Abstract
Only a few studies comparing flea composition on the coast and in the mountains have been conducted. We investigated differences in flea communities infesting small mammals in selected habitats in northern, central, and southern Poland. We predicted (1) a greater number of flea species in the southeastern Poland and a lower number in the north, (2) a greater number of flea species in fertile and wet habitats than in poor and arid habitats, and (3) a low similarity of flea species between flea communities in western and eastern Poland. We found a negative effect of increasing latitude on flea species richness. We suppose that the mountains providing a variety of environments and the limits of the geographic ranges of several flea subspecies in southeastern Poland result in a higher number of flea species. There was a positive effect of increasing wetness of habitat on flea species richness. We found a high diversity in flea species composition between western and eastern Poland (beta diversity = 11) and between central and eastern Poland (beta diversity = 12). Re-colonization of Poland by small mammals and their ectoparasites from different (western and eastern) refugees can affect on this high diversity of flea species.
Keywords: Alpha and beta diversity, Biogeography, Fleas, Latitude, Small mammals, Poland
Introduction
Species composition of fleas is affected by a variety of abiotic (e.g., temperature, humidity, precipitation, and elevation or structure of the substrate) and biotic factors (e.g., host species, its age, sex, behavior, and habitat preferences) (Marshall 1981; Krasnov et al. 2007, 2010b; Pawelczyk et al. 2004). Hosts with similar habitat requirements and diet can be infected by similar or even identical flea species (Krasnov et al. 2006b; Klimpel et al. 2007). The composition of flea species on host species is determined not only by host-flea relations but also by host-habitat relations. Therefore, a habitat for fleas is not a particular host or a group of hosts but rather a particular host or a group of hosts inside a particular habitat (Krasnov et al. 2006b). In addition, flea species composition varies less (1) among populations of the same host species than among different host species and (2) among habitats of the same type than among different habitats (Poulin and Valtonen 2002; Krasnov et al. 2006b).
Social species living in large family groups, like many rodents, are characterized by a higher prevalence of parasites than less social species like shrews, which are more solitary (Rychlik 1998; Karbowiak et al. 2005; Oguge et al. 2009; Krasnov et al. 2010a). In a more social animal, contacts between individuals are much more frequent which promotes the exchange of fleas. Similarly, increased densities have the same effect as increasing the number of contacts between individuals in population. So, high densities of fleas should mirror high host densities (Rödl 1979; Laakkonen 2000).
Flea species composition depends on the depth and stability of the host’s burrow (Krasnov et al. 2006a). Deep and permanent burrows with a constant microclimate seem to be a better habitat for fleas than the shallow and temporary burrows. As a result, the hosts living in deep and permanent burrows may have higher number of flea species and higher prevalence than hosts living in ephemeral and ground burrows (Krasnov et al. 2004, 2010a).
This study aimed to find differences in the flea species composition of small mammals in selected habitats of the Baltic coast, the central lowlands, and mountains of Poland. Due to the biogeography rule, the biodiversity of plant and animal species decreases with increasing latitude (Rohde 1992; Rosenzweig 1992; Krasnov et al. 2004; Pavoine and Bonsall 2011). Additionally, due to the fact that in Poland we can find the limits of the geographic ranges of several flea species and subspecies (Skuratowicz 1967; Bartkowska 1973, 1977), we predicted that (1) a greater number of flea species will be noted in the southeastern Poland and a lower number in the north. Moreover, fauna of small mammals is usually richer in fertile and wet habitats (e.g., Aulak 1970). We thus predicted that (2) a greater number of flea species will be recorded in fertile and wet habitats and a lower number in poor and arid habitats. There are some proofs that after the last glaciation, Poland was re-colonized by small mammals and their ectoparasites from different (western and eastern) refugees (Michaux et al. 2004, 2005; Deffontaine et al. 2005; Nieberding et al. 2008). Therefore, we expected (3) a low similarity of flea species between flea communities in western and eastern Poland.
Materials and methods
Study area
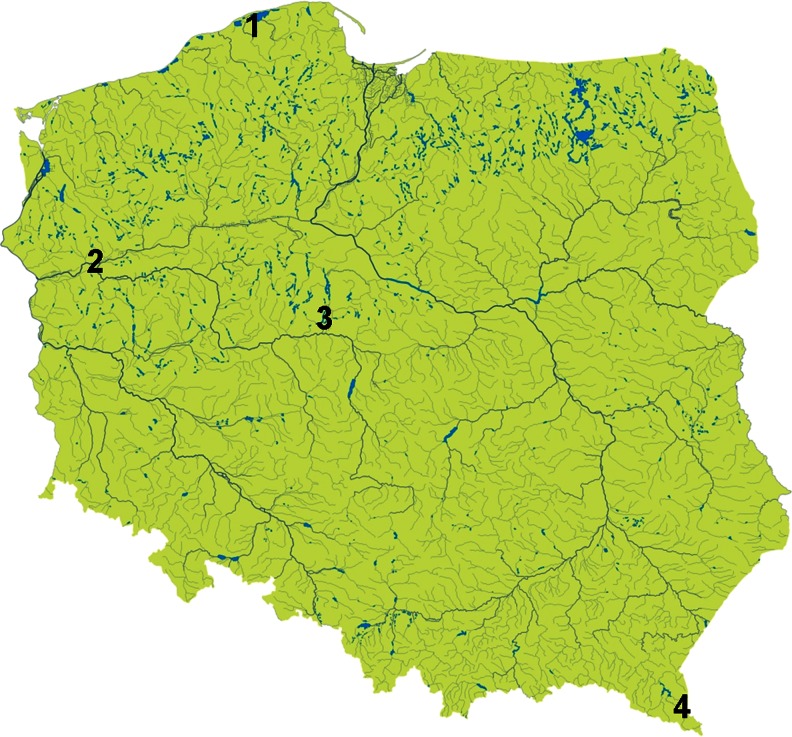
In total, 19 study plots were located in selected habitats of four regions: the Baltic coast (Słowiński National Park), the western lowlands (Gorzowska Forest), the central lowlands (Konin lakes area), and the mountains in the southeast (Bieszczady Mountains) of Poland (Fig. 1). In the Słowiński National Park (SNP), we established seven study plots. Plots S1–S4 were located in the central part of the park and were investigated for 2 weeks in late July and August 2010 (Table 4 in Appendix). Plots S5–S7 were located on the spit separating the Baltic Sea from the Gardno Lake (the western part of the park) and were studied in September 2011 (1 week). In the Gorzowska Forest, the study was conducted in July 2010 on two plots and in the Konin lakes area on eight plots from August to October 2011. In the Bieszczady Mountains, research was conducted for 5 days in August 2011 on two study plots near Lutowiska village.
Fig. 1.
The location of the study plots: 1 Słowiński National Park, 2 Gorzowska Forest, 3 Konin lakes area, 4 Bieszczady Mountains
Table 4.
Description of the 19 study plots in the Słowiński National Park, Gorzowska Forest, Konin lakes area and in the Bieszczady Mountains, including Habitat richness and humidity, and trapping effort
| Plot no. | Latitude and longitude | Plot description | Wetness | Richness | Trapping season | Number and layout of traps | Trapping effort [trap-hours] |
|---|---|---|---|---|---|---|---|
| Słowiński National Park | |||||||
| S1 | 54° 41′ 59.74″ N, 17° 18′ 31.39″ E | Community similar to Pomeranian fertile beech forest Melico-Fagetum on moist soil; dominant plants: Molinia cerulea, Fagus silvatica, Quercus sessilis | 2 | 1 | July 2010 | Grid: 3 parallel lines of 10 box traps | 4,350 |
| S2 | 54° 41′ 52.96″ N, 17° 18′ 47.83″ E | Peat alder forest Sphagno squarrosi-Alnetum, wet (water from 20 cm below ground surface to 20 cm deep); dominant plants: Pteridium aquilinum, Molinia cerulea, Alnus glutinosa, Betula pubescens | 3 | 5 | July 2010 | Grid: 3 parallel lines of 10 box traps | 2,700 |
| S3 | 54° 41′ 40.00″ N, 17° 12′ 25.50″ E | Reeds Phalaridetum arundinaceae, wet (water 0–30 cm deep); dominant plants: Phalaris arundinacea, Carex pseudocyperus, Glyceria maxima | 5 | 4 | Aug 2010 | Grid: 3 parallel lines of 10 box traps | 1,185 |
| S4 | 54° 42′ 4.95″ N, 17° 12′ 38.43″ E | Alder forest (shore of Dołgie Lake) and a mosaic of plant communities Iridetum pseudacori and Cicuto-Caricetum pseudocyperi (water 20–30 cm deep); dominant plants: Carex pseudocyperus, Iris pseudoacorus, Carex nigra, Alnus glutinosa, Betula pubescens | 5 | 4 | Aug 2010 | Grid: 2 parallel lines of 15 box traps | 1,125 |
| S5 | 54° 40′ 17.39″ N, 17° 05′ 19.60″ E | Seaside pine forest Empetro nigri-Pinetum | 3 | 4 | Sep 2011 | Grid: 3 parallel lines of 10 box traps | 3,600 |
| S6 | 54° 40′ 29.63″ N, 17° 03′ 55.27″ E | Wet meadow | 4 | 2 | Sep 2011 | Grid: 3 parallel lines of 10 (14) box traps | 3,600 |
| S7 | 54° 40′ 23.92″ N, 17° 04′ 00.11″ E |
White dune Elymo- Ammophiletun arenariae |
1 | 1 | Sep 2011 | Grid: 2 parallel lines of 15 box traps | 3,600 |
| Gorzowska Forest | |||||||
| G1 | 52° 41′ 22.18″ N, 15° 03′ 43.92″ E | Wet meadow with individual trees and shrubs and lush herbaceous vegetation (herbs and grass) | 3 | 4 | July 2010 | Grid: 8 parallel lines of 8 box traps | 36,864 |
| G2 | 52° 41′ 58.14″ N, 15° 04′ 9.87″ E | Beech forest | 2 | 2 | July 2010 | Grid: 8 parallel lines of 8 box traps | 36,864 |
| Konin lakes area | |||||||
| K1 | 52° 18′ 9.17″ N, 18° 19′ 11.50″ E | Three microhabitats: lake bank with lush vegetation, herbs and bulrush; channel leading eutrophic water to a nearby wetland and ecotone between rather dry and wet meadow (southwestern bank of Licheńskie Lake) | 3 | 5 | Aug 2011 | Grid: 3 parallel lines of 10 box traps | 1,290 |
| K2 | 52° 18′ 25.80″ N, 18° 18′ 21.98″ E | Dry aspect of alder forest separated with a causeway from the eastern bank of Pątnowskie Lake | 1 | 1 | Aug 2011 | Grid: 3 parallel lines of 10 box traps | 1,200 |
| K3 | 52° 17′ 54.63″ N, 18° 11′ 42.22″ E | Two microhabitats: bank of a small forest lake with sedges and herbs, and moist alder forest (without standing water between the clumps) with dense stand (700 m west of Gosławskie Lake) | 4 | 5 | Aug 2011 | Grid: 3 parallel lines of 10 box traps | 600 |
| K4 | 52° 17′ 43.48″ N, 18° 11′ 5.14″ E | Three microhabitats: forest lake bank overgrowing with the willow bushes and bulrush; wet (but without standing water between the clumps) alder forest and mixed forest on the side of the hill (500 m north of Skąpe Lake) | 3 | 4 | Aug 2011 | Grid: 2 parallel lines of 10 box traps and a separate line (C) of 10 box traps | 600 |
| K5 | 52° 15′ 54.40″ N, 18° 15′ 25.93″ E | The bank of artificial and polluted lake including three microhabitats: steep bank overgrown with reeds, quite moist open plateau with blackberry and broom bushes and a steep slope overgrown with herbs and with a loose trees and grasses | 3 | 3 | October 2011 | Grid: 3 parallel lines of 10 box traps | 1,080 |
| K6 | 52° 16′ 40.35′ N, 18° 16′ 14.44″ E | An anthropogenic and polluted environment (backwaters near the “Gosławice” Power); included three microhabitats: reeds growing on wetlands bank, sewer bank with lush rush and slope descending to this sewer covered with herbaceous vegetation with a few birches | 4 | 3 | October 2011 | Grid: 3 parallel lines of 10 box traps | 1,080 |
| K7 | 52° 19′ 25.30″ N, 18° 21′ 7.98″ E | Three microhabitats: a large glacial lake bank with a narrow strip of riparian vegetation, steep slope covered with mature trees and the plateau with lush grassy places, old pines and younger oaks and maples (eastern bank of Licheńskie Lake) | 2 | 2 | October 2011 | Grid: 3 parallel lines of 10 box traps | 1,860 |
| K8 | 52° 20′ 12.32″ N, 18° 21′ 28.07″ E | Two microhabitats: large glacial lake bank with a narrow strip of riparian vegetation with reeds and pine forest with herbaceous vegetation (northeastern bank of Licheńskie Lake) | 2 | 2 | October 2011 | Grid: 3 parallel lines of 10 box traps | 1,860 |
| Bieszczady Mountains (in/near Lutowiska village) | |||||||
| B1 | 49° 14′ 51.70″ N, 22° 40′ 14.84″ E | Pine forest with dense undergrowth dominated by blackberry | 2 | 4 | Aug 2011 | Grid: 3 parallel lines of 10 box traps | 2,070 |
| B2 | 49° 15′ 0.77″ N, 22° 41′ 24.67″ E | Banks of a stream running through wet meadow with individual shrubs and lush herbaceous vegetation (reeds and herbs) | 4 | 3 | Aug 2011 | Grid: 3 parallel lines of 10 box traps | 1,680 |
Trapping procedure
Small mammals were captured in wooden live traps, which were usually arranged in three parallel lines with 10 traps in each line (except S4, S7, G1, G2, and K4; see Table 4 in Appendix). The first line was located directly along the shoreline (traps were set within 1 m from water) or in the wettest habitat. The next two lines were set at distances about 10 and 20 m from the first line. The traps were spaced at approximately 10-m intervals within lines so it can be assumed that each study plot covered approximately 0.30 ha. Only in Gorzowska Forest traps were arranged in eight lines with eight traps in each line and covered 0.49 ha. We placed food (oat flakes and minced beef) in the traps as bait and as provision for captured animals. Trapping sessions were carried out at night for 5–10 days in the Słowiński National Park and for 3–4 days in the Bieszczady Mountains. Within the Konin lakes area, the study was carried out non-stop for 2 days. Traps were set in the afternoon and checked every 3–4 h in areas inhabited by shrews or every 5–6 h in areas inhabited only by rodents. In Gorzowska Forest, traps were active non-stop and checked twice a day for five consecutive days. The captured animals’ species were determined and they weighed. We recorded also their age, sex, and reproductive activity and individually marked by ear tagging (Gorzowska Forest) or cutting a small patch of fur (other locations). Then, mammals were placed in a canvas bag for 2–3 min to collect fleas (e.g., Haas and Walton 1973, Paramasvaran et al. 2009, Zuo et al. 2011). The collected fleas were placed in a vial with alcohol, and mammals were released at the place of capture.
Data analysis
We analyzed the relationship between (1) the latitude and number of flea species and (2) between habitat richness and number of flea species using generalized linear mixed models implemented via “lme4” package (Bates et al. 2011). In both analyses, we used a number of flea species as response variable and location as random factor. In analysis (1) latitude, abundance of small mammals, number of small mammal species, and abundance of fleas were implemented as fixed factors, whereas in analysis (2) we used habitat wetness and habitat fertility, abundance of small mammals, number of small mammal species, and abundance of fleas as fixed factors. In both analyses we used Poisson error distribution, and we began with a model containing all the above mentioned explanatory variables and determined the structure of the final model through backward stepwise elimination of non-significant factors using likelihood ratio test. For the purpose of analysis (2), we categorized all habitats into five classes of wetness (1–least wet, 5–most wet) and five classes of fertility (1–least fertile, 5–most fertile). Habitats were assigned to fertility classes based on undergrowth density and the thickness of the litter and to wetness categories based on differences in plant communities. All the analyses were conducted in R software (R Development Core Team 2012).
We measured the alpha and beta diversity to find differences in species composition between flea communities in western and eastern Poland. Alpha diversity refers to the diversity within particular area (or ecosystem) and is usually expressed by the number of species (i.e., species richness) in that area. In turn, beta diversity refers to the total number of species that are unique to each of the areas (or ecosystems) being compared (Whittaker 1972, Harrison et al. 2004).
Results
Small mammals captured
In the Słowiński National Park, we captured 60 small mammals belonging to four rodent and three shrew species in 2010 and 34 mammals belonging to five rodent and two shrew species in 2011 (see Table 5 in Appendix). In the Konin lakes area, we captured 187 small mammals belonging to four rodent and two shrew species. In the Gorzowska Forest, we recorded 125 rodents belonging to two species, and in the Bieszczady Mountains, 75 small mammals belonging to five rodents and three shrews.
Table 5.
Species and numbers of all small mammals captured in selected habitats of the Baltic coast, the central lowlands, and mountains of Poland
| Słowiński National Park | Gorzowska Forest | Konin lakes area | Bieszczady Mountains | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mammal species | Code | S1 | S2 | S3 | S4 | S5 | S6 | S7 | G1 | G2 | K1 | K2 | K3 | K4 | K5 | K6 | K7 | K8 | B1 | B2 |
| Apodemus agrarius | Agr | – | – | 1 | 1 | – | 1 | 3 | – | – | 19 | – | 2 | 2 | 14 | 28 | 5 | 4 | – | 5 |
| Apodemus flavicollis | Afl | 4 | 16 | 2 | 1 | – | – | 1 | 49 | 31 | – | 1 | 4 | 9 | 14 | 1 | 3 | 1 | 10 | 23 |
| Apodemus sylvaticus | Asl | – | – | – | – | – | – | – | – | – | – | – | – | – | 4 | – | – | – | – | – |
| Micromys minutus | Mmn | – | – | – | – | 1 | 12 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Microtus agrestis | Mgr | – | – | – | – | 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Microtus arvalis | Mar | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 5 |
| Microtus oeconomus | Moe | – | 1 | 10 | 11 | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Myodes glareolus | Mgl | – | 3 | – | – | 1 | – | – | 44 | 11 | 12 | 3 | 23 | 4 | 11 | 4 | – | – | 14 | – |
| Neomys fodiens | Nfd | – | – | – | 1 | – | – | – | – | – | 2 | – | 1 | 1 | – | 2 | – | – | – | 2 |
| Sorex araneus | Sar | – | 1 | 1 | 1 | 1 | – | – | – | – | 2 | 1 | 1 | 1 | 1 | 6 | 1 | – | 3 | 11 |
| Sorex minutus | Smn | – | 1 | 2 | 3 | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Total | 4 | 22 | 16 | 18 | 12 | 18 | 4 | 93 | 42 | 35 | 5 | 31 | 17 | 44 | 41 | 9 | 5 | 27 | 48 | |
Fleas collected
In total, on all study plots, we collected 634 individuals of fleas belonging to 17 species and subspecies. In the Słowiński National Park, we collected 18 individuals belonging to three species of fleas in 2010 and 65 fleas belonging to eight species in 2011 (Table 1). In the Gorzowska Forest, we found 294 fleas belonging to five species, and in the Bieszczady Mountains, we collected 110 fleas belonging to seven species and subspecies. In the Konin lakes area, we recorded 147 fleas belonging to 13 species (Table 2).
Table 1.
Fleas collected from small mammals in the Słowiński National Park, Gorzowska Forest, and the Bieszczady Mountains
| Area/Plot no. | Słowiński National Park | Gorzowska Forest | Bieszczady Mountains | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S5 | S6 | S7 | G1 | G2 | B1 | B2 | ||||||||||||||||
| Flea species/ subspecies | Afl | Afl | Mgl | Afl | Agr | Moe | Mmn | Mgl | Mgr | Sar | Moe | Agr | Afl | Mgl | Afl | Mgl | Afl | Mgl | Sar | Smn | Sar | Agr | Afl | Mar | Mgr |
| Ctenophthalmus agyrtes agyrtes | 4 | 2 | 1 | 1 | 1 | – | – | – | 4 | – | – | – | 39 | 52 | 24 | 11 | – | – | – | – | – | – | – | – | – |
| Ctenophthalmus agyrtes kleinschmidtianus | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | – | – | 1 | 5 | 27 | – | 3 | |
| Ctenophthalmus bisoctodentatus | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Ctenophthalmus solutus | – | – | – | – | – | – | – | – | – | – | – | – | 28 | 6 | 15 | 2 | – | – | – | – | – | – | – | – | – |
| Ctenophthalmus uncinatus | – | – | – | – | – | – | – | – | – | – | – | – | 4 | 47 | – | 6 | 1 | 1 | – | – | – | – | – | 1 | – |
| Doratopsylla dasycnema cuspis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 17 | – | – | – | – |
| Doratopsylla dasycnema dasycnema | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hystrichopsylla talpae orientalis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – |
| Hystrichopsylla talpae talpae | – | – | – | – | – | – | – | – | 8 | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Megabothris turbidus | – | – | 1 | – | – | 1 | – | 3 | 9 | – | – | 1 | 25 | 27 | 3 | 4 | 2 | 10 | – | – | – | 2 | 8 | – | – |
| Megabothris walkeri | – | – | – | – | – | 7 | – | – | 23 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Palaeopsylla soricis | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | 2 | 3 | 18 | – | – | 1 | – |
| Peromyscopsylla bidentata | – | – | – | – | – | – | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Peromyscopsylla silvatica | – | – | – | – | – | – | – | – | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Total | 4 | 2 | 2 | 1 | 1 | 8 | 1 | 3 | 57 | 1 | 2 | 1 | 96 | 132 | 43 | 23 | 5 | 13 | 3 | 5 | 36 | 7 | 36 | 2 | 3 |
Afl Apodemus flavicollis, Agr Apodemus agrarius,MarMicrotus arvalis, MglMyodes glareolus, MgrMicrotus agrestis, MmnMicromys minutus, MoeMicrotus oeconomus, Nfd Neomys fodiens, Sar Sorex araneus, Smn Sorex minutus
Table 2.
Fleas collected from small mammals in the Konin lake area. Mammal species codes as in Table 5
| Plot no. | K1 | K2 | K3 | K4 | K5 | K6 | K7 | K8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flea species/subspecies | Agr | Mgl | Mgl | Agr | Afl | Mgl | Nfd | Afl | Agr | Afl | Asl | Mgl | Agr | Mgl | Sar | Afl | Sar | Agr |
| Ctenophthalmus agyrtes agyrtes | 7 | 2 | – | 1 | 1 | 1 | – | 1 | 3 | 1 | – | 1 | 8 | 4 | – | 1 | – | 1 |
| Ctenophthalmus agyrtes peusianus | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – |
| Ctenophthalmus assimilis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – |
| Ctenophthalmus bisoctodentatus | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ctenophthalmus solutus | – | – | – | – | – | – | – | 4 | 4 | 1 | – | – | – | – | – | – | – | – |
| Ctenophthalmus uncinatus | 1 | 1 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – |
| Doratopsylla dasycnema dasycnema | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| Hystrichopsylla talpae talpae | – | – | – | – | – | 1 | – | 1 | 1 | – | – | – | 1 | – | – | – | 2 | – |
| Megabothris turbidus | 20 | 20 | 2 | 1 | 2 | 8 | 1 | 2 | 1 | 2 | – | 3 | 4 | – | – | 1 | – | 1 |
| Megabothris walkeri | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – |
| Nosopsyllus fasciatus | – | – | – | – | – | – | – | – | 5 | – | 2 | – | 9 | – | – | – | – | – |
| Palaeopsylla soricis | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 2 | – | 1 | – |
| Peromyscopsylla bidentata | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – |
| Total | 30 | 23 | 3 | 2 | 3 | 12 | 1 | 8 | 15 | 4 | 2 | 4 | 22 | 6 | 3 | 2 | 4 | 3 |
Factors influencing diversity of fleas
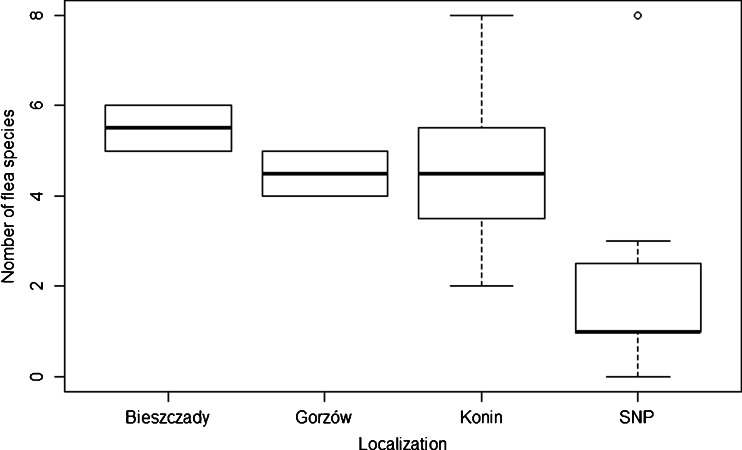
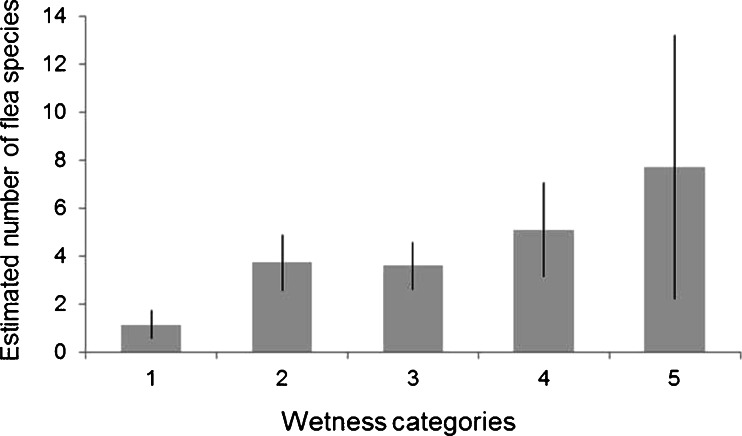
The final model in analysis (1) revealed in backward procedure included only latitude as an explanatory variable and the effect of increasing latitude on flea species richness was negative (z 4,18 = −2.50, p = 0.01; Fig. 2). In the case of analysis (2), the final model included habitat wetness and habitat fertility as an explanatory variables. The differences in flea species number between habitats of different wetness categories were significant with increasing number of flea species along with the increasing humidity of habitat (Fig. 3). We found no pattern in relationship between number of flea species and habitat fertility.
Fig. 2.
Number of flea species in different geographical localizations (with Bieszczady being outermost South and SNP farthest North). Boxes denote 25th, 50th, and 75th percentiles; whiskers represent the lowest and highest datum within the 1.5 interquartile range of the lower and upper quartile
Fig. 3.
Number of flea species in habitats of different wetness categories. The differences between category 1 and categories 3 and 4 are significant (p < 0.05), and the difference between categories 1 and 2 is approaching significance (p = 0.08). Whiskers indicate standard errors
The alpha diversity (species richness) for western Poland was 10, 13 for central, and 7 for eastern Poland. We found a low similarity of flea species/subspecies between western and eastern Poland (beta diversity = 11) and between central and eastern Poland (beta diversity = 12). The flea species composition of western and central Poland was more similar (beta diversity = 5; Table 3).
Table 3.
Alpha and beta diversity for flea species/subspecies infesting small mammals in western (Słowiński National Park and Gorzowska Forest), central (Konin lakes area), and eastern (Bieszczady Mountains) Poland
| Flea species/subspecies | Western Poland | Central Poland | Eastern Poland |
|---|---|---|---|
| Ctenophthalmus agyrtes agyrtes | x | x | |
| Ctenophthalmus agyrtes kleinschmidtianus | x | ||
| Ctenophthalmus agyrtes peusianus | x | ||
| Ctenophthalmus assimilis | x | ||
| Ctenophthalmus bisoctodentatus | x | x | |
| Ctenophthalmus solutus | x | x | |
| Ctenophthalmus uncinatus | x | x | x |
| Doratopsylla dasycnema cuspis | x | ||
| Doratopsylla dasycnema dasycnema | x | x | |
| Hystrichopsylla talpae orientalis | x | ||
| Hystrichopsylla talpae talpae | x | x | |
| Megabothris turbidus | x | x | x |
| Megabothris walkeri | x | x | |
| Nosopsyllus fasciatus | x | ||
| Palaeopsylla soricis | x | x | x |
| Peromyscopsylla bidentata | x | x | |
| Peromyscopsylla silvatica | x | ||
| Alpha diversity | 10 | 13 | 7 |
| Beta diversity | Western vs. central Poland: 5 | Central vs. eastern Poland: 12 | Western vs. eastern Poland: 11 |
Discussion
In accordance with our predictions, we recorded the inverse relationship between latitude and the number of flea species. In the same time, we found no effect of flea abundance, small mammal abundance, and number of small mammal species on number of flea species recorded. Overall, a well-known rule of biogeography is that the biodiversity of plant and animal species decreases with the increase of the distance from the equator (Rohde 1992; Rosenzweig 1992; Krasnov et al. 2004; Pavoine and Bonsall 2011). On the other hand, Krasnov et al. (2007) reported that the number of flea species in the region increases with the average elevation. This is likely not caused directly by the variation in altitude, but rather the presence of mountains, which presumably provide a variety of environments within the region, possibly resulting in a higher number of flea species. This relationship has been confirmed for Palaeoarctic realm (including Tatry Mountains), where they conducted their research, but have not been confirmed for Nearctic realm. Generally, this relationship is concerned for ectoparasites, which are susceptible to environmental factors that vary with the latitude (Krasnov et al. 2007). In contrast, endoparasites due to their stable habitat (inside the host body) do not show this relationship (Rohde and Heap 1998). Our studies were conducted in the Palearctic realm, and two study plots were located in the mountains. So, the high number of flea species in the Bieszczady Mountains can be explained by the availability of more diverse environments in this region. In addition, in Poland, we can find the northwestern limits of the geographic ranges of several flea species, which may also result in a high number of species in the southeastern part of the country (Bartkowska 1977). On the Baltic coast, the most abundant flea was Megabothris walkeri reaching the southern range in Poland. In the Bieszczady Mountains, we found two flea subspecies which reach their western geographic ranges in this region: Hystrichopsylla talpae orientalis and Doratopsylla dasycnema cuspis. Other authors also recorded these fleas in mountain areas (Skuratowicz 1967; Bartkowska 1973, 1977).
Nevertheless, the number of flea species not always is lower at higher latitudes. Krasnov et al. (2004) found a positive correlation between latitude and the number of flea species in the Palearctic realm. They suggest that the reason may be many species of mammals living in regions of temperate climate inhabit deeper and more permanent burrows than those of mammals from warmer regions, which in turn are more preferred by fleas (Krasnov et al. 2004, 2006a, 2010a) because they spend a large part of their life in the host nest. Our research was conducted only in Poland, i.e., only under temperate climate, so generally most small mammals living here can possess deeper burrows than mammals living in tropical regions. However, sandy grounds on the Baltic coast likely hinder digging deep burrows (Haitlinger 1972), which could result in observed lower number of flea species in the north.
There are some proofs that after the last glaciation, Poland was re-colonized by small mammals and their ectoparasites from different (western and eastern) refugees (Michaux et al. 2004, 2005; Deffontaine et al. 2005; Wójcik et al. 2010). Therefore, we expected differences in species composition between flea communities in western and eastern Poland. The results of our study confirmed our predictions. We found a low similarity between western and eastern Poland (11 species and subspecies). In the Bieszczady Mountains, we found four flea species/subspecies (H. talpae orientalis, Ctenophthalmus agyrtes kleinschmidtianus, Ctenophthalmus bisoctodentatus, and Doratopsylla dasycnema cuspis) which were absent in western Poland. Hystrichopsylla talpae orientalis, D. dasycnema cuspis, and C. agyrtes kleinschmidtianus reach their western geographic ranges in this region of Poland. In turn, seven flea species/subspecies were found only in western Poland. We found Peromyscopsylla silvatica only in western Poland, but Bartkowska (1973) found it in Tatry Mountains (southern Poland). In turn, Skuratowicz (1967) indicates that this species occurs in Pomeranian region. Similarly, we reported a low similarity of flea species between central and eastern Poland (12 species/subspecies).
Aulak (1970) indicates that usually fauna of small mammals is richer in fertile and wet habitats. Thus, we supposed that a greater number of flea species could be recorded in fertile and wet habitats and lower in poor and arid habitats. The results of our study confirmed our predictions about the relationship between wetness of habitat and the number of flea species. However, this was not due to the increased number of host species or host abundance on richer habitats as we found no effect of habitat fertility or habitat wetness on number of small mammal species recorded or on small mammal abundance. The increased number of flea species recorded in more wet habitats could be due to the fact that humidity has a strong influence on the development of flea larvae (Skuratowicz 1967, Krasnov et al. 2006a) and relative humidity within burrows is one of the main factors influencing flea development and survival (Krasnov et al. 2001, Osacar-Jimenez et al. 2001). Therefore, more humid habitats could be suitable for bigger number of flea species than arid ones. On the other hand, we found no relationship between richness of habitat and the number of flea species.
Short term of our study undoubtedly had a negative impact on the number of captured mammals and collected fleas. It is probable that, with larger sample sizes and sampling in more localities, some of our predictions and observed trends would obtain stronger support. So, further studies are required to accurately determine the differentiation in flea species composition of small mammals in selected regions of Poland. Due to the fact that in Poland fleas spend the most of their time in host nests (Skuratowicz 1967; Krasnov et al. 2010a), fleas should be collected from both small mammals captured in live traps as well as from host nests. Additionally, it would be desirable to investigate more study plots in Pomerania region and highlands in order to increase the range of data allowing for determination of the impact of both latitude and longitude on flea communities.
Acknowledgments
We are very grateful to A. Stachowiak, P. Kardynia, N. Osten-Sacken, D. Matuszyk, and W. Eichert for help in field works; to K. Woźniak (director of SNP), I. Izydorek, and D. Staniaszek (from SNP) for allowing us to conduct our studies in the Park; to S. Pagacz and J. Witczuk for allowing us to conduct studies near Lutowiska village; and to R. Zwolak and S. Dziemian for access to research material collected in Gorzowska Forest. Our special thanks go to A. Brzeg and M. Wojterska for the botanical consultations, to B. Piłacińska for the help in determining some rodents, to R. Bajaczyk for the help in determining some flea species, and to S. von Merten and R. Zwolak for the advice and their comments on the manuscript. The research was supported by grant no. SFRH/BD/ 31602/2006 from the Science and Technology Foundation (Portuguese Ministry of Science, Technology and Higher Education) and the budget of the Department of Systematic Zoology (Faculty of Biology AMU, Poznań).
Ethical standards
All handling of animals was done with permissions received from the Local Ethical Committee for the Animal Experiments in Poznań, the General Director for Environmental Protection, and from the Polish Minister of the Environment.
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix
References
- Aulak W. Small mammal communities of the Białowieża National Park. Acta Theriol. 1970;15:465–515. doi: 10.4098/AT.arch.70-32. [DOI] [Google Scholar]
- Bartkowska K. Siphonaptera Tatr Polskich. Fragm Faun. 1973;19:227–283. [Google Scholar]
- Bartkowska K. Z badań nad Siphonaptera w Beskidach Zachodnich. Wiad Parazytol. 1977;23:219–220. [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B (2011) lme4: linear mixed-effects models using S4 classes. R package version 0.999375-38. http://CRAN.R-project.org/package=lme4. Accessed 25 Oct 2013
- Deffontaine V, Libois R, Kotlik P, Sommer R, Nieberding C, Paradis E, Searle JB, Michaux JR. Beyond the Mediterranean peninsulas: evidence of central European glacial refugia for a temperate forest species, the bank vole (Clethrionomys glareolus) Mol Ecol. 2005;14:1727–1739. doi: 10.1111/j.1365-294X.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- Haas GE, Walton DW. Fleas (Siphonaptera) infesting small mammals from the Western Oriental Region. Korean J Parasitol. 1973;11:102–107. doi: 10.3347/kjp.1973.11.2.102. [DOI] [PubMed] [Google Scholar]
- Haitlinger R. Drobne ssaki bezleśnych wydm nadmorskich i ich fauna pcheł. Prz Zool. 1972;16:231–237. [Google Scholar]
- Harrison I, Laverty M, Sterling E (2004) Alpha, beta, and gamma diversity. http://cnx.org/content/m12147/1.2/. Accessed 29 Jul 2004
- Karbowiak G, Rychlik L, Nowakowski W, Wita I. Natural infections of small mammals with blood parasites on the borderland of boreal and temperate forest zones. Acta Theriol. 2005;50:31–42. doi: 10.1007/BF03192616. [DOI] [Google Scholar]
- Klimpel S, Förster M, Schmall G. Parasites of two abundant sympatric rodent species in relation to host phylogeny and ecology. Parasitol Res. 2007;100:867–875. doi: 10.1007/s00436-006-0368-8. [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV. Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med Vet Entomol. 2001;15:249–258. doi: 10.1046/j.0269-283x.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Shenbrot GI, Khokhlova IS, Degen AA. Flea species richness and parameters of host body, host geography and host ‘milieu’. J Anim Ecol. 2004;73:1121–1128. doi: 10.1111/j.0021-8790.2004.00883.x. [DOI] [Google Scholar]
- Krasnov BR, Shenbrot GI, Khokhlova IS, Poulin R. Is abundance a species attribute? An example with haematophagous ectoparasites. Oecologia. 2006;150:132–140. doi: 10.1007/s00442-006-0498-9. [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Stanko M, Miklisova D, Morand S. Habitat variation in species composition of flea assemblages on small mammals in central Europe. Ecol Res. 2006;21:460–469. doi: 10.1007/s11284-005-0142-x. [DOI] [Google Scholar]
- Krasnov BR, Shenbrot GI, Khokhlova IS, Poulin R. Geographical variation in the ‘bottom-up’ control of diversity: fleas and their small mammalian hosts. Glob Ecol Biogeogr. 2007;16:179–186. doi: 10.1111/j.1466-8238.2006.00273.x. [DOI] [Google Scholar]
- Krasnov BR, Matthee S, Lareschi M, Korallo-Vinarskaya NP, Vinarski MV. Co-occurrence of ectoparasites on rodent hosts: null model analyses of data from three continents. Oikos. 2010;119:120–128. doi: 10.1111/j.1600-0706.2009.17902.x. [DOI] [Google Scholar]
- Krasnov BR, Mouillot D, Shenbrot GI, Khokhlova IS, Vinarski MV, Korallo-Vinarskaya NP, Poulin R. Similarity in ectoparasite faunas of Palaearctic rodents as a function of host phylogenetic, geographic or environmental distances: which matters the most? Int J Parasitol. 2010;40:807–817. doi: 10.1016/j.ijpara.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Laakkonen J. Microparasites of three species of shrews from Finnish Lapland. Ann Zool Fenn. 2000;37:37–41. [Google Scholar]
- Marshall AG. The ecology of ectoparasitic insects. London: Academic; 1981. [Google Scholar]
- Michaux JR, Libois R, Paradis E, Filippucci MG. Phylogeographic history of the yellow-necked fieldmouse (Apodemus flavicollis) in Europe and in the Near and Middle East. Mol Phylogenet Evol. 2004;32:788–798. doi: 10.1016/j.ympev.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Michaux JR, Libois R, Filippucci MG. So close and so different: comparative phylogeograpgy of two small mammal species, the Yellow-necked fieldmouse (Apodemus flavicollis) and the Woodmouse (Apodemus sylvaticus) in the Western Palearctic region. Hered. 2005;94:52–63. doi: 10.1038/sj.hdy.6800561. [DOI] [PubMed] [Google Scholar]
- Nieberding CM, Durette-Desset MC, Vanderpoorten A, Casanova JC, Ribas A, Deffontaine V, Feliu C, Morand S, Libois R, Michaux JR. Geography and host biogeography matter for understanding the phylogeography of a parasite. Mol Phylogenet Evol. 2008;47:538–554. doi: 10.1016/j.ympev.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Oguge NO, Durden LA, Keirans JE, Balami HD, Schwan TG. Ectoparasites (sucking lice, fleas, ticks) of small mammals in southeastern Kenya. Med Vet Entomol. 2009;23:387–392. doi: 10.1111/j.1365-2915.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osacar-Jimenez JJ, Lucientes-Curdi J, Calvete-Margolles C. Abiotic factors influencing the ecology of wild rabbit fleas in north-western Spain. Med Vet Entomol. 2001;15:157–166. doi: 10.1046/j.1365-2915.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- Paramasvaran S, Sani RA, Hassan L, Krishnasamy M, Jeffery J, Oothuman P, Salleh I, Lim KH, Sumarni MG, Santhana RL. Ectoparasite fauna of rodents and shrews from habitats in Kuala Lumpur and the states of Selangor and Negeri Sembilan, Malaysia and its public health significance. Trop Biomed. 2009;26:303–311. [PubMed] [Google Scholar]
- Pawelczyk A, Bajer A, Behnke JM, Gilbert FS, Siński E. Factors affecting the component community structure of haemoparasites in common voles (Microtus arvalis) from the Mazury Lake District region of Poland. Parasitol Res. 2004;92:270–284. doi: 10.1007/s00436-003-1040-1. [DOI] [PubMed] [Google Scholar]
- Pavoine S, Bonsall MB. Measuring biodiversity to explain community assembly: a unified approach. Biol Rev. 2011;86:792–812. doi: 10.1111/j.1469-185X.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- Poulin R, Valtonen T. The predictability of helminth community structure in space: a comparison of fish populations from adjacent lakes. Int J Parasitol. 2002;32:1235–1243. doi: 10.1016/S0020-7519(02)00109-1. [DOI] [PubMed] [Google Scholar]
- Rödl P. Investigation of the transfer of fleas among small mammals using radioactive phosphorus. Folia Parasitol. 1979;26:265–274. [PubMed] [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos. 1992;65:514–527. doi: 10.2307/3545569. [DOI] [Google Scholar]
- Rohde K, Heap M. Latitudinal differences in species and community richness and in community structure of metazoan endo- and ectoparasites of marine teleost fish. Int J Parasitol. 1998;28:461–474. doi: 10.1016/S0020-7519(97)00209-9. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ML. Species diversity gradients: we know more and less than we thought. J Mammal. 1992;73:715–730. doi: 10.2307/1382191. [DOI] [Google Scholar]
- Rychlik L. Evolution of social systems in shrews. In: Wójcik JM, Wolsan M, editors. Evolution of shrews. Białowieża: Mammal Research Institute, Polish Academy of Sciences; 1998. pp. 347–406. [Google Scholar]
- Skuratowicz W (1967) Klucze do oznaczania owadów Polski. Część XXIX. Pchły - Siphonaptera (Aphaniptera). Polskie Towarzystwo Entomologiczne, Państwowe Wydawnictwo Naukowe, Warszawa
- Whittaker RH. Evolution and measurement of species diversity. Taxon. 1972;21:213–251. doi: 10.2307/1218190. [DOI] [Google Scholar]
- Wójcik JM, Kawałko A, Markova S, Searle JB, Kotlik P. Phylogeographic signatures of northward post-glacial colonization from high-latitude refugia: a case study of bank voles using museum specimens. J Zool. 2010;281:249–262. [Google Scholar]
- Zuo XH, Guo XG, Zhan YZ, Wu D, Yang ZH, Dong WG, Huang LQ, Ren TG, Jing YG, Wang QH, Sun XM, Lin SJ. Host selection and niche differentiation in sucking lice (Incecta: Anoplura) among small mammals in southwestern China. Parasitol Res. 2011;108:1243–1251. doi: 10.1007/s00436-010-2173-7. [DOI] [PubMed] [Google Scholar]