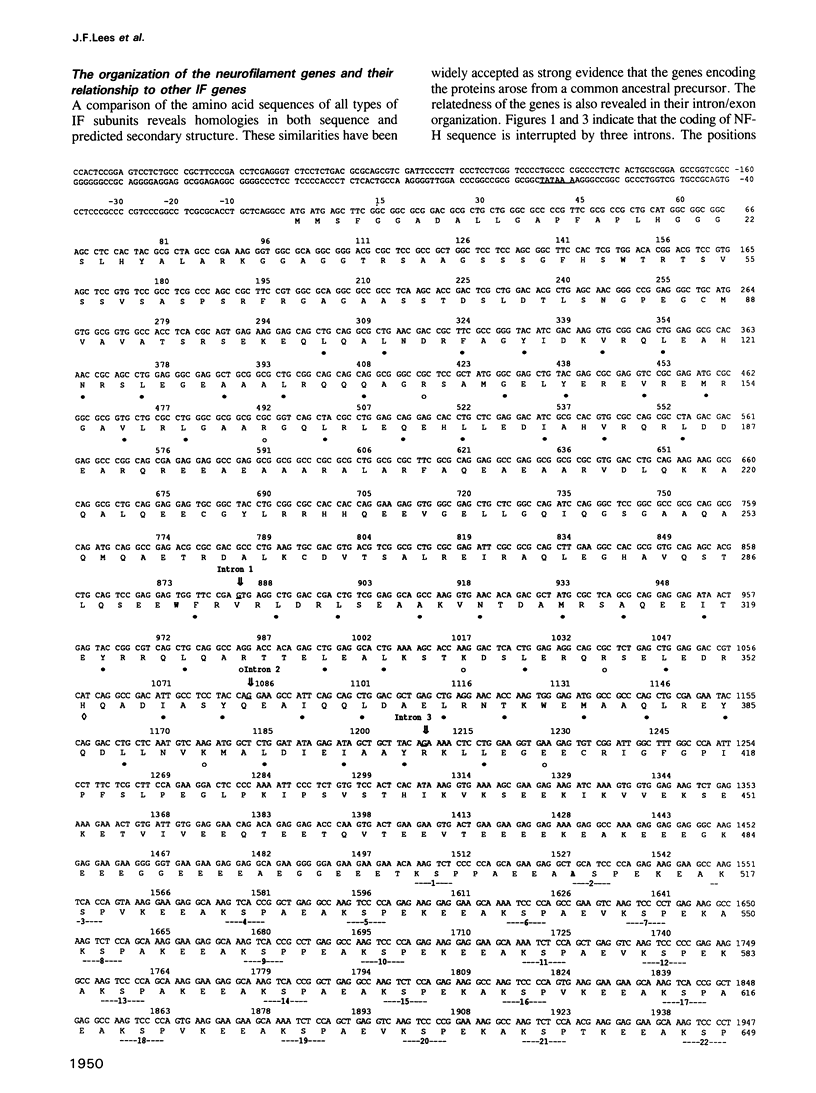
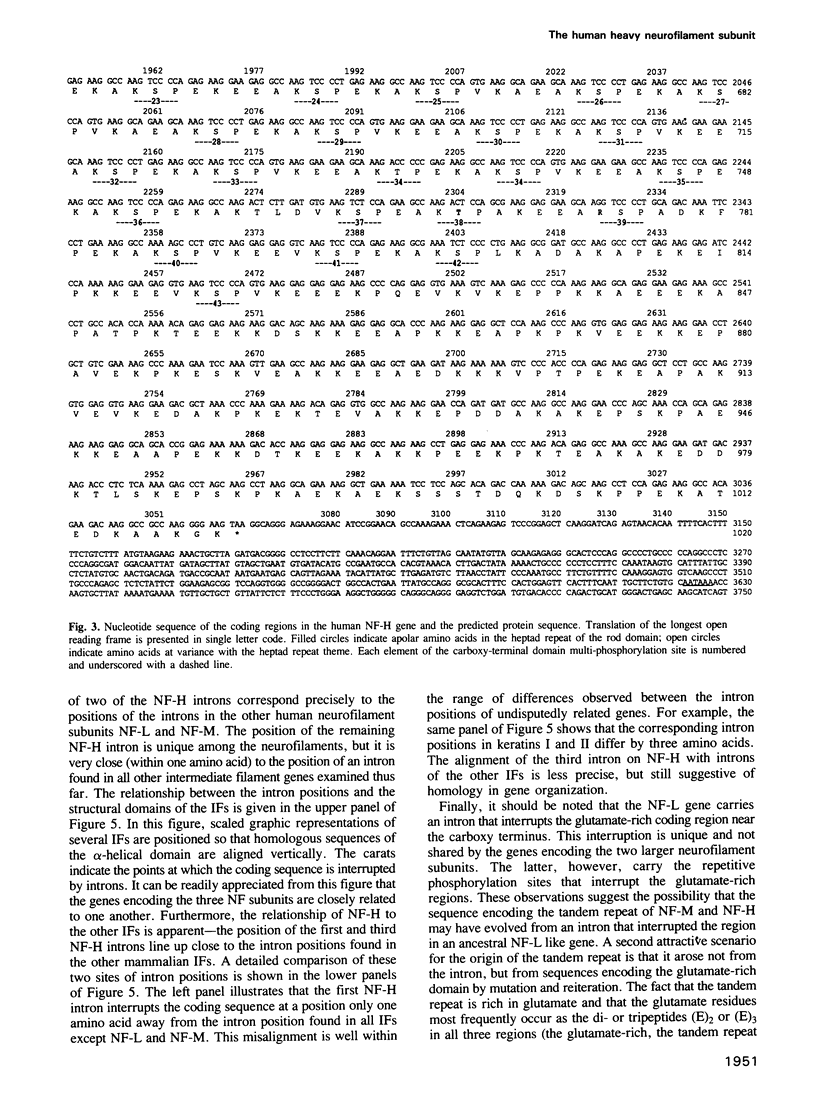
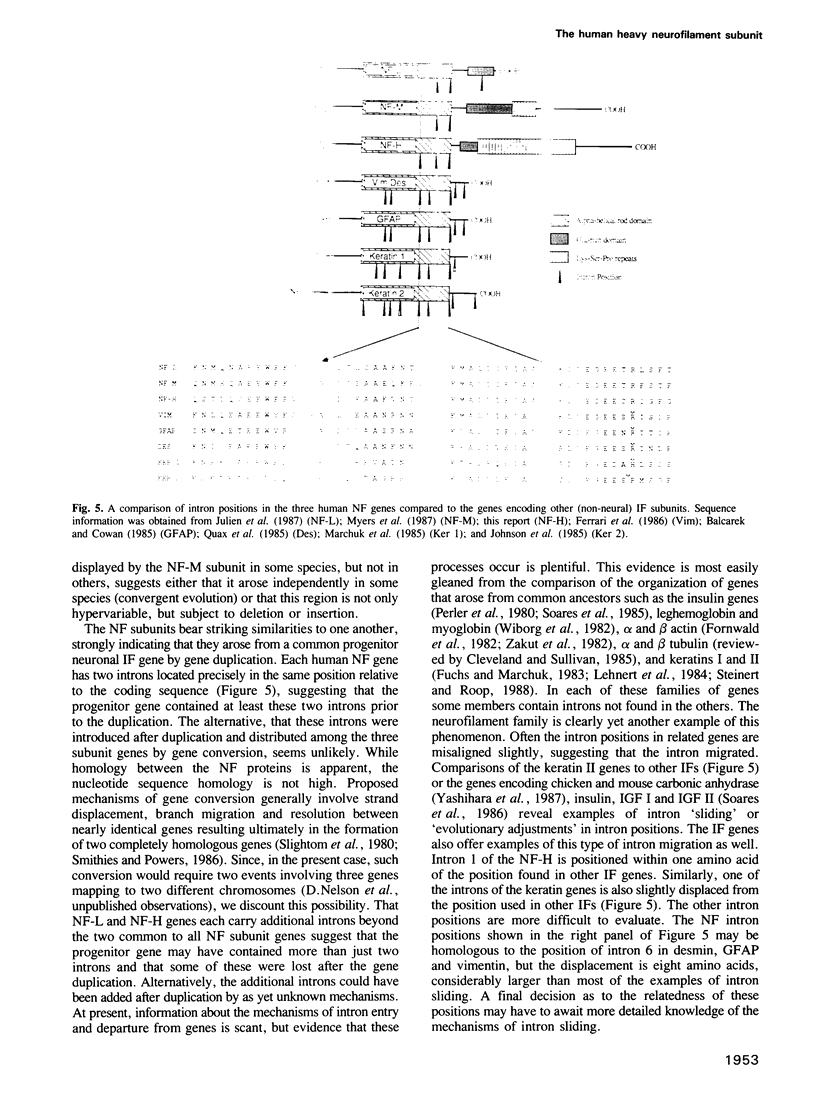
Genomic clones for the largest human neurofilament protein (NF-H) were isolated, the intron/exon boundaries mapped and the entire protein-coding regions (exons) sequenced. The predicted protein contains a central region that obeys the structural criteria identified for alpha-helical 'rod' domains typically present in all IF protein components: it is approximately 310 amino acids long, shares amino acid sequence homology with other IF protein rod domains and displays the characteristic heptad repeats of apolar amino acids which facilitate coiled-coil interaction. Nevertheless, anomalies are noted in the structure of the NF-H rod which could explain observations of its poor homopolymeric assembly in vitro. The protein segment on the carboxy-terminal side of the human NF-H rod is uniquely long (greater than 600 amino acids) compared to other IF proteins and is highly charged (greater than 24% Glu, greater than 25% Lys), rich in proline (greater than 12%) and impoverished in cysteine, methionine and aromatic amino acids. Its most remarkable feature is a repetitive sequence that covers more than half its length and includes the sequence motif, Lys-Ser-Pro (KSP) greater than 40 times. Together with the recent identification of the serine in KSP as the main target for NF-directed protein kinases in vivo, this repetitive character explains the massive phosphorylation of the NF-H subunit that can occur in axons. The human NF-H gene has three introns, two of which interrupt the protein-coding sequence at identical points to introns in the genes for the two smaller NF proteins, NF-M and NF-L.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcarek J. M., Cowan N. J. Structure of the mouse glial fibrillary acidic protein gene: implications for the evolution of the intermediate filament multigene family. Nucleic Acids Res. 1985 Aug 12;13(15):5527–5543. doi: 10.1093/nar/13.15.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. S., DiLullo C. Slow posttranslational modification of a neurofilament protein. J Cell Biol. 1985 May;100(5):1799–1804. doi: 10.1083/jcb.100.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Eagles P. A. Neurofilaments from ox spinal nerves. Isolation, disassembly, reassembly and cross-linking properties. Biochem J. 1983 Nov 1;215(2):227–237. doi: 10.1042/bj2150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Carden M. J., Trojanowski J. Q., Schlaepfer W. W., Lee V. M. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987 Nov;7(11):3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin T. K., Eagles P. A., Maggs A. The proteolytic digestion of ox neurofilaments with trypsin and alpha-chymotrypsin. Biochem J. 1983 Nov 1;215(2):239–252. doi: 10.1042/bj2150239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Cohlberg J. A., Hajarian H., Sainte-Marie S. Discrete soluble forms of middle and high molecular weight neurofilament proteins in dilute aqueous buffers. J Biol Chem. 1987 Dec 15;262(35):17009–17015. [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz J. K., Franke W. W. Cloning of cDNA and amino acid sequence of a cytokeratin expressed in oocytes of Xenopus laevis. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6475–6479. doi: 10.1073/pnas.83.17.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M. Construction and characterization of a genomic library in lambda. Methods Enzymol. 1987;152:190–199. doi: 10.1016/0076-6879(87)52020-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Marchuk D. Type I and type II keratins have evolved from lower eukaryotes to form the epidermal intermediate filaments in mammalian skin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5857–5861. doi: 10.1073/pnas.80.19.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Fischer S., Vandekerckhove J., Damme J. V., Plessmann U., Weber K. Protein-chemical characterization of NF-H, the largest mammalian neurofilament component; intermediate filament-type sequences followed by a unique carboxy-terminal extension. EMBO J. 1985 Jan;4(1):57–63. doi: 10.1002/j.1460-2075.1985.tb02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Fischer S., Plessmann U., Weber K. Neurofilament architecture combines structural principles of intermediate filaments with carboxy-terminal extensions increasing in size between triplet proteins. EMBO J. 1983;2(8):1295–1302. doi: 10.1002/j.1460-2075.1983.tb01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. The complete amino acid sequence of the major mammalian neurofilament protein (NF-L). FEBS Lett. 1985 Mar 25;182(2):475–478. doi: 10.1016/0014-5793(85)80357-4. [DOI] [PubMed] [Google Scholar]
- Geisler N., Vandekerckhove J., Weber K. Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett. 1987 Sep 14;221(2):403–407. doi: 10.1016/0014-5793(87)80964-x. [DOI] [PubMed] [Google Scholar]
- Georges E., Mushynski W. E. Chemical modification of charged amino acid moieties alters the electrophoretic mobilities of neurofilament subunits on SDS/polyacrylamide gels. Eur J Biochem. 1987 Jun 1;165(2):281–287. doi: 10.1111/j.1432-1033.1987.tb11439.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Glicksman M. A., Willard M. B. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984 Apr;98(4):1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J. P., Grosveld F., Yazdanbaksh K., Flavell D., Meijer D., Mushynski W. The structure of a human neurofilament gene (NF-L): a unique exon-intron organization in the intermediate filament gene family. Biochim Biophys Acta. 1987 Jun 6;909(1):10–20. doi: 10.1016/0167-4781(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Meyer D., Flavell D., Hurst J., Grosveld F. Cloning and developmental expression of the murine neurofilament gene family. Brain Res. 1986 Dec;387(3):243–250. doi: 10.1016/0169-328x(86)90030-6. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983 Mar 25;258(6):4019–4025. [PubMed] [Google Scholar]
- Kaufmann E., Geisler N., Weber K. SDS-PAGE strongly overestimates the molecular masses of the neurofilament proteins. FEBS Lett. 1984 May 7;170(1):81–84. doi: 10.1016/0014-5793(84)81373-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek R. J., Phillips L., Katz M. J., Autilio-Gambetti L. Function and evolution of neurofilament proteins. Ann N Y Acad Sci. 1985;455:462–478. doi: 10.1111/j.1749-6632.1985.tb50429.x. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert M. E., Jorcano J. L., Zentgraf H., Blessing M., Franz J. K., Franke W. W. Characterization of bovine keratin genes: similarities of exon patterns in genes coding for different keratins. EMBO J. 1984 Dec 20;3(13):3279–3287. doi: 10.1002/j.1460-2075.1984.tb02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Eyer J. Properties of highly viscous gels formed by neurofilaments in vitro. A possible consequence of a specific inter-filament cross-bridging. Biochem J. 1987 Jul 1;245(1):93–101. doi: 10.1042/bj2450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E., Liem R. K., D'Eustachio P., Cowan N. J. Structure and evolutionary origin of the gene encoding mouse NF-M, the middle-molecular-mass neurofilament protein. Eur J Biochem. 1987 Jul 1;166(1):71–77. doi: 10.1111/j.1432-1033.1987.tb13485.x. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Anomalous placement of introns in a member of the intermediate filament multigene family: an evolutionary conundrum. Mol Cell Biol. 1986 May;6(5):1529–1534. doi: 10.1128/mcb.6.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Genetics, evolution, and expression of the 68,000-mol-wt neurofilament protein: isolation of a cloned cDNA probe. J Cell Biol. 1985 Mar;100(3):843–850. doi: 10.1083/jcb.100.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., McCrohon S., Fuchs E. Complete sequence of a gene encoding a human type I keratin: sequences homologous to enhancer elements in the regulatory region of the gene. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1609–1613. doi: 10.1073/pnas.82.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatani S., Winkles J. A., Sargent T. D., Dawid I. B. Stage-specific keratins in Xenopus laevis embryos and tadpoles: the XK81 gene family. J Cell Biol. 1986 Nov;103(5):1957–1965. doi: 10.1083/jcb.103.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano E. W., Chin S. S., Colman D. R., Liem R. K. Complete amino acid sequence and in vitro expression of rat NF-M, the middle molecular weight neurofilament protein. J Neurosci. 1987 Aug;7(8):2590–2599. [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Quax W., van den Broek L., Egberts W. V., Ramaekers F., Bloemendal H. Characterization of the hamster desmin gene: expression and formation of desmin filaments in nonmuscle cells after gene transfer. Cell. 1985 Nov;43(1):327–338. doi: 10.1016/0092-8674(85)90038-8. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Robinson P. A., Wion D., Anderton B. H. Isolation of a cDNA for the rat heavy neurofilament polypeptide (NF-H). FEBS Lett. 1986 Dec 15;209(2):203–205. doi: 10.1016/0014-5793(86)81111-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Smithies O., Powers P. A. Gene conversions and their relation to homologous chromosome pairing. Philos Trans R Soc Lond B Biol Sci. 1986 Jan 29;312(1154):291–302. doi: 10.1098/rstb.1986.0008. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Schon E., Henderson A., Karathanasis S. K., Cate R., Zeitlin S., Chirgwin J., Efstratiadis A. RNA-mediated gene duplication: the rat preproinsulin I gene is a functional retroposon. Mol Cell Biol. 1985 Aug;5(8):2090–2103. doi: 10.1128/mcb.5.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M. B., Turken A., Ishii D., Mills L., Episkopou V., Cotter S., Zeitlin S., Efstratiadis A. Rat insulin-like growth factor II gene. A single gene with two promoters expressing a multitranscript family. J Mol Biol. 1986 Dec 20;192(4):737–752. doi: 10.1016/0022-2836(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. doi: 10.1146/annurev.cb.01.110185.000353. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara C. M., Lee J. D., Dodgson J. B. The chicken carbonic anhydrase II gene: evidence for a recent shift in intron position. Nucleic Acids Res. 1987 Jan 26;15(2):753–770. doi: 10.1093/nar/15.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut R., Shani M., Givol D., Neuman S., Yaffe D., Nudel U. Nucleotide sequence of the rat skeletal muscle actin gene. Nature. 1982 Aug 26;298(5877):857–859. doi: 10.1038/298857a0. [DOI] [PubMed] [Google Scholar]
- Zopf D., Hermans-Borgmeyer I., Gundelfinger E. D., Betz H. Identification of gene products expressed in the developing chick visual system: characterization of a middle-molecular-weight neurofilament cDNA. Genes Dev. 1987 Sep;1(7):699–708. doi: 10.1101/gad.1.7.699. [DOI] [PubMed] [Google Scholar]