Abstract
BACKGROUND
Injury to saphenous vein grafts during surgical preparation may contribute to the subsequent development of intimal hyperplasia, the primary cause of graft failure. Surgical skin markers currently used for vascular marking contain gentian violet and isopropanol that damage tissue and impair physiologic functions. Brilliant blue FCF (FCF) is a nontoxic dye alternative that may also ameliorate preparation-induced injury.
METHODS
Porcine saphenous vein (PSV) was used to evaluate the effect of FCF on physiologic responses in a muscle bath. Cytotoxicity of FCF was measured using human umbilical venous smooth muscle cells (HUVSMC). Effect of FCF on the development of intimal hyperplasia was evaluated in organ culture using PSV. Intracellular calcium fluxes and contractile responses were measured in response to agonist and inhibitors in rat aorta and human saphenous vein (HSV).
RESULTS
Marking with FCF did not impair smooth muscle contractile responses and restored stretch injury-induced loss in smooth muscle contractility of PSV. Gentian violet has cytotoxic effects on HUVSMC while FCF is nontoxic. FCF inhibited intimal thickening in PSV in organ culture. 2′(3′)-O-(4-Benzoylbenzoyl)adenosine-5′-triphosphate-induced contraction and intracellular calcium flux were inhibited by FCF, oxidized ATP, KN62, and brilliant blue G, suggesting that FCF may inhibit the purinergic receptor P2X7.
CONCLUSIONS
Our studies indicated that FCF is a non-toxic marking dye for vein grafts that ameliorates vein graft injury and prevents intimal thickening, possibly due to P2X7 receptor inhibition. FCF represents a non-toxic alternative for vein graft marking and a potentially therapeutic approach to enhance outcome in autologous transplantation of HSV into the coronary and peripheral arterial circulation.
Keywords: Vein graft failure, intimal hyperplasia, FCF, P2X7
INTRODUCTION
Approximately 1,000,000 aortocoronary and peripheral vascular bypass procedures are performed annually using human saphenous vein (HSV). However, outcomes from these procedures remain limited by high rates of vein graft failure, with a per patient rate of 39% and 45% at one year in the PREVENT III 1 and the PREVENT IV trials of infrainguinal and aortocoronary bypass,2 respectively. The leading cause of vein graft failure is intimal hyperplasia,3 a process characterized by pathologic narrowing of the lumen, graft stenosis, and ultimately graft failure,4 leading to substantial morbidity, reintervention, limb loss, myocardial infarction, and death. Despite significant efforts to prevent intimal hyperplasia, no therapeutics, techniques, or devices, have been demonstrated to prevent this process in humans.
HSV undergoes a series of surgical manipulations during the time of explantation to be prepared for implantation into the arterial circulation. Common intraoperative vein graft preparation includes warm ischemia times in non-buffered, non-physiologic storage solutions (e.g. normal saline)5; marking with a surgical skin marker for orientation; and mechanical injury, such as stretching and traction during harvest and pressure distention. These current means of vein graft preparation techniques are injurious to the conduits, resulting in cellular dysfunction, increased oxidative stress, and promotes the development of intimal hyperplasia of HSV.6–9 Collectively, less injurious means of preparing HSV prior to autologous transplantation may improve outcomes of the procedures.
The off-label use of surgical skin markers to prevent twisting and kinking on implantation critically impairs smooth muscle and endothelial function in HSV.8 These markers generally contain 10% gentian violet with 50% isopropanol as the solvent. These dyes have been shown to reduce vascular function;10,11 however they continue to be used for marking conduits. We previously demonstrated that the food dye Brilliant Blue FCF (FCF) enhances endothelial-dependent relaxation in HSV and restores contractility in HSV that were otherwise considered functionally non-viable possibly by inhibiting the purinergic receptor P2X7 (P2X7R).12 FCF, a highly water soluble dye, is structurally related to Brilliant Blue G (BBG) which has been shown to ameliorate stretch induced injury in the spinal cord via P2X7R antagonism.13 In this current investigation, we hypothesized that FCF can be utilized as a nontoxic alternative for vein graft marking and restores stretch-induced loss of physiological function in a porcine model. We compared the biocompatibility of FCF and other marking dyes on physiologic function as well as the development of intimal hyperplasia in an organ culture model. The findings of these studies suggest that FCF is not only nontoxic but may also be a beneficial component of the vein graft preparation that mitigates detrimental effects of stretch-induced injury during bypass procedures.
METHODS
Materials
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless specified otherwise.
Human saphenous vein (HSV) procurement
HSV were obtained after approval from the Institutional Review Boards of Vanderbilt University Medical Center and the Tennessee Valley Veterans Affairs Medical Center from patients undergoing coronary artery bypass grafting procedures. Method of vein harvest (open or endoscopic) and graft preparation (including hydrostatic distention, marking with a surgical skin marker, and placement in storage solution) was at the discretion of the surgical team. Surgical remnant segments were transported to laboratory in heparinized PlasmaLyte (HP; 10 unit heparin/mL PlasmaLyte) for experimentation within 30 minutes of collection. Areas showing visible signs of injury were not used in experimentation.
Animal procedures
Animal procedures followed study protocols approved by the Vanderbilt Institutional Animal Care and Use Committee and adhered to National Institute of Health guidelines for care and use of laboratory animals.
Porcine saphenous vein (PSV) procurement
Immediately after euthanasia, greater saphenous veins (n=6) were procured from adult Yorkshire pigs (Oak Hill Genetics, Ewing, IL) using an open harvest method and transported in HP to laboratory for immediate experimentation.
Rat aorta procurement
Immediately after euthanasia, aortae were gently dissected from adult female Sprague-Dawley rats (n=8), collected in HP at room temperature and used immediately for experimentation.
Mechanical stretch injury of PSV
After harvest, unmanipulated PSV segments were reserved as control vessels. Additional segments were stretched to 200% the resting length as previously described.7 This amount of stretch is equivalent to the extent of passive stretch that PSV segments would tolerate. Stretched tissues were then subjected to different treatments prior to measurement of physiologic responses.
Measurement of physiologic responses
To determine effect of surgical skin marker or dyes on contractile responses, untreated or stretched PSV segments were either left untreated, painted on the extravascular surface with a surgical skin marker (Aspen surgical Products, Caledonia, MI) or a cotton swab saturated with a solution of FCF (2.6 mM), or incubated in HP containing methylene blue (MB; 1%), isopropanol (IPA; 50%), brilliant blue G (BBG, a structural analog of FCF; 50 μM) or allura red (Red, another food coloring dye; 50 μM) for 15min. Endothelium was denuded in order to assess smooth muscle function prior to suspension in the muscle bath.
Rings from PSV segments were suspended in a muscle bath containing a bicarbonate buffer (120 mM sodium chloride, 4.7 mM potassium chloride, 1.0 mM magnesium sulfate, 1.0 mM monosodium phosphate, 10 mM glucose, 1.5 mM calcium chloride, and 25 mM sodium bicarbonate, pH 7.4) equilibrated with 95% O2/5% CO2 at 37°C for 1 hr at a resting tension of 1g, manually stretched to 3–4 times the resting tension, and maintained at resting tension for an additional 1 hr. This produced the maximal force tension relationship as previously described.12 Force measurements were obtained using the Radnoti force transducer (model 159901A) interfaced with a PowerLab data acquisition system and Chart software (AD Instruments). After equilibration, the rings were contracted with 110 mM potassium chloride (with equimolar replacement of sodium chloride in bicarbonate buffer) to determine smooth muscle functional viability. Contractile functions were compared to either the unmarked, the non-stretched or stretched segments from the same vessel of the same animal.
Measurement of dye cytotoxicity
Primary human umbilical venous smooth muscle cells (HUVSMC; ScienCell, CA) were cultured per manufacturer’s instruction and treated with reagents or dye at indicated concentrations diluted in medium. Stock solution of gentian violet (GV) was prepared as 10% with 50% IPA as solvents. For phase contrast imaging, cells seeded in 6-well plates were treated for 10 min. Cells were then washed to remove dyes and images were taken before and after trypan blue (0.4%) staining. For cytotoxicity assay, cells seeded in 96-well plates were treated 2–12hr. Cytotoxicity of the dyes was evaluated by measuring the release of dead-cell protease using the CytoTox-Glo assay kit (Promega, CA). The percentage of dead cells was determined. Each data point represents the average of triplicate wells of each treatment for each independent experiment. Cytotoxicity was determined by comparing to the untreated cells.
Organ culture
Rings (1–2 mm) were cut from PSV segments. Two rings were fixed in 10% neutral buffered formalin to measure basal (pre-culture) intimal thickness. Additional rings were either cultured without any dye (control) or in the presence of FCF (50 μM), BBG (50μM) or Red (50μM) in RPMI 1640 medium supplemented with 30% FBS, 1% L-glutamine and 1% penicillin/streptomycin for 14 days at 37°C in an atmosphere of 5% CO2 in air. Tissues were fixed, paraffin-embedded, sectioned and stained using Verhoeff-Van Gieson (VVG) to allow the visualization of the internal elastic lamina. Measurements of intimal thickness were made on transverse sections of each vessel using a Zeiss Axiovert 200M microscope (Carl Zeiss) with a computerized image analysis system (Zeiss software and Adobe Photoshop) as described previously.9 Intimal thickness of treated rings were compared to the control, cultured rings of the same vessel from the same animal.
Measurement of purinergic receptor-induced contraction and cytosolic calcium ion flux
Rat aorta was dissected free of fat and connective tissue, sectioned into 1-mm rings, and suspended in a FluoroPlex Tissue Bath Fluorometry System (IonOptix LLC, Milton, MA and Radnoti Glass Technology Inc., Monrovia, CA), which enables fluorescence ion recording in parallel with force measurement. Rings were loaded with 10 μM Fura-2 AM ester (Invitrogen, Carlsbad, CA) and 0.01% Pluronic F-127 (Invitrogen) in the bicarbonate buffer as described previously,14 and then either left untreated or treated with FCF (50 μM), P2X7R antagonists periodate oxidized sodium salt (oATP; 50 μM), KN-62 (10 μM), or BBG (50 μM) for 30 min prior to contraction with the P2X7R agonist 2′ (3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate (BzATP; 100 μM). Force and calcium fluorescence were measured continuously for 15 min after the addition of BzATP. Changes in intracellular calcium concentrations ([Ca2+]i) was determined as previously described.14 Contractile responses and [Ca2+]i were compared to the untreated ring from the same vessel of the same animal.
Rings from HSV segments were suspended and equilibrated in the muscle bath and contracted with 110 mM KCl as described above to determine smooth muscle viability. Tissues produced <0.025 ×105 N/m2 were considered non-viable and were not used in further studies.7 Viable rings were then either left untreated or treated with 50 μM FCF for 30 min prior to contraction with 100 μM BzATP. BzATP-induced contraction was expressed as the percentage of maximum KCl-induced contraction. Contractile responses were compared to the untreated rings from the same vessel of the same patient.
Immunohistochemistry of HSV
Antigen retrieval of formalin fixed tissue sections were performed using citrate buffer (pH 6) at 95°C for 12 min. After pre-incubation with 5% goat serum to block non-specific sites, sections were incubated with primary antibodies against P2X7R (Alomone, Israel) overnight at 4°C. The sections were then incubated with Alexa 568-tagged anti-rabbit antibodies (Invitrogen, CA) for 1hr. Controls were performed by pre-absorbing the primary antibody with the immunogen peptide. Immunostaining was examined under a fluorescence microscope.
Statistical analysis
Contractile responses were defined by stress, calculated using force generated by tissues as follows: Stress (×105 N/m2) = Force (g) × 0.0987/area, where area = wet weight (mg)/at maximal length (mm)]/1.055. Sample size (n) was determined with α = 0.05 and power = 0.9 using PS program version 3.0.43 15 (http://biostat.mc.vanderbilt.edu/PowerSampleSize) based on prior studies7,8,12,16. Data were reported as mean responses ± standard deviation of the mean. The statistical significance (P value) and achieved power of each experiment was determined using GraphPad Prizm version 5.0 and G*Power version 3.1.9.2 (www.gpower.hhu.de/en.html), respectively. Paired t-tests were used for experiments with dependent (matched-pairs) samples, i.e. different techniques used on samples of the same tissue from the same patient/animal. One-way ANOVA test followed by Tukey’s Multiple Comparison post-tests was used for experiments with independent groups.. A P value <0.05 and power ≥0.9 was considered statistically significant.
RESULTS
FCF did not impair functional responses in PSV
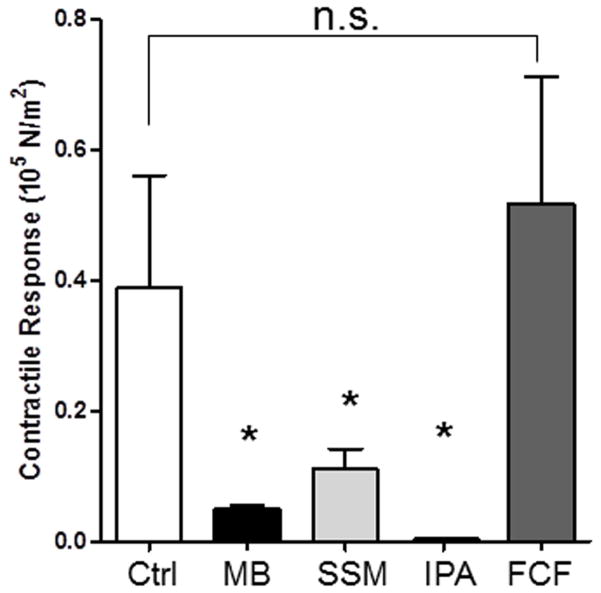
Smooth muscle functional viability (contraction in response to a depolarizing KCl stimulus) was significantly reduced in PSV marked with methylene blue (0.051±0.005 ×105N/m2 vs. 0.220±0.066 ×105N/m2 in control; P=.04, n=3; Figure 1) and surgical skin marker (0.111±0.031×105N/m2 vs.0.255±0.095 ×105N/m2 in control; P=0.03, n=5; Figure 1) or treated with 50% isopropanol (0.0002±0.00002 ×105N/m2 vs. 255±0.095 ×105N/m2 in control; P=0.03, n=3]. Topically applied FCF had no effect on smooth muscle physiologic responses in PSV (0.564±0.19 ×105N/m2 vs. 453±0.167 ×105N/m2 in control); P=0.12, n=10; Figure 1) suggesting that FCF is non-toxic to the tissue.
Figure 1. FCF does not impair contractility in porcine saphenous veins.
Segments of porcine saphenous veins (n=3–15) were either left untreated (Ctrl), marked with a surgical skin marker (SSM) or a solution of FCF (2.6 mM, in 5% propylene glycol and water) with a cotton swab in a longitudinal line and incubated in at room temperature in PlasmaLyte for 15min, or treated with 50% isopropanol (IPA) or 1% methlyene blue (MB) for 15 min treated Segments were then incubated, cut into rings, suspended in a muscle bath and treated with KCl (110 mM). Force generated was converted to stress. The error bars show the standard deviation. *P< 0.05 in paired t-tests.
Cytotoxicity of marking dyes and FCF
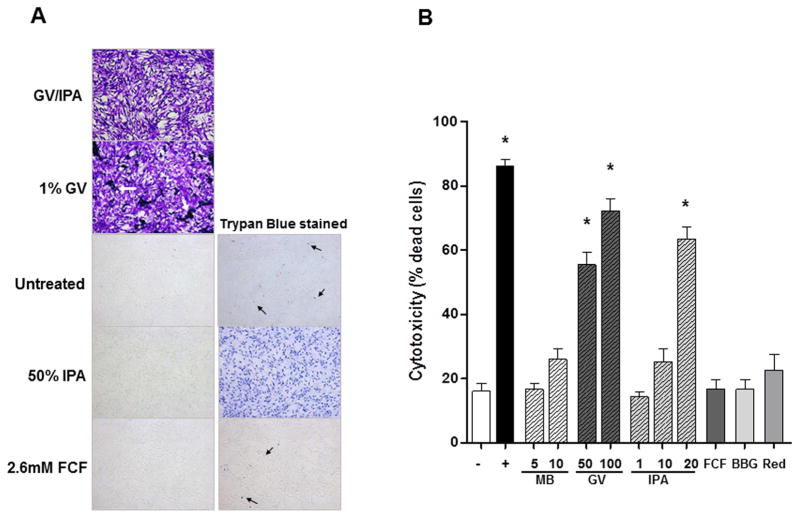
Upon exposure to 1% gentian violet (25 mM, a concentration that is 10 times lower than surgical skin markers) and 50% isopropanol for 5 min, either alone or combined, HUVSMC essentially underwent cytological fixation and preservation, and adhered to the bottom of the wells (Figure 2A). Trypan blue dye exclusion test on IPA-treated cells suggested cells are rendered non-viable by the chemical constituents of surgical skin markers (Figure 2A). Likewise, application of 1% (or 30mM) menthylene blue immediately lysed cells. The intense blue staining precluded the ability to perform trypan blue dye extclusion test or cytoxicity assay (data not shown). Solubility of GV is poor in non-organic solvent as evident by the precipitation of dye in wells where cells where treated with 1% GV (with effective concentration of IPA at 5%; Figure 2A). Exposure to 2.6mM FCF (concentration used for extravascular marking of PSV) for 5 min did not result cell death compare to untreated cells (Figure 2A). Cytotoxic effect of a considerably low concentration of GV (0.002% or 50μM), FCF (50μM), BBG (50μM) or allura red (50μM) was not observed after short-term (2 hr) treatment (data not shown). After 12hr-treamtent (n=7), 10mM MB (a concentration that is 3 times lower than clinical use) did not result in significant cell death (26.0±8.5% vs 16.1±6.3%; P>005). Both gentian violet (55.4±10.1% for 50uM and 72.1±10.6% for 100μM) and 20% IPA (63.5±9.1%) were cytotoxic to HUVSMC Figure 2B; P<0.05 vs untreated cells). The cytotoxicity of GV was not due to residual IPA (0.01%) in the diluted dye as 1% IPA was not toxic to the cells (14.3±4.3% vs 16.1±6.3% in untreated cells, P>0.05). Prolonged treatment with 50μM FCF (16.77±8.0%), BBG (22.6±13.1%) or allura red (16.62±8.2%) remained non-toxic to the cells (Figure 2B; P>.05 vs untreated cells).
Figure 2. Cytotoxicity of vein graft marking dyes on human umbilical venous smooth muscle cells (HUVSMC).
(A) Phase contract images of HUVSMC either left untreated or treated with gentian violet (GV; 1%) dissolved in 50% isopropanol (GV/IPA), 50% IPA, 1% GV, or 2.6mM FCF for 10 min (left panels) and then with stained with trypan blue (right panels). Minimal dead cells were detected in untreated and FCF-treated cells (black arrows) whereas all cells took up trypan blue after brief exposure to 50% IPA. GV alone also chemically fixed the cells and could not be washed off. Because the cells were already stained, no trypan blue staining was performed on these cells. White arrows indicated precipitation of GV due to low organic solvent content. (B) HUVSMC seeded in 96-well plates were left untreated (−) or treated for 12 hrs with staurosporine (+; 800nM), MB (5 and 10mM), GV (50 and 100μM); IPA (1–20%); FCF (50μM); BBG (50μM); and allura red (Red; 50μM). Cytotoxicity of the treatments were evaluated by measuring release of protease in the medium using a dead-cell protease assay and expressed as % dead cell of each well. Each data point represents an average of triplicate wells for each treatment in each independent experiment. Data represents mean±SD; n=5–7; *P<0.05 in Tukey’s Multiple Comparison tests.
FCF restored functional responses in PSV impaired by mechanical stretch injury
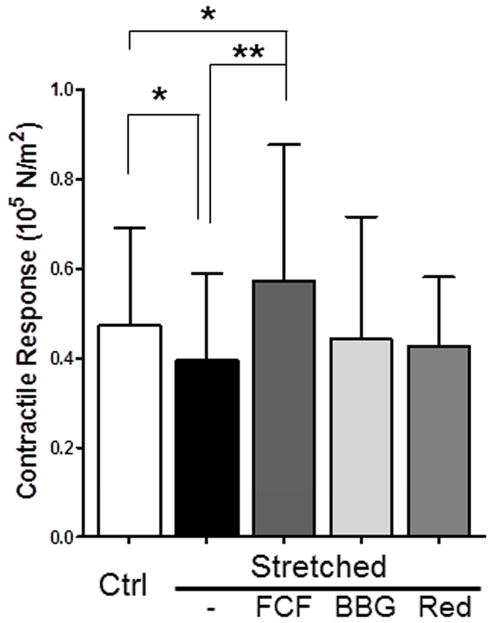
We previously showed that longitudinal stretch impairs functional viability in PSV.13 We used this model of stretch injury to determine the effect of FCF on restoring smooth muscle contractile responses. Compared with control segments, PSV subjected to stretch injury generated significantly less contractile force in response to110mM KCl (0.473±0.22×105N/m2 vs 0.396±0.19 ×105N/m2 in control, P =.02, n=10; Figure 3). Treatment with a topical application of FCF restored the contractile response of stretch-injured PSV (0.573±0.101×105N/m2 vs 0.389±0.2×105N/m2 in stretched, P =.005, n=9; Figure 3). Treatment with BBG (0.442±0.101×105N/m2 vs 0.351±0.21×105N/m2 in stretched; P=.09, n=7) or allura red (0.427±0.069×105N/m2 vs 0.417±0.19×105N/m2 in stretched; P=0.8, n=5) did not restore smooth muscle contractile response (Figure 3).
Figure 3. FCF restores functional viability after stretch injury in porcine saphenous vein.
Pig saphenous veins were either untreated (ctrl; n=5–10) or stretched to twice their resting length (stretched; n=10). Stretched segments were then either left untreated, marked with a solution of FCF (2.6 mM, in 5% propylene glycol and water) using a cotton swab in a longitudinal line, or treated in 50μM brilliant blue G (BBG) or 50μM Allura red (Red). The segments were then incubated at room temperature for 15 min in PlasmaLyte and cut into rings, suspended in a muscle bath and treated with KCl (110 mM). Force generated was converted to stress. Results are presented as mean±SD. *P< .05, **P<.005 in paired t-tests.
FCF reduced intimal thickening in cultured PSV
Since injury leads to the development of intimal hyperplasia, the effect of FCF on the development of intimal hyperplasia was determined in an organ culture model. PSV were either left untreated (control) or treated in the continuous presence of dyes in organ culture. After 14 days in organ culture, intima thickness increased 8.4±6.8% from 31.71± 8.2μm to 39.6±8.9 μm (P<.0001; n=22) in untreated (control) rings. Intimal thickening was significantly reduced in segments of FCF-treated PSV compared to untreated tissues (32.4±6.5 μm vs 39.8±9.4 μm P <.0001, n=21; Figure 4) with a 1.2±4.9% increase over the pre-cultured rings, suggesting that treatment with FCF have an inhibitory effect on neointimal thickening ex vivo. The presence of BBG (44.7±10.6 μm vs. 39.4±9.2 μm; P=0.03; n=19) or allura red (44.8±9.2 μm vs. 40.4±9.5 μm; P=0.02; n=18) during organ culture led to increased intimal thickness when compared to the untreated PSV (Figure 4), implicating that these dyes did not prevent intimal hyperplasia and accelerated the processes that contributed to intimal thickening.
Figure 4. FCF inhibits intimal thickening in porcine saphenous veins in organ culture.
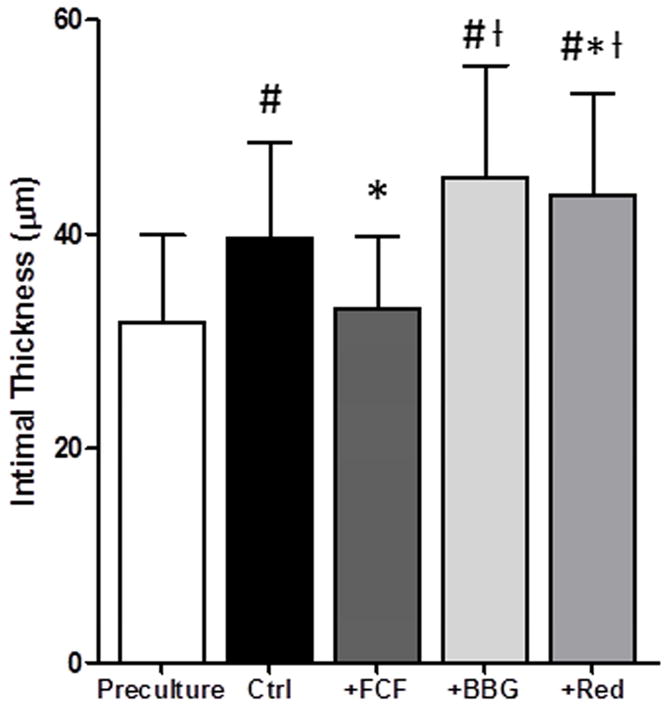
Rings from pig saphenous veins were either left untreated (ctrl) or treated in the presence of FCF (50μM); BBG (50μM); and allura red (Red; 50μM) in organ culture for 14 days. Veins were stained using Verhoff Van Gieson stain and intimal layer thickening was measured. Results are presented as mean±SD. #P<0.05 vs pre-culture; *P<.05 vs control; ƚP<.05 vs FCF in paired t-tests.
FCF inhibited P2X7 purinergic receptor-induced contraction and cytosolic Ca2+ fluxes in rat aorta
Spinal cord stretch injury has been shown to be ameliorated by treatment with BBG and the mechanism is thought to be via P2X7R antagonism.13 We hypothesized that FCF restores stretch-injured PSV by targeting the P2X7R. In addition, P2X7R activation leads to increases in intracellular calcium concentrations [Ca2+]i.17 We therefore measured force generation and [Ca2+]i concurrently in response to BzATP, a P2X7R agonist that is known to elicit a contraction.16,18 We chose to use rat aorta because the thin nature of the arterial wall that allows penetration of the fluorochrome and the reproducibility of the results. Pretreatment with FCF blocked BzATP-induced contraction (0.006 ±0.003×105 N/m2 vs 0.019±0.006× 105N/m2 in control; P=.002; n=8; Figure 5A). Additionally, FCF blocked BzATP-induced calcium ion flux (0.50 ±0.032 A.U. vs 0.016±0.023 A.U. in control; P=.0004; n=8; Figure 5B). FCF inhibition of BzATP-induced contraction and Ca2+ fluxes was comparable to those mediated by known P2X7R antagonists oATP (0.006±0.007×105 N/m2 vs 0.019 ±0.007 ×105N/m2 in control; P=.005; n=4; and 0.039±0.022 A.U. vs 0.135 ±0.021A.U. in control; P=.004; n=4), KN-62 (0.004±0.002×105 N/m2 vs 0.043 ±0.05×105N/m2 in control; P=.005; n=4 and 0.066±0.008 A.U. vs 0.128 ±0.01 A.U. in control; P<.001; n=4) and BBG (0.003±0.0006 ×105 N/m2 vs 0.042 ±0.05×105N/m2 in control; P=.005; n=4 and 0.05 ±0.014 A.U. vs 0.15±0.03 A.U. in control; P=.02; n=4), inferring that FCF is likely an antagonist of the P2X7R in vascular tissues (Figure 5A and B).
Figure 5. FCF inhibits P2X7R-mediated cytosolic Ca2+ fluxes in rat aorta.
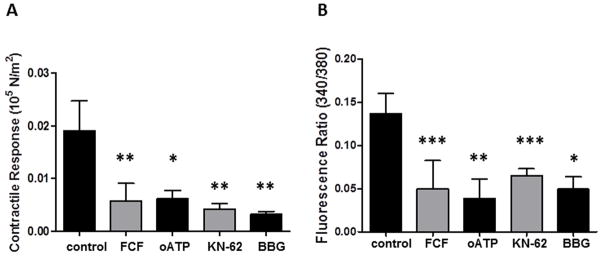
Rat aortic rings (n=4–8) were suspended in the FluoroPlex muscle bath, either left untreated (control) or treated with FCF or other P2X7R antagonists oATP, KN62 or BBG prior to contraction with BzATP. Concurrent force generation (A) and cytosolic Ca2+ flux (B) were measured. Results are presented as mean±SD. *P<0.0, **P<0.001. ***P<0.0001 vs to control in paired t-tests.
FCF inhibited P2X7 purinergic receptor-induced contraction in HSV
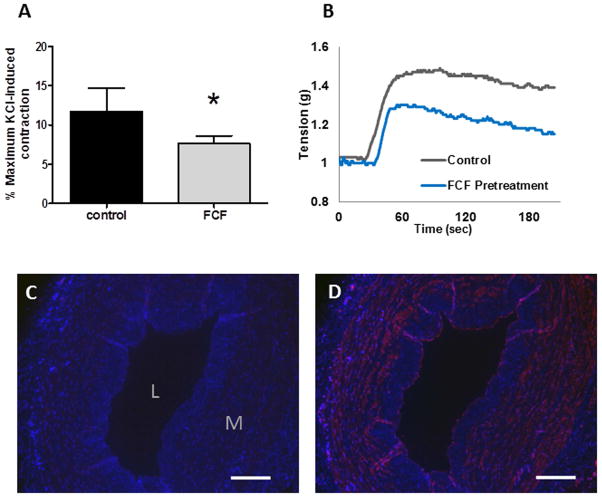
To determine whether FCF interferes with P2X7R in HSV, BzATP-induced contraction was measured. Pre-incubation with FCF significantly reduced contraction to BzATP when compared to the control rings (7.7 ± 0.9% vs 11.8 ± 2.9% of maximum KCl-induced contraction in control; P=.006; n=6; Figure 6A and B) suggesting that FCF inhibits P2X7R activation in HSV. Expression of P2X7R in HSV was confirmed by immunohistochemistry (Figure 6C and D).
Figure 6. FCF inhibits P2X7R-mediated contraction in human saphenous vein.
A. Rings of HSV (n=6) were suspended in the muscle bath, either left untreated (control) or treated with 50μM FCF for 30min prior to contraction with BzATP. B. Representative muscle bath force tracings of BzATP-induced contraction in HSV. Results are presented as mean±SD. *P=0.006 vs control in a paired t-test. Expression of P2X7R in HSV as detected by immunohistochemistry using pre-absorbed (C) or normal P2X7R-specific antibody (D). P2X7R were stained red. Scale bar = 200 μm; L= lumen, M=medial.
DISCUSSION
Intimal hyperplasia is the leading cause of vein graft failure. While the specific inciting events remain uncertain, there is general agreement that this process is a ‘response to injury’ initiated by vein graft harvest, surgical preparation, implantation, reperfusion, and exposure to arterial hemodynamics.19,20 Surgical dissection of the vein graft causes significant mechanical stretch injury and subsequent preparation of the vein graft are deleterious to vein grafts and optimization of these techniques may reduce intimal hyperplasia.6
Surgical skin markers, commonly used to mark vein grafts, are not approved by the FDA to use on vein tissue. We have previously showed that these marking pens reduce viability and impair physiologic function of the HSV and such detrimental effects are attributable by the gentian violet dye and isopropanol.10 We sought an alternative to the off-label use of surgical skin marker and identified FCF as a non-toxic dye that improves endothelial and smooth muscle functional responses in HSV, suggesting that the FCF dye has pharmacologic properties.12 In the current study, we demonstrated further that FCF can be utilized to mark vein graft and may have additional benefits in restoring stretch-injury resulting from vein harvest and preparation. Similar to our earlier study using HSV,8 surgical skin marker essentially abolished contractile responses in PSV (Figure 1). Exposure to another common dye, methylene blue, and 50% isopropanol, the solvent found in surgical skin markers resulted in 60–100% loss in contractile responses (Figure 1). In contrast, extravascular marking with FCF did not affect the contractile responses in PSV (Figure 1). Since contractile response to 110mM KCl correlates with cellular viability in vascular tissues,7 cellular death likely occurs within the vein graft soon after vein graft marking. Gentian violet is widely used for medicinal purpose and is commonly used as staining dye for biological samples. However, it is a clastogenic dye that induces chromosomal aberrations and mitotic anomalies in various types of cultured mammalian cells at a low concentration of 5μM.21 Likewise, isopropanol is commonly used as cell and tissue fixative in cytohistological preparations and is converted to acetone by an endogenous enzyme.22 Prolonged exposure to alcohols and acetone disrupts plasma membrane and damages intracellular structures.23 Hence, exposing the graft to isopropanol is essentially fixing the conduits. This is analogous to cryopreservation of saphenous vein used in bypass procedures, causes substantial damages. The patency of cryopreserved veins are dismal at 27% and 17% at 1 and 3 years.24 In this study, exposure to 1% gentian violet or 50% isopropanol chemically fixed the HUVSMC with complete loss of viability as evident by trypan blue dye exclusion test (Figure 2A). When HUVSMC were treated with 2.6mM FCF, concentration that was used for extravascular application on PSV, no cell death was observed (Figure 2A). Cytotoxicity of gentian violet on HUVSMC was further confirmed by treating cells with 0.002% or 50μM gentian violet, a concentration that is 5,000 times lower than that found in surgical skin markers. (Figure 2B). Exposure to GV caused over 50% cell death in HUVSMC, levels that are comparable to staurosporine, an inducer of apoptosis that was used as a positive control for the assay (Figure 2B). Conversely, HUVSMC treated with comparable concentration (50μM) of FCF, BBG or allura red demonstrated no cytotoxicity (Figure 2B). Taken together, these data suggest that the off-label used dye, gentian violet, is particularly toxic to the conduit and that other non-toxic dyes are available for vein graft marking.
In a previous study, we showed that mechanical stretching of PSV reduced contractility of the vein. In the current study the topical application of FCF restored functional viability after stretch injury in PSV (Figure 3). This was not observed with allura red or BBG (Figure 3), suggesting that the pharmacologic properties are unique to FCF. Moreover, FCF prevented neointimal thickening whereas BBG or allura red promoted the development of intimal hyperplasia in organ cultured PSV (Figure 4). Taken together, while BBG and allura red are biocompatible as marking dyes, they lack the pharmacologic properties that are unique to FCF and may promote intimal hyperplasia.
FCF inhibited the selective P2X7R agonist BzATP-induced smooth muscle force (Figure 5A) and calcium ion flux (Figure 5B) to levels comparable to other known antagonists of the receptor,16,25,26 implying that FCF is an antagonist of the P2X7R. Additionally, the evidence that FCF inhibited BzATP-induced contraction and that P2X7R is expressed in HSV supports a potential role of P2X7R in the response to injury in saphenous veins (Figure 6).
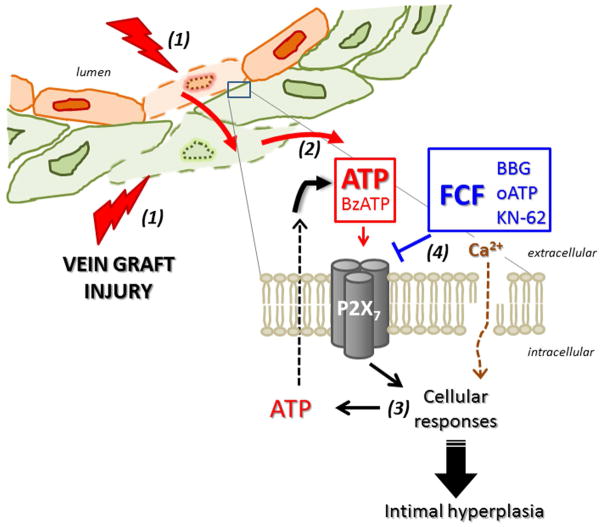
Vein graft injury incurred during graft preparation is a sufficient stimulus for the development of intimal hyperplasia in HSV ex vivo, suggesting a crucial role for early injury in the cellular processes that contribute to the development of intimal hyperplasia.6,12 Given that tissue damage, particularly that caused by stretching, leads to extracellular ATP release,13,27–29 it can be envisioned that injury to the vein leads to release of ATP from damaged cells that activates the P2X7R in neighboring cells. Increases in intracellular calcium ensues and result in further ATP release thus propagating the injury response (Figure 7).17 The findings of the current study offer evidence that deleterious effects of harvest-induced injury can be ameliorated by treatment with FCF. The mechanism for the pharmacologic properties of FCF may be due to inhibition of the P2X7 purinergic receptor (Figure 7). Hence, it is conceivable that intervening P2X7R activation during the period of explantation is a clinically relevant approach to preventing intimal hyperplasia and vein graft failure.
Figure 7. Model of P2X7R activation during vein graft preparation injury.
Surgical harvest and preparation cause vein graft injury (1), leading to release of ATP (2). ATP activates the P2X7 receptor on neighboring cells, propagating the response to injury (3). FCF may mitigate the effect of P2X7R activation (4) by inhibiting membrane pore formation, [Ca2+]i flux, and additional release of extracellular ATP. (Agonists, red; inhibitors, blue)
POTENTIAL LIMITATIONS
While our stretch injury model of PSV recapitulated the potential injury to the vein grafts, these tissues came from healthy animals. The model system used for these experiments has the advantage of more homogeneity and greater reproducibility compared to HSV. Additionally, large effect sizes were observed in studies that evaluated the toxicity of marking dyes on PSV as well as P2X7R blockade in rat aortae and achieved high statistical power (≥0.9) with relatively small sample sizes, supporting that the findings were unlikely due to false negative or the lack of power. Moreover, the mechanistic links between P2X7R blockade and restoration of smooth muscle injury or reduction in intimal thickening requires further evaluation in in vivo models. Aside from changes in calcium ion flux, it remains to be determined whether treatment with FCF affects downstream events elicited by P2X7R activation that have been characterized in other cell types. In addition, while the culturing medium for PSV contains serum, the organ culture model lacks in vivo elements (pressure, flow, and exposure to blood components).
CONCLUSIONS
We have demonstrated that FCF is a non-toxic alternative to the current off-label use of surgical skin marker for vein graft marking. FCF treatment during vein graft preparation inhibits the injury response in vascular tissues and inhibits intimal hyperplasia in vitro. Further work is needed to address the therapeutic properties of FCF in animal models and better characterize the mechanism of action of FCF. Treatment with FCF represents a new approach to vein graft marking and possibly a therapeutic clinical alternative to the off-label use of surgical skin markers during preparation of vein graft conduits.
CLINICAL RELEVANCE.
Saphenous veins remain the most commonly used conduits for bypass procedures. Current use of surgical skin markers to orient conduits impairs physiologic functions of the vein grafts. FCF represents an alternative marking dye that has pharmacologic properties as well as a potential therapeutic approach to prevent vein graft injury and enhance outcome.
Acknowledgments
This study was also supported in part with resources and materials from the VA Tennessee Valley Healthcare System.
FUNDING SOURCES
This study was supported by NIH R01HL70715-09 and a VA Biomedical Laboratory Research and Development Grant (CB), and NIH R01HL105731-01A1 (JC).
Footnotes
DISCLOSURES
Drs. Brophy and Cheung-Flynn disclose that they have a financial relationship with Vasoprep Surgical.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. Journal of vascular surgery. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. discussion 51. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 3.Clowes AW, Reidy MA. Prevention of stenosis after vascular reconstruction: pharmacologic control of intimal hyperplasia--a review. J Vasc Surg. 1991;13:885–91. doi: 10.1067/mva.1991.27929. [DOI] [PubMed] [Google Scholar]
- 4.LoGerfo FW, Quist WC, Cantelmo NL, Haudenschild CC. Integrity of vein grafts as a function of initial intimal and medial preservation. Circulation. 1983;68:II117–24. [PubMed] [Google Scholar]
- 5.Harskamp RE, Alexander JH, Schulte PJ, Brophy CM, Mack MJ, Peterson ED, et al. Vein Graft Preservation Solutions, Patency, and Outcomes After Coronary Artery Bypass Graft Surgery: Follow-up From the PREVENT IV Randomized Clinical Trial. JAMA Surgery. 2014;149:798–805. doi: 10.1001/jamasurg.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osgood MJ, Hocking KM, Voskresensky, Li FD, Komalavilas P, Cheung-Flynn J, et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. Journal of Vascular Surgery. 2013 doi: 10.1016/j.jvs.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocking KM, Brophy C, Rizvi SZ, Komalavilas P, Eagle S, Leacche M, et al. Detrimental effects of mechanical stretch on smooth muscle function in saphenous veins. Journal of Vascular Surgery. 2011;53:454–60. doi: 10.1016/j.jvs.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagle S, Brophy CM, Komalavilas P, Hocking K, Putumbaka G, Osgood M, et al. Surgical skin markers impair human saphenous vein graft smooth muscle and endothelial function. Am Surg. 2011;77:922–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Li FD, Eagle SE, Brophy CM, Hocking KM, Osgood MJ, Komalavilas P, Cheung-Flynn J. Pressure control during preparation of saphenous veins. JAMA Surgery. 2013 doi: 10.1001/jamasurg.2013.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DA, Rubin JW, Zumbro GL, Tackett RL. The use of methylene blue as an extravascular surgical marker impairs vascular responses of human saphenous veins. J Thorac Cardiovasc Surg. 1995;109:21–9. doi: 10.1016/S0022-5223(95)70417-5. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker K, Rubin J, Zumbro GL, Tackett R. Evans blue and gentian violet: alternatives to methylene blue as a surgical marker dye. J Thorac Cardiovasc Surg. 1996;112:542–4. doi: 10.1016/s0022-5223(96)70286-6. [DOI] [PubMed] [Google Scholar]
- 12.Voskresensky IV, Wise ES, hocking KM, Li Fandong, Osgood MJ, Komalavilas P, Brophy C, Cheung-Flynn J. Brilliant Blue FCF as an Alternative Dye for Saphenous Vein Graft Marking. Effect on Conduit Function. JAMA Surgery. 2014 doi: 10.1001/jamasurg.2014.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hocking KM, Baudenbacher FJ, Putumbaka G, Venkatraman S, Cheung-Flynn J, Brophy CM, et al. Role of cyclic nucleotide-dependent actin cytoskeletal dynamics:Ca(2+)](i) and force suppression in forskolin-pretreated porcine coronary arteries. PLoS One. 2013;8:e60986. doi: 10.1371/journal.pone.0060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Controlled clinical trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 16.Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res. 1998;83:196–203. doi: 10.1161/01.res.83.2.196. [DOI] [PubMed] [Google Scholar]
- 17.Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D’Alimonte I, et al. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7:2533–7. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- 18.Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca(2+)](i) changes and depolarization in GH3 cells. Br J Pharmacol. 2000;130:1843–52. doi: 10.1038/sj.bjp.0703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Conte MS. Technical factors in lower-extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009;22:227–33. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Au W, Pathak S, Collie CJ, Hsu TC. Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutation research. 1978;58:269–76. doi: 10.1016/0165-1218(78)90019-8. [DOI] [PubMed] [Google Scholar]
- 22.Kapp RW, Jr, Bevan C, Gardiner TH, Banton MI, Tyler TR, Wright GA. Isopropanol: summary of TSCA test rule studies and relevance to hazard identification. Regul Toxicol Pharmacol. 1996;23:183–92. doi: 10.1006/rtph.1996.0042. [DOI] [PubMed] [Google Scholar]
- 23.Hoetelmans RW, Prins FA, Cornelese-ten Velde I, van der Meer J, van de Velde CJ, van Dierendonck JH. Effects of acetone, methanol, or paraformaldehyde on cellular structure, visualized by reflection contrast microscopy and transmission and scanning electron microscopy. Appl Immunohistochem Mol Morphol. 2001;9:346–51. doi: 10.1097/00129039-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Chang CK, Scali ST, Feezor RJ, Beck AW, Waterman AL, Huber TS, et al. Defining utility and predicting outcome of cadaveric lower extremity bypass grafts in patients with critical limb ischemia. Journal of Vascular Surgery. 2014 doi: 10.1016/j.jvs.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. British journal of pharmacology. 2009;157:1203–14. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Molecular pharmacology. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- 27.Volonte C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets. 2012;11:705–21. doi: 10.2174/187152712803581137. [DOI] [PubMed] [Google Scholar]
- 28.Dale N. A classic review on extracellular ATP and its signalling functions that helped to define the field’s agenda for many years. Biochem J. 2012;2012:1–6. [Google Scholar]
- 29.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]