Abstract
Urticaria has a documented association with the prodromal phases of hepatitis A, B and, although still contentious, likely hepatitis C. Despite the documented association there are few actual reported cases of urticaria occurring with hepatitis A infection and in all of the cases reported so far the urticaria preceded the diagnosis of hepatitis A and was acute rather than chronic. We describe a case of urticaria occurring following acute infection with hepatitis A, which persisted beyond 6 weeks and therefore was by definition chronic. Although chronic urticaria has been reported to be associated with other forms of viral hepatitis, to the best of our knowledge this has not been reported previously with hepatitis A.
Background
Urticaria is a ubiquitous syndrome that occurs quite commonly and is associated with a range of aetiological factors, including autoimmune conditions and infections. It is characterised by pruritic elevated erythematous lesions, described as wheals, that can occur anywhere on the skin and range in size from millimetres to large confluent areas. Urticaria has a documented association with the prodromal phases of hepatitis A, B and, although still contentious, hepatitis C as well.1–4 Despite the documented association there are few actual reported cases of urticaria occurring with hepatitis A infection; 2 patients of 130 in a foodborne outbreak in Navy recruits in 1974,5 a single case in 27-year-old Caucasian homosexual man6 and in two paediatric patients.7 In all of these cases the urticaria preceded the diagnosis of hepatitis A and was acute rather than chronic.
We describe a case of urticaria occurring following acute infection with hepatitis A which persisted beyond 6 weeks and therefore was by definition chronic. Although chronic urticaria has been reported to be associated with other forms of viral hepatitis, to the best of our knowledge this has not been reported previously with hepatitis A.
Case presentation
A middle-aged woman was referred to hospital by her general practitioner, with a 10 day history of fevers, rigours, nausea, lethargy and malaise. She had returned 1 month beforehand from a holiday to Egypt and Turkey. She had been well during her trip, except for a brief episode of diarrhoea. The patient had not had any pretravel vaccinations.
There was no significant medical history, and no significant findings on initial clinical examination. Liver function tests were deranged, with prominent transaminitis: bilirubin 44 (2–20), alkaline phosphatase 203 (30–115), γ-glutamyl transpeptidase 286 (0–45), alanine transaminase (ALT) 4090 (0–45), aspartate transaminase (AST) 3810 (0–41) and lactate dehydrogenase 1440 (80–250). Initial coagulation profile showed a prothrombin time of 22 (12–15), international normalised ratio 1.8 (0.9–1.2) and activated partial thromboplastin time 44 (26–41).
Serological testing ordered by her general practitioner on the morning of her admission revealed a positive IgM and IgG for hepatitis A. Testing on admission to hospital for hepatitis B, C and E was negative. Negative serology was also obtained for Entamoeba histolytica and salmonella.
Ultrasound of her liver was reported as ‘normal with no focal lesion or biliary dilatation’.
She was admitted for supportive management with intravenous fluids and antiemetics.
The patient's liver function tests continued to deteriorate, with a peak ALT of 6690 and AST of 5000 on day 2 of her admission. Jaundice developed on day 4 of admission and deepened over the following 3 days. Her bilirubin climbed to 198 on the final day of admission. The patient was able to maintain an oral intake on day 6, and reported feeling much improved. She was discharged on day 7. Neither during the prodrome nor during admission, were there any cutaneous manifestations of disease other than jaundice.
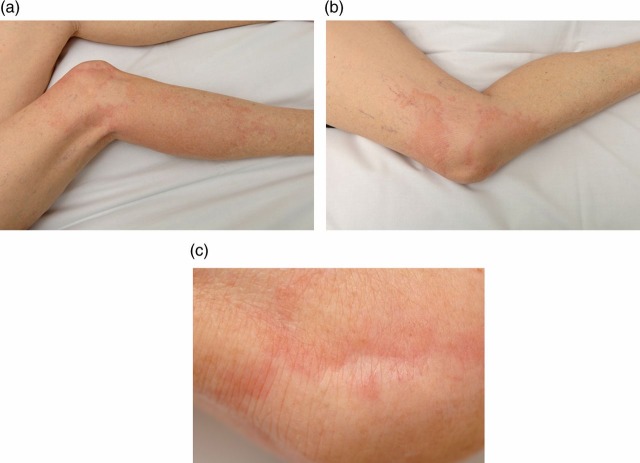
When reviewed in outpatient clinic 10 days after discharge, the patient had developed prominent patchy urticaria of both legs and arms (see figure 1A–C). Symptom control was achieved with regular over-the-counter oral antihistamines.
Figure 1.
(A–C) Photographs of urticaria affecting the lower limbs of a patient following confirmed hepatitis A infection.
Further history did not reveal any prior history of urticaria, allergy or atopy or any autoimmune conditions, nor was there a family history of the same.
Outcome and follow-up
The urticaria improved gradually over the next 8–10 weeks, though lethargy persisted. Autoimmune screening, including erythrocyte sedimentation rate, C reactive protein, antinuclear antibody, extractable nuclear antigen, antineutrophil cytoplasmic antibodies (P-ANCA and C-ANCA), double-stranded DNA and IgE were all within normal limits. When reviewed in clinic approximately 5 months after discharge, all symptoms had resolved and liver function tests had normalised.
Discussion
Urticaria is a common problem affecting up to 25% of the population at some point in their lifetime.8 Pruritic wheals are the result of degranulation of mast cells and basophils, with release of inflammatory mediators predominantly histamine. There are many factors associated with the development of urticaria, including reactions to food and food additives, medications, autoimmune and connective tissue diseases and infections. Although infections have been implicated as a cause for urticaria for more than 100 years, the pathogenesis of mast cell activation in response to infection remains to be elucidated.9 There is mounting evidence that infection with Helicobacter pylori is associated with chronic urticaria, and that successful treatment of this infection can result in the resolution of urticaria.10 11 Other organisms implicated in chronic urticaria include many bacterial species such as Streptococcus, Staphylococcus and Yersinia, and parasites including Blastocystis hominis and Giardia lamblia.11
Hepatitis B is an established cause of chronic urticaria.12 The link between hepatitis C and urticaria remains tenuous, with many authors reporting it as a cause,4 13 8 while many others refute the association.14 15
We were able to identify a total of five cases of urticaria associated with hepatitis A in the medical literature.5–7 All occurred in the prodromal period of infection, and were acute rather than chronic, as opposed to the chronic urticaria occurring postinfection reported in this case. None of these cases included any images of the cutaneous manifestations to allow future comparisons, so our case report is unique in this regard also. In a large case series attributed to a foodborne outbreak, as many as 14% of subjects infected with hepatitis A demonstrated evanescent rashes immediately prior to or during the acute manifestations of the infection,5 although once again no images were included in this article.
Similar manifestations occurring during the prodromal period of hepatitis B infection have been speculated to be due to circulating immune complexes formed by viral antigens and antibodies,16 and one could speculate about a similar mechanism in this case. The possibility that circulating immune complexes do occur during hepatitis A infection is supported by a number of observations, including the detection of serum anticomplementary activity in early acute phase serum,5 17 18 the finding of the virus and anti-hepatitis A antibodies in the same acute phase specimen,19 demonstration of depressed serum complement levels,20 and the deposition of hepatitis A virus in glomeruli in experimental animals.19 Such a phenomenon could also potentially explain other extrahepatic manifestations that have been reported with hepatitis A, including arthralgia in as many as 10% of cases reported during a large outbreak.5
Ideally, further investigation could have been undertaken to ascertain a potential mechanism of urticaria and firmly link the urticaria to the acute hepatitis A infection. However, this was unable to be undertaken for a number of reasons. First, our patient's symptoms although quite concerning initially, were managed effectively with simple antihistamines so she was reluctant to submit to further investigation, particularly anything invasive including a skin biopsy. In addition, the uniqueness of the case was not fully recognised for some time and once it was there were no stored serum samples available for further testing, and the patient declined further phlebotomy.
As this is yet to be reported in the literature, it could be that urticaria may occur as a sequel to hepatitis A infection more frequently than the lack of reports suggests, and it may be under-recognised as a cause for this very common condition. Given this report, it may well be that the association is recognised in future cases, and if so the opportunity may arise to investigate further and potentially elucidate a mechanism to explain this phenomenon.
Learning points.
Urticaria is relatively common and there are a number of associated aetiologies including a variety of infections.
There is a documented association with urticaria and hepatitis A, B and likely hepatitis C; however, the association is with the prodromal phases of the viral infection, is acute rather than chronic urticaria and particularly with hepatitis A, there are few case reports in the literature.
We herein report a case of chronic urticaria occurring following acute infection with hepatitis A which to the best of our knowledge has not been previously reported and may represent an as yet unrecognised cause for this relatively common condition.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Cribier B. Urticaria and hepatitis. Clini Rev Allergy Immunol 2006;30:25–9. [DOI] [PubMed] [Google Scholar]
- 2.Parsons ME, Russo GG, Millikan LE. Dermatologic disorders associated with viral hepatitis infections. Int J Dermatol 1996;35:77––81.. [DOI] [PubMed] [Google Scholar]
- 3.McElgunn PS. Dermatologic manifestations of hepatitis B virus infection. J Am Acad Dermatol 1983;8:539––48.. [DOI] [PubMed] [Google Scholar]
- 4.Reichel M, Mauro TM. Urticaria and hepatitis C. Lancet 1990;336:822–3. [DOI] [PubMed] [Google Scholar]
- 5.Routenberg JA, Dienstag JL, Harrison WO, et al. Foodborne outbreak of hepatitis A: clinical and laboratory features of acute and protracted illness. Am J Med Sci 1979;278:123–37. [PubMed] [Google Scholar]
- 6.Scully LJ, Ryan AE. Urticaria and acute hepatitis A virus infection. Am J Gastroenterol 1993;88:277–8. [PubMed] [Google Scholar]
- 7.Dollberg S, Berkun Y, Gross-Kieselstein E. Urticaria in patients with hepatitis A virus infection. Pediatr Infect Dis J 1991;10:702–3. [DOI] [PubMed] [Google Scholar]
- 8.Siddique N, Pereira BN, Hasan Arshad S. Hepatitis C and urticaria: cause and effect? Allergy 2004;59:668. [DOI] [PubMed] [Google Scholar]
- 9.Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol 2004;4:387–96. [DOI] [PubMed] [Google Scholar]
- 10.Federman DG, Kirsner RS, Moriarty JP, et al. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol 2003;49:861–4. [DOI] [PubMed] [Google Scholar]
- 11.Wedi B, Raap U, Wieczorek D, et al. Urticaria and infections. Allergy, Asthma, Clin Immunol 2009;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaida GA, Goldman MA, Bloch KJ. Testing for hepatitis B virus in patients with chronic urticaria and angioedema. J Allergy Clin Immunol 1983;72:193–8. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa K, Yaoita H, Tsuda F, et al. Hepatitis C virus infection in patients with urticaria. J Am Acad Dermatol 1996;35(2 Pt 1):195–8. [DOI] [PubMed] [Google Scholar]
- 14.Smith R, Caul EO, Burton JL. Urticaria and hepatitis C. Br J Dermatol 1997;136:980. [DOI] [PubMed] [Google Scholar]
- 15.Llanos F, Raison-Peyron N, Meunier L, et al. Hepatitis C virus infection in patients with urticaria. J Am Acad Dermatol 1998;38:646. [DOI] [PubMed] [Google Scholar]
- 16.Dienstag JL, Rhodes AR, Bhan AK, et al. Urticaria associated with acute viral hepatitis type B: studies of pathogenesis. Ann Intern Med 1978;89:34–40. [DOI] [PubMed] [Google Scholar]
- 17.Provost PJ, Ittensohn OL, Villarejos VM, et al. A specific complement-fixation test for human hepatitis a employing CR326 virus antigen. Diagnosis and epidemiology. Proc Soc Exp Biol Med 1975;148:962–9. [DOI] [PubMed] [Google Scholar]
- 18.Chang LW, O'Brien TF. Australia antigen serology in the Holy Cross football team hepatitis outbreak. Lancet 1970;1:59–61. [DOI] [PubMed] [Google Scholar]
- 19.Mathiesen LR, Drucker J, Lorenz D, et al. Localization of hepatitis A antigen in marmoset organs during acute infection with hepatitis A virus. J Infect Dis 1978;138:369–77. [DOI] [PubMed] [Google Scholar]
- 20.Baer GM, Walker JA. Studies of an outbreak of acute hepatitis A: I. Complement level fluctuation. J Med Virol 1977;1:1–7. [DOI] [PubMed] [Google Scholar]