Abstract
Background
Synthetic cannabinoids are touted as legal alternatives to cannabis, at least when first released, and routine urine cannabinoid screening methods do not detect these novel psychoactive substances. Synthetic cannabinoids are widely available, are a major public health and safety problem, and a difficult challenge for drug testing laboratories. We evaluated performance of the NMS JWH-018 direct ELISA kit to sensitively, selectively, and rapidly screen urinary synthetic cannabinoids.
Materials/ Methods
The NMS ELISA kit targeting the JWH-018 N-(5-hydroxypentyl) metabolite was utilized to screen 2492 urine samples with 5 and 10µg/L cutoffs. A fully validated LC-MS/MS method for 29 synthetic cannabinoids markers confirmed all presumptive positive and negative results. Performance challenges at ±25 and ±50% of cutoffs determined intra- and inter-plate imprecision around proposed cutoffs.
Result
The immunoassay was linear from 1–500µg/L with intra- and inter-plate imprecision of ≤8.2% and <14.0%, respectively. No interferences were present from 93 common drugs of abuse, metabolites, co-administered drugs, over-the-counter medications or structurally similar compounds, and 19 of 73 individual, synthetic cannabinoids (26%) exhibited moderate to high cross-reactivity to JWH-018 N-(5-hydroxypentyl) metabolite. Sensitivity, specificity, and efficiency results were 83.7%, 99.4% and 97.6% and 71.6%, 99.7% and 96.4%, with the 5 and 10µg/L urine cutoffs, respectively.
Conclusion
This high throughput immunoassay exhibited good diagnostic efficiency and documented that the NMS JWH-018 direct ELISA is a viable method for screening synthetic cannabinoids in urine targeting the JWH-018 N-(5-hydroxypentyl) and related analytes. Optimal performance was achieved with a matrix-matched 5µg/L urine cutoff.
Keywords: Synthetic cannabinoids, ELISA, immunoassay
1. Introduction
Synthetic cannabinoids are marketed as natural, herbal mixtures not for human consumption, but are abused as novel psychoactive substances. Synthetic cannabinoids are touted as legal alternatives to cannabis, at least when first released, and are not detected in routine urine drug screening methods, making them attractive to those subject to urine drug tests1. Synthetic cannabinoids bind to the same CB1 and CB2 cannabinoid receptors as Δ9-tetrahydrocannabinol (THC)2. CB1 agonists primarily located in the central nervous system are responsible for psychotropic effects, while CB2 receptor agonists are important to immune function and analgesia3. Some synthetic cannabinoids have greater affinity for CB1 receptors as compared to THC, resulting in more intense and prolonged reactions4,5. The 2012 DAWN Report, U.S. Substance Abuse and Mental Health Services Administration (SAMHSA), stated that synthetic cannabinoids were responsible for 28,500 emergency room visits in 20116. The inconsistent composition and potency of synthetic cannabinoids herbal mixtures contributes to reported unpredictable effects. Reported adverse effects include seizures, psychosis, paranoia, altered mental status, intense anxiety, hallucinations, increased blood pressure and heart rates, panic attacks, nausea and vomiting7–11.
In response to these adverse effects and high abuse potential, the Synthetic Drug Abuse Prevention Act was passed in July 2012. While 26 synthetic compounds are currently classified as Schedule I drugs under the Controlled Substances Act, cannabimimetic agents, any chemical that mimics cannabis’ effects via CB1 receptors, also are banned12. This led to the rapid development and introduction of novel psychoactive substances with modified chemical structures.
Synthetic cannabinoids are an increasingly popular recreational drug13. According to the 2012 Monitoring The Future survey, 11.4% of 12th graders used synthetic cannabinoids in the last year; making it the second most popular illegal drug among American teenagers, after cannabis14. A similar trend also was noted among young adults. In a sampling of students (n=852) from a large American university, 8% reported smoking synthetic cannabinoids at least once in their lifetime15.
As a result of this recent popularity, several qualitative16–21 and quantitative22–26 methods were developed utilizing liquid chromatography tandem mass spectrometry (LC-MS/MS) to detect one or more synthetic cannabinoids in urine. Gas chromatography mass spectrometry (GC-MS)17, 18 and tandem MS (GC-MS/MS)19 methods also were published.
Identification and quantification of synthetic cannabinoids and their metabolites in biological specimens is an ongoing challenge for laboratories, as their constantly changing structures require new antibody production for commercial immunoassays to stay current. Mass spectrometric analytical methods for identification and confirmation are hampered by lack of commercially available reference standards. Furthermore, these methods must be updated and validated to include the latest synthetic cannabinoids, creating a cycle where drug detection lags behind the newly emerging synthetic cannabinoids. Griffiths et al. commented that synthetic cannabinoids are a transient product that is challenging the existing models of drug control27.
A rapid, inexpensive screening procedure for synthetic cannabinoids in multiple biological matrices is important for clinical, forensic, drug treatment, driving under the influence, and workplace drug testing programs. Enzyme Linked Immunosorbent Assays (ELISA) screening techniques are flexible, easily automated and have the required sensitivity and specificity that has made this an increasingly popular option for drug testing laboratories28–30.
The aim of this study was to evaluate the National Medical Services (NMS) JWH-018 direct ELISA kit as a screening method for the detection of synthetic cannabinoids in 2492 urine samples. All presumptive positive and negative results were confirmed by a validated LC-MS/MS method for 29 synthetic cannabinoid markers to determine sensitivity, specificity and efficiency.
2. Materials and Methods
2.1 ELISA Instrumentation
The qualitative, automated analysis of synthetic cannabinoids was performed on a Freedom EVO 100 platform configured with a microtiter plate washer and reader. (Tecan Group, San Jose, CA). The Freedom EVO 100 is a flexible liquid handling system for low to medium throughput applications. The 8-channel liquid handling arm and robotic manipulator arm enhance workstation capabilities allowing for fully automated ELISA plate processing.
2.2 NMS Synthetic Cannabinoids Microplate ELISA Kit
The NMS JWH-018 direct ELISA kit contained all components required for analysis with the exception of calibrators and controls. Each kit contained coated 96-well microtiter plates, drug enzyme conjugate, tetramethylbenzidine (TMB) substrate, 0.2N HCl stop solution, and Phosphate Buffered Saline (PBS). The assay was performed without modification according to the manufacturer’s instructions. All kit components were stored at 4°C until analysis.
Briefly, a 20µL aliquot (blank, cutoff, control or unknown) and 100µL drug enzyme conjugate were added to the coated microtiter plate and incubated at room temperature for 60min. After incubation, the plate was washed five times with 300µL PBS using an automatic plate washer. After washing, plates were manually inverted and slapped dry to remove residual liquid from the wells. The liquid handling arm added 100µL TMB substrate to initiate a colorimetric reaction. After 30 min, 100µL acid stop solution was added and the absorbance (450nm) immediately measured with a plate reader. Total analysis time was approximately 2h.
2.3 Calibrators, Controls and Performance Challenges
JWH-018 N-(5-hydroxypentyl) metabolite was purchased from Cayman Chemicals (Ann Arbor, MI). Stock standard solutions of cutoff calibrators and controls were prepared from different ampoules by diluting with appropriate volumes of methanol. Cutoff calibrators (5 and 10µg/L) were prepared by fortifying drug-free urine and PBS with stock calibrator solution. Working performance challenges at ±25% and ±50% of each cutoff were prepared by fortifying drug-free urine with stock control solution. Positive controls at 10 and 20µg/L were prepared by fortifying drug-free urine with stock control solution to evaluate assay performance at 5 and 10µg/L.
2.4 Specimens
We tested 20,017 authentic anonymous urine specimens from the Department of Defense (DoD) drug testing laboratories, which previously screened negative for cannabinoids, cocaine, amphetamines, phencyclidine and opiates, during routine urinalysis testing. Specimens were collected from all around the world from July 2011 through June 2012 and were stored at room temperature before initial immunoassay analyses, as per DoD protocol. Specimens were analyzed with the Randox Drugs of Abuse V biochip array technology for synthetic cannabinoids. Presumptive positive (1,424) and randomly selected negative (1,068) specimens were stored at 4 – 7°C before this ELISA determination and LC-MS/MS confirmation. This project was funded under an inter-agency agreement between the DoD Drug Demand Reduction Initiative and the National Institute on Drug Abuse, National Institutes of Health.
2.5 Method Development
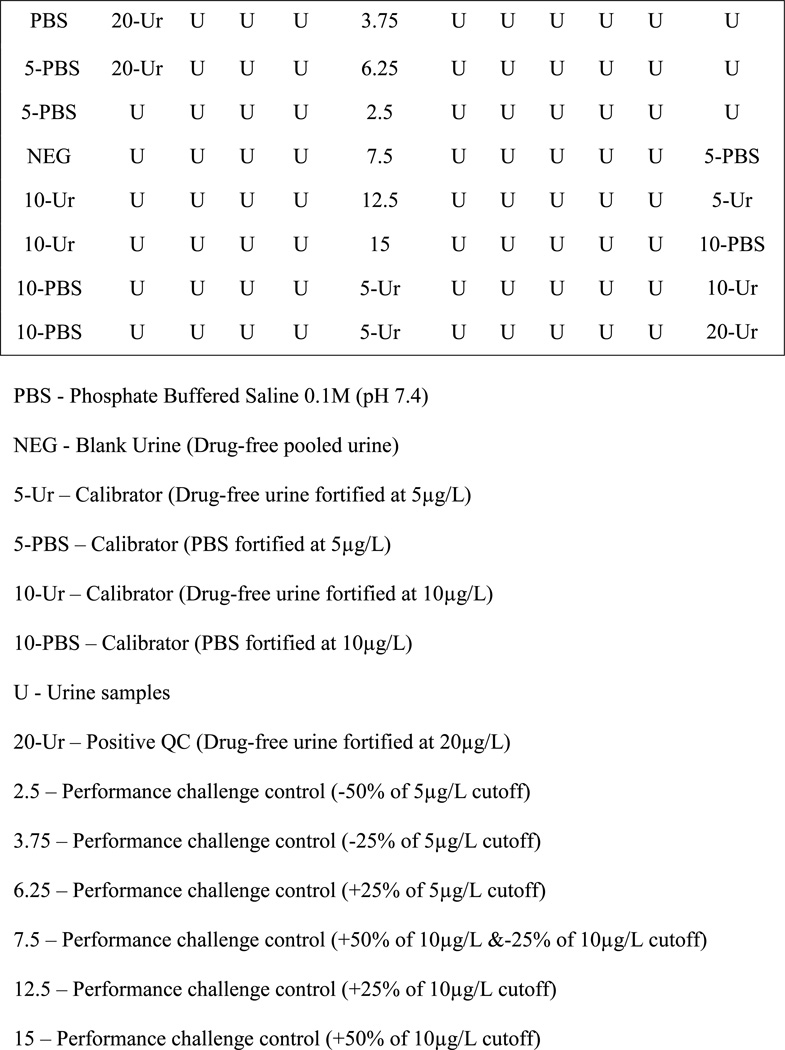
Analysis was performed according to the manufacturer’s instructions, although additional experiments were performed to optimize assay performance. The recommended cutoff calibrator was 5µg/L JWH-018 N-(5-hydroxypentyl) metabolite in PBS. Our plate configuration allowed evaluation of two cutoff concentrations (5 and 10µg/L) in two matrices, PBS and urine. Negative and positive (10 and 20µg/L) matrix matched urine controls were always run at the beginning and end of each plate; with urine performance challenges at ±25 and ±50% of each cutoff included in the middle to evaluate the assay’s ability to correctly classify concentrations near decision points (Figure 1). Single absorbances of each cutoff at the end of the plate were used to assess drift. Specimens were presumptive positive when absorbance at 450nm was less than or equal to that of the averaged cutoff calibrators in the first column of the plate.
Figure 1. Representative 96-well plate layout utilized in the validation of NMS direct ELISA kit for screening synthetic cannabinoids in urine targeting the JWH-018 N-(5-hydroxypentyl) metabolite.
PBS - Phosphate Buffered Saline 0.1M (pH 7.4)
NEG - Blank Urine (Drug-free pooled urine)
5-Ur – Calibrator (Drug-free urine fortified at 5µg/L)
5-PBS – Calibrator (PBS fortified at 5µg/L)
10-Ur – Calibrator (Drug-free urine fortified at 10µg/L)
10-PBS – Calibrator (PBS fortified at 10µg/L)
U - Urine samples
20-Ur – Positive QC (Drug-free urine fortified at 20µg/L)
2.5 – Performance challenge control (−50% of 5µg/L cutoff)
3.75 – Performance challenge control (−25% of 5µg/L cutoff)
6.25 – Performance challenge control (+25% of 5µg/L cutoff)
7.5 – Performance challenge control (+50% of 10µg/L &−25% of 10µg/L cutoff)
12.5 – Performance challenge control (+25% of 10µg/L cutoff)
15 – Performance challenge control (+50% of 10µg/L cutoff
2.6 Analytical Validation and Acceptance Criteria
The method was validated by determining limit of detection (LOD), linearity, intra- and inter-plate imprecision, inter-read imprecision, plate drift, cross-reactivity, interference, carryover and matrix effect.
The LOD was determined from absorbances of 9 negative urine samples from 9 drug-free volunteers. Mean absorbances and standard deviations were evaluated and LOD calculated by subtracting 3 times absorbance SD from the mean absorbance of the 9 negative urine samples (A0).
Linearity was investigated using a non-linear regression model and expressed as the coefficient of determination (r2). We initially evaluated linearity with triplicate analysis of the following JWH-018 N-(5-hydroxypentyl) concentrations: 0.25, 0.5, 1, 2.5, 5, 7.5, 10, 25, 50, 100, 250 and 500µg/L in pooled negative urine on three different days. Absorbances were plotted against concentration using an exponential (two phase decay) function. To characterize linearity, the natural logarithms of concentration and absorbance also were plotted.
In-house controls, prepared in pooled negative urine, were used to evaluate intra-plate imprecision at 1, 2.5, 5, 7.5 and 10µg/L JWH-018 N-(5-hydroxypentyl). Seven replicates of each control level were assayed on a single plate. For inter-plate imprecision, in-house performance challenges at ±25 (3.75 and 6.25µg/L) and ±50% (2.5 and 7.5µg/L) of the cutoff level (5µg/L) were assayed in singlicate on 35 plates over 2 weeks. Similarly, inter-plate absorbances also were monitored at ±25 and ±50% (5, 7.5, 12.5 and 15µg/L) of the 10µg/L cutoff. To monitor inter-read imprecision, one plate consisting of a full calibration curve (1–500µg/L) was read 7 times over 30 min to determine any variation in absorbance after addition of stop solution. Mean absorbances and standard deviations were calculated and imprecision expressed as percent coefficient of variation (%CV).
Variations in absorbance as a function of physical location on the 96-well plate (drift) were monitored across all plates (n=35). Duplicate absorbances for the 5 and 10µg/L cutoffs in PBS (column 1) and duplicate 10µg/L urine cutoff calibrators in column 1 were averaged and compared to singlicate absorbance determinations from column 12. Average absorbances for the 5µg/L urine cutoff (column 6) were compared to single absorbance determinations in column 12 using the formula; % Drift = [(Absorbance column 12 − Average cutoff absorbance)/ Average cutoff absorbance]*100.
Negative pooled urine samples were individually fortified with each available synthetic cannabinoid at 500µg/L and analyzed (Tables 1a & 1b). Single absorbances from 73 individual synthetic cannabinoids were compared with blank, negative and averaged 5µg/L urine calibrator (n=2) absorbances. Initial estimates of cross-reactivity were based upon these preliminary absorbances. Absorbances ≥ the negative sample were classified as null, while absorbances between the blank and the 5µg/L urine calibrator were said to have <1% cross reactivity. No further testing was performed on these compounds. However, if absorbances were <5µg/L calibrator, samples were diluted with negative urine and re-analyzed in duplicate for comparison against a calibration curve (1 – 250µg/L). Cross-reactivity (%) was calculated as 100*(apparent concentration from the calibration curve) / (analyte concentration).
Table 1.
| a. Synthetic cannabinoids (n=42) with <0.4% cross-reactivity to the NMS JWH-018 N-(5-hydroxypentyl) metabolite ELISA kit | ||
|---|---|---|
| Synthetic Cannabinoid |
Concentration (µg/L) |
Cross-reactivity % |
| JWH-203 | 500 | Negative |
| JWH-203 2-hydroxyindole metabolite | 500 | Negative |
| CP 47,497 | 500 | Negative |
| CP 47, 497 C7-hydroxy metabolite | 500 | Negative |
| CP 47, 497 C8 homolog | 500 | Negative |
| CP 47, 497 C8 homolog-C8 hydroxy metabolite | 500 | Negative |
| JWH-250 5-hydroxyindole metabolite | 500 | Negative |
| URB754 | 500 | Negative |
| JWH-018 2-hydroxyindole metabolite | 500 | Negative |
| AKB848 | 500 | Negative |
| JWH-007 | 500 | Negative |
| STS-135 | 500 | Negative |
| JWH-251 | 500 | Negative |
| JWH-018 adamantyl analog | 500 | Negative |
| JWH-018 adamantyl carboxamide | 500 | Negative |
| XRL-11 | 500 | Negative |
| HU-210 | 500 | <1 |
| UR-144 | 500 | <1 |
| UR-144 N-pentanoic acid metabolite | 500 | <1 |
| UR-144 N-(5-hydroxypentyl) metabolite | 500 | <1 |
| JWH-210 5-hydroxyindole metabolite | 500 | <1 |
| JWH-250 | 500 | <1 |
| JWH-250 N-pentanoic acid metabolite | 500 | <1 |
| JWH-250 N-(5-hydroxypentyl) metabolite | 500 | <1 |
| JWH-250 N-(4-hydroxypentyl) metabolite | 500 | <1 |
| JWH-210 | 500 | <1 |
| RCS-4 | 500 | <1 |
| RCS-4 N-pentanoic acid metabolite | 500 | <1 |
| RCS-8 | 500 | <1 |
| JWH-019 5-hydroxyindole metabolite | 100 | 0.1 |
| JWH-018 7-hydroxyindole metabolite | 100 | 0.1 |
| JWH-073 4-hydroxyindole metabolite | 100 | 0.1 |
| JWH-019 | 100 | 0.1 |
| JWH-398 | 100 | 0.1 |
| JWH-022 | 100 | 0.1 |
| AM2201 N-(4-fluoropentyl) isomer | 100 | 0.2 |
| JWH-081 | 100 | 0.2 |
| JWH-122 | 100 | 0.2 |
| RCS-4 N-(5-hydroxypentyl) metabolite | 100 | 0.3 |
| RCS-4 2 methoxy isomer | 100 | 0.3 |
| JWH-073 | 100 | 0.3 |
| JWH-015 | 100 | 0.4 |
| b. Synthetic cannabinoids (n=31) with >0.5% cross-reactivity to the NMS JWH-018 N-(5-hydroxypentyl) metabolite ELISA kit at 10–100µg/L. | ||
|---|---|---|
| Synthetic Cannabinoid |
Concentration (µg/L) |
Cross-reactivity % |
| AM694 | 100 | 0.5 |
| JWH-073 7-hydroxyindole metabolite | 100 | 0.5 |
| WIN 55,212-2 | 100 | 1 |
| JWH-210 N-(4-hydroxypentyl) metabolite | 100 | 1.2 |
| JWH-073 5-hydroxyindole metabolite | 100 | 1.3 |
| JWH-210 N-(5-hydroxypentyl) acid metabolite | 100 | 2 |
| JWH-073 6-hydroxyindole metabolite | 100 | 2.5 |
| AM2201 6-hydroxyindole metabolite | 100 | 3.4 |
| JWH-081 N-(5-hydroxypentyl) metabolite | 100 | 5.8 |
| JWH-210 N-pentanoic acid metabolite | 100 | 7.1 |
| JWH-200 5-hydroxyindole metabolite | 100 | 11 |
| JWH-200 6-hydroxyindole metabolite | 100 | 12.3 |
| JWH-018 5-hydroxyindole metabolite | 10 | 2 |
| JWH-018 | 10 | 2 |
| JWH-018 6-hydroxyindole metabolite | 10 | 2 |
| MAM2201 | 10 | 2 |
| AM2201 | 10 | 6 |
| JWH-398 N-(5-hydroxypentyl) metabolite | 10 | 11 |
| JWH-122 N-(5-hydroxypentyl) metabolite | 10 | 11 |
| MAM2201 N-pentanoic acid metabolite | 10 | 13 |
| JWH-398 N-pentanoic acid metabolite | 10 | 14 |
| AM1220 | 10 | 21 |
| JWH-073 N-butanoic acid | 10 | 53 |
| JWH-018 N-(5-hydroxypentyl)-β-D-glucuronide | 10 | 56 |
| AM2201 N-(4-hydroxypentyl) metabolite | 10 | 60 |
| JWH-018 N-(4-hydroxypentyl) metabolite | 10 | 79 |
| JWH-073 N-(4-hydroxybutyl) metabolite | 10 | 130 |
| JWH-073 N-(3-hydroxybutyl) metabolite | 10 | 133 |
| JWH-019 N-(6-hydroxyhexyl) metabolite | 10 | 136 |
| JWH-018 N-pentanoic acid | 10 | 249 |
| JWH-200 | 10 | 271 |
Immunoassay interferences can alter antibody binding and affect concentrations by increasing or decreasing signal response. Methanolic stock solutions of 93 common drugs of abuse, metabolites, co-administered drugs, over-the-counter medications and structurally similar compounds (Table 2) were added to conical centrifuge tubes and evaporated to dryness at 37°C under nitrogen. Analytes were reconstituted with 5mL blank urine for a final concentration of 1000µg/L. Additional challenges including; ethanol (5mg/mL), NaCl (40g/L), ascorbic acid (4g/L) and urine specimens at different pH values (<4 and >8) also were evaluated. Interference samples (1000µg/L in urine) were aliquotted into duplicate tubes and one set fortified with JWH-018 N-(5-hydroxypentyl) metabolite (5µg/L) to evaluate signal suppression. Drug-free urine was also fortified at 5µg/L, and analyzed in triplicate in columns 1 and 6. Mean absorbances (n=6) and associated standard deviations were calculated to determine normal absorbance range for samples containing 5µg/L of JWH-018 N-(5-hydroxypentyl). Signal suppression was considered present when absorbances of interference challenges were outside of this normal range.
Table 2.
Exogenous compounds (n=93) fortified in blank urine at 1000µg/L to investigate interferences and fortified into a low JWH-018 N-(5-hydroxypentyl) cutoff (5µg/L) to investigate potential signal suppression.
| 2C-B | diazepam | nicotine |
|---|---|---|
| 11-OH-THC | diphenhydramine | nitrazepam |
| 6-acetylcodeine | ecgonine | norbenzoylecgonine |
| 6-acetylmorphine | ecgonine ethyl ester | norbuprenorphine |
| 7-aminoclonazepam | ecgonine methyl ester | norcocaethylene |
| 7-aminoflunitrazepam | EDDP | norcocaine |
| 7-aminonitrazepam | EMDP | norcodeine |
| acetaminophen | ephedrine | norcotinine |
| acetylsalicylic acid | ethylamphetamine | nordiazepam |
| alprazolam | flunitrazepam | norfluoxetine |
| amphetamine | fluoxetine | normorphine |
| anhydroecgonine methyl ester | flurazepam | noroxycodone |
| BDB | HMA | noroxymorphone |
| benzoylecgonine | HMMA | oxazepam |
| bromazepam | hydrocodone | oxycodone |
| brompheniramine | hydromorphine | oxymorphone |
| buprenorphine | ibuprofen | paroxetine |
| caffeine | imipramine | pentazocine |
| cannabigerol | ketamine | phentermine |
| cannabidiol | lorazepam | p-hydroxyamphetamine |
| cannabinol | MBDB | p-hydroxybenzoylecgonine |
| cathinone | MDA | p-hydroxycocaine |
| chlorpheniramine | MDEA | p-hydroxymethamphetamine |
| clomipramine | MDMA | p-methoxyamphetamine |
| clonazepam | methadone | p-methoxymethamphetamine |
| clonidine | methaphetamine | propoxyphene |
| cocaethylene | m-hydroxybenzoylecgonine | pseudoephedrine |
| cocaine | m-hydroxycocaine | temazepam |
| codeine | morphine | THC |
| cotinine | morphine-3-glucuronide | THCCOOH |
| dextromethorphan | morphine-6-glucuronide | trans-3’-hydroxycotinine |
2C-B: 4-bromo-2,5-dimethoxyphenethylamine
11-OH-THC: 11-hydroxy-Δ9-tetrahydrocannabinol
BDB: 3,4-(methylenedioxyphenyl)-2-butanamine
EDDP: 2-ethylidene-1,5-dimethyl-3-3-diphenylpyrrolidine
EMDP: 2-ethylidene-5-methyl-3-3-diphenylpyraline
HMA: 4-hydroxy-3-methoxyamphetamine
HMMA: 4-hydroxy-3-methoxymethamphetamine
MBDB: n-methyl-1-(3,4-methylenedioxyphenyl)-2-butanamine
MDA: 3,4-methylenedioxyamphetamine
MDEA: 3,4- methylenedioxyethylamphetamine
MDMA: 3,4-methylenedioxymethamphetamine
THC: Δ9-tetrahydrocannabinol
THCCOOH: 11-nor-9-carboxy-Δ9-tetrahydrocannabinol
To investigate carryover, pooled negative urine samples (n=4) were fortified with JWH-018 N-(5-hydroxypentyl) metabolite at 750µg/L. Samples from the same pool of blank urine were analyzed before and after each carryover sample. Carryover was evaluated by determining if the blanks before and after were statistically different.
Absorbances of cutoff calibrators prepared in urine and PBS were assessed to evaluate matrix effects. Blank urine samples (pH 5.0 – 6.0) and PBS (pH 7.4) were fortified with JWH-018 N-(5-hydroxypentyl) metabolite at 5 and 10µg/L and analyzed in duplicate at the beginning of each plate. (n=35 plates)
2.7 LC-MS/MS Analysis
The qualitative LC-MS/MS synthetic cannabinoid method was fully validated and previously published16. Briefly, authentic urine (100µL) was fortified with internal standards and ammonium acetate buffer to adjust pH, prior to hydrolysis with beta-glucuronidase. Samples were extracted/precipitated with acetonitrile, vortexed and centrifuged at 15000g and 4°C to produce a supernatant suitable for LC-MS/MS analysis. The method was fully validated with good analytical recovery (53–95%), low matrix effect (95–122%) and LOD’s between 0.5 and 10µg/L for all analytes.
2.8 Diagnostic Efficiency
Urine specimens (n=2492) were analyzed by immunoassay and the reference LC-MS/MS method to evaluate ELISA performance. True-positive (TP), true-negative (TN), false-positive (FP), and false-negative (FN) results were determined by comparing ELISA and LC-MS/MS results. A sample was considered positive if the analytical result was greater than or equal to the specified LC-MS/MS cutoff. A sample was considered TP if the immunoassay and LC-MS/MS were positive, and if both results were negative, the sample was TN. A positive immunoassay result and a negative LC-MS/MS result for all synthetic cannabinoids was considered a FP. A negative immunoassay result and an LC-MS/MS positive result for at least one of the synthetic cannabinoid analytes was considered a FN. Sensitivity of the immunoassay at a specific cutoff was calculated as TP/(TP + FN) × 100 and specificity as TN/(TN + FP) × 100. Efficiency was calculated as (TP +TN)/total number × 100.
3. Results
We present assay performance criteria for the NMS JWH-018 direct ELISA kit as a screening method for the detection of synthetic cannabinoids in urine. Additional performance characteristics (sensitivity, specificity and efficiency) were determined by comparing ELISA results to the LC-MS/MS reference method.
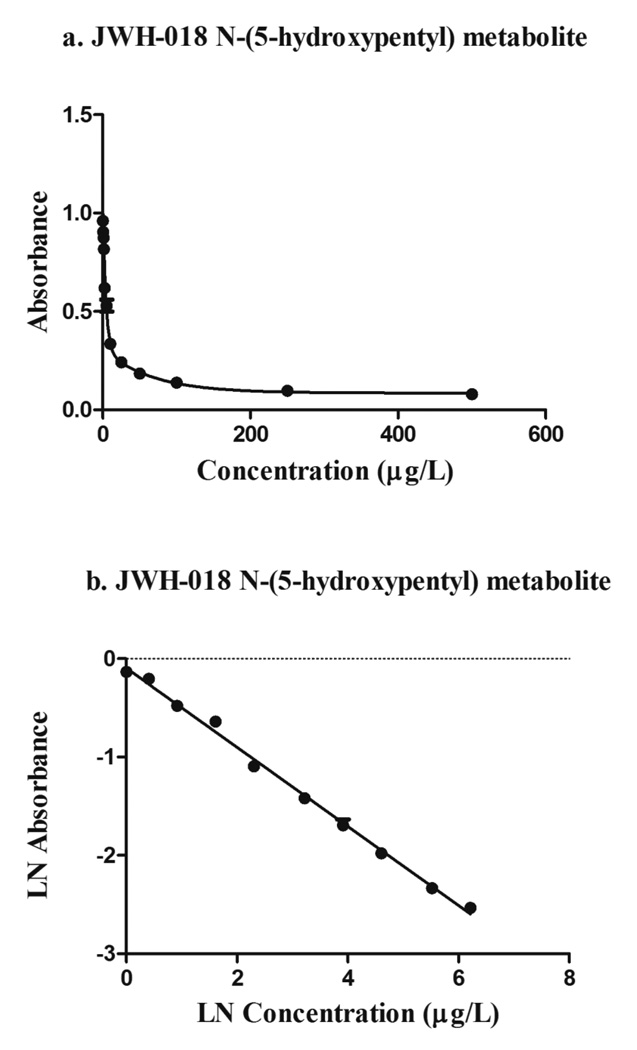
Detection limits were determined after three separate experiments with drug-free urine. Daily LODs of 0.7, 0.5 and 0.8µg/L were empirically obtained (n=9); an average LOD of 0.7µg/L was reported. The calibration curve (0.25 – 500µg/L) was constructed by plotting absorbances of each calibrator (n=3) against concentration. The non-linear regression model using an exponential (two phase decay) function is shown in Figure 2a. The natural logarithms of mean absorbance vs. concentration exhibited linearity from 1–500µg/L, with a coefficient of determination of 0.993 (Figure 2b).
Figure 2. Linearity evaluation.
a) Non-linear calibration curve of JWH-018 N-(5-hydroxypentyl) metabolite in urine (0.25–500µg/L)
b) Linear regression line of JWH-018 N-(5-hydroxypentyl) metabolite in urine (1–500µg/L) r2=0.993
Of the 73 synthetic cannabinoids analyzed, 29 showed zero or low (<1%) cross-reactivity at 500µg/L (Table 1a), while 26% (19 compounds) presented moderate to high cross-reactivity at 10µg/L (Table 1b). The NMS JWH-018 direct ELISA kit showed significant cross-reactivity with metabolites of other synthetic cannabinoids including JWH-200, JWH-073 N-(3-hydroxybutyl) metabolite, JWH-073 N-(4-hydroxybutyl) metabolite, JWH-019 N-(6-hydroxyhexyl) metabolite and AM-2201 N-(4-hydroxypentyl) metabolite.
Interference was evaluated by analyzing 93 common drugs of abuse, metabolites, co-administered drugs, over-the-counter medications and structurally similar compounds (Table 2). No samples fortified at 1000µg/L exhibited a positive result. Likewise, interference samples fortified with JWH-018 N-(5-hydroxypentyl) metabolite (5µg/L) exhibited absorbances similar to mean (n=6) absorbances for the 5µg/L cutoff. There was no significant difference (p=0.27) between the absorbances of blank urine specimens (n=4) analyzed before and after 750µg/L carryover samples (n=4).
Intra-plate imprecision (n=7) was ≤8.2% CV from 1–10µg/L (Table 3). Inter-plate imprecision was evaluated using single absorbances (n=35) from performance challenges at ±25 and ±50% of each cutoff (5 and 10µg/L). Inter-plate imprecision was between 9.0 – 14.0% CV (Table 3). Inter-read imprecision was evaluated with absorbance readings from a single plate containing one replicate of each level of the calibration curve (1, 2.5, 5, 7.5, 10, 25, 50, 100, 250 and 500µg/L). Absorbance readings were taken immediately after adding the stop solution and at 5 min intervals. For all calibrators, imprecision was ≤4% and absolute % difference from initial absorbance was between 4.9 to 10.5%.
Table 3.
Intra-plate imprecision (n=7) for JWH-018 N-(5-hydroxypentyl) concentrations (1–10 µg/L). Inter-plate imprecision (n=35) for JWH-018 metabolite using positive controls (10 & 20µg/L) and performance challenge concentrations at ±25% and ±50% of each cutoff. Inter-read imprecision for a representative calibration curve (1–500µg/L) for JWH-018 N-(5-hydroxypentyl) in urine.
| Urine JWH-018 metabolite µg/L |
Intra-plate (n=7) Absorbance % |
Inter-plate (n=35) Absorbance % |
Inter-read (n=7) Absorbance % |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | CV | Mean (SD) | CV | Mean (SD) | CV | |
| 1 | 0.827 (0.051) | 6.2 | - | - | 0.902 (0.024) | 2.7 |
| 2.5 | 0.613 (0.027) | 4.2 | 0.787 (0.092) | 11.6 | 0.727 (0.020) | 2.8 |
| 3.75 | - | - | 0.630 (0.079) | 12.5 | - | - |
| 5 | 0.511 (0.036) | 7.0 | 0.594 (0.067) | 11.2 | 0.474 (0.015) | 3.2 |
| 6.25 | - | - | 0.557 (0.067) | 12.0 | - | - |
| 7.5 | 0.469 (0.038) | 8.2 | 0.555 (0.078) | 14.0 | 0.407 (0.014) | 3.5 |
| 10 | 0.327 (0.020) | 5.5 | 0.388 (0.035) | 9.0 | 0.374 (0.011) | 3.1 |
| 12.5 | - | - | 0.351 (0.035) | 10.0 | - | - |
| 15 | - | - | 0.321 (0.031) | 9.8 | - | - |
| 20 | - | - | 0.284 (0.037) | 13.0 | - | - |
| 25 | - | - | - | - | 0.222 (0.007) | 3.3 |
| 50 | - | - | - | - | 0.157 (0.006) | 3.7 |
| 100 | - | - | - | - | 0.118 (0.005) | 4.0 |
| 250 | - | - | - | - | 0.087 (0.003) | 3.7 |
| 500 | - | - | - | - | 0.070 (0.001) | 2.1 |
For replicates (n=2) positioned in the same column (in consecutive wells) the median % agreement (range) for 5 and 10µg/L PBS cutoff calibrator absorbances was 8 (−18 to 35) and 10 (−11to 39) respectively. Several absorbances (n=6) had differences >23%. Similarly, median % differences (range) for fortified urine calibrators (5 and 10µg/L) were 5 (−25 to 23) and 8 (−7 to 31), respectively. Again, the highest value in these ranges occurred only once. This highlights the necessity of using duplicate cutoff samples and monitoring expected absorbances and separation rates of calibrators and controls. Absorbances in the last column were almost always higher than initial averaged values, but despite these differences, positive urine controls (10 and 20µg/L) at the end of the plate were always positive for both cutoffs (5 and 10µg/L) in urine and PBS.
A significant difference was observed between JWH-018 N-(5-hydroxypentyl) metabolite solutions prepared in urine and PBS at 5 and 10µg/L. At the same concentration, solutions prepared in PBS had higher absorbances, and greater Δ absorbance differences than those prepared in urine (Table 4). Despite similar mean absorbances for the 5µg/L urine (0.583 ± 0.066) and 10µg/L PBS cutoffs (0.486 ± 0.090), the differences were significant (p=1.63e-06, n=35).
Table 4.
Matrix effect of NMS JWH-018 N-(5-hydroxypentyl) metabolite direct ELISA cutoffs prepared in urine and phosphate buffered saline (PBS), evaluated in duplicate across 35 plates.
| Matrix | Cutoff µg/L |
Absorbance (Mean ± SD) |
C.V. (%) |
|---|---|---|---|
| PBS | 5 | 0.752 ± 0.083 | 11.0 |
| 10 | 0.486 ± 0.090 | 18.5 | |
| Urine | 5 | 0.583 ± 0.066 | 11.2 |
| 10 | 0.402 ± 0.045 | 11.3 |
The frequency of each averaged cutoff to classify these urine performance challenge concentrations as positive or negative based on a single absorbance from each plate (n=35) are presented in Table 5. Initial testing found significant differences (p<0.05) between all challenge concentrations (n=6) and the 5µg/L urine cutoff. However, further evaluation of performance challenges (n=35) around the 5µg/L cutoff showed significant differences between the negative challenges (2.5 and 3.75µg/L), but not for positive challenges (6.25 and 7.5µg/L) above the cutoff. Based on similar absorbances and the response of the calibration curve, these results are more typical and reflect daily differences in assay sensitivity. Interestingly, the 5µg/L urine cutoff showed better performance below the cutoff, while the 5µg/L PBS cutoff was better for concentrations above. The best agreement between performance challenge results obtained and expected was for the 10µg/L urine cutoff.
Table 5.
Performance challenge classifications (n=35) at ±25% and ±50% of each evaluated cutoff (5 & 10 µg/L) in each evaluated matrix (Urine & Phosphate Buffered Saline) across 35 plates.
| Cutoffs (µg/L) |
JWH-018 N-(5-hydroxypentyl) metabolite Performance Challenge concentration (µg/L) in urine |
Correct classification (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 3.75 | 5.0 | 6.25 | 7.5 | 12.5 | 15 | Negative | Positive | |||
| −50% | −25% | +25% | +50% | ||||||||
| 5-PBS | 25/35 (−) | 5/35 (−) | - | 34/35 (+) | 32/35 (+) | - | - | 71 | 14 | 97 | 91 |
| 5-UR | 35/35 (−) | 30/35 (−) | - | 24/35 (+) | 23/35 (+) | - | - | 100 | 86 | 69 | 66 |
| 10-PBS | - | - | 29/35 (−) | - | 27/35 (−) | 33/35 (+) | 35/35 (+) | 83 | 77 | 94 | 100 |
| 10-UR | - | - | 35/35 (−) | - | 35/35 (−) | 34/35 (+) | 34/35 (+) | 100 | 100 | 97 | 97 |
(−) = Negative result
(+) = Positive result
PBS = Phosphate Buffered Saline
UR= Urine
4. Diagnostic Assay Performance
The designation of individual specimens as TP, TN, FP, and FN and the performance characteristics of the NMS JWH-018 direct ELISA kit immunoassay versus LC–MS/MS cutoffs are summarized in Table 6. As expected, the lower 5µg/L cutoffs (PBS and urine) exhibited higher overall sensitivity (90.7% and 83.7%), yet produced the greatest numbers (29 and 14) of FP results. Specificity and efficiency for the 5µg/L cutoffs in PBS and urine were similar, with both greater than 97.6%. Raising the ELISA cutoff from 5 to 10µg/L resulted in an 12.5% and 12.1% loss of assay sensitivity for PBS and urine, respectively. FP results could be due to cross-reactivities with low concentrations of multiple synthetic cannabinoid metabolites, or of analytes not included in the confirmatory method, while FN results may be explained by differences in ELISA cutoff concentrations and LC-MS/MS LOD’s (0.5 – 10µg/L).
Table 6.
Diagnostic performance of NMS JWH-018 N-(5-hydroxypentyl) metabolite direct ELISA for 2492 urine samples. All samples confirmed by LC-MS/MS.
| Cutoff | 5 µg/L in PBS |
5 µg/L in Urine |
10 µg/L in PBS |
10 µg/L in Urine |
|---|---|---|---|---|
| True Positive | 262 | 242 | 226 | 207 |
| True Negative | 2174 | 2189 | 2195 | 2196 |
| False Positive | 29 | 14 | 8 | 7 |
| False Negative | 27 | 47 | 63 | 82 |
| Sensitivity % | 90.7 | 83.7 | 78.2 | 71.6 |
| Specificity % | 98.7 | 99.4 | 99.6 | 99.7 |
| Efficiency % | 97.8 | 97.6 | 97.2 | 96.4 |
5. Discussion
A major source of drift across the plate is the different timing for reactions between antibody and antigens (drug-free and conjugate). In an effort to minimize this effect, we optimized the liquid handling parameters to include a pause step during the addition of the conjugate. This ensured that each sample was in contact with the antibodies for a similar time. However, plate drift was still present but reduced and generally more pronounced for lower drug concentrations. Inter-plate variability also was observed, suggesting the need to calibrate each plate when monitoring forensic urine tests. When comparing the 5µg/L cutoffs prepared in urine and PBS, we observed higher absorbances in buffer than in urine. It appears that the conjugate binds better with the antibodies in the presence of buffer, or in the absence of matrix elements.
Cross-reactivity is advantageous in drug screening assays as it enhances the detection of compounds with similar chemical structures making the screening process more practical and less expensive, an important characteristic for synthetic cannabinoids analysis. We observed some common structural characteristics when reviewing the cross-reactivity of 73 synthetic cannabinoids in the NMS JWH-018 direct ELISA kit. It appears that hydroxyl substituents on the alkyl side chain increased cross-reactivity. For compounds tested, the general order of reactivity was: pentanoic acid > 5-hydroxypentyl > 4-hydroxypentyl > 5-hydroxypentyl glucuronide > parent compound. However, reactivity decreased significantly when the hydroxyl substituent was on the indole moiety. These data may be useful for predicting cross-reactivity of new synthetic cannabinoids. However, it is important to emphasize that these conclusions are restricted to this assay and this targeted metabolite. However, more detailed experiments with a complete database of synthetic cannabinoids would be necessary to definitively document these effects, and allow the prediction of cross-reactivity results based on chemical structures. This highlights a limitation of all synthetic cannabinoid immunoassays, as newer compounds (PB-22, RCS-4, RCS-8, XRL-11 and AKB48) may not react with current ELISA kit antibodies. These FN results are a reality, especially when laboratories are continually faced with newly emerging abused synthetic cannabinoids.
If we consider that FN results are of greater concern in a screening assay, then the 5µg/L PBS cutoff had the best performance (Table 6). The higher sensitivity of the 5µg/L PBS may be explained by the absence of matrix effect, as cutoff solutions were prepared in buffer. While there are advantages of preparing calibrators in buffer or synthetic urine (stability, separation) these solutions only approximate matrix effects. The urine performance challenge classifications of samples at ±25% of the cutoff showed correct identification more frequently for matrix matched cutoffs than buffered prepared cutoffs; with the 10µg/L urine cutoff performing best. However, increasing the cutoff reduced sensitivity and yielded the most FN samples. Based on our results, we recommend the use of a 5µg/L cutoff in urine when using the NMS JWH-018 direct ELISA kit as a screening method for the detection of synthetic cannabinoids in urine.
ACKNOWLEGMENTS
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health and the Department of Defense through an interagency agreement. We thank National Medical Services for generously providing the immunoassay analysis kits.
References
- 1.Dresen S, Kneisel S, Weinmann W, et al. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46:163–171. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 2.Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 4.Brents LK, Reichard EE, Zimmerman SM, et al. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devane WA, Breuer A, Sheskin T, et al. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35:2065–2069. doi: 10.1021/jm00089a018. [DOI] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. [Google Scholar]
- 7.Harris CR, Brown A. Synthetic cannabinoid intoxication: a case series and review. J Emerg Med. 2013;44:360–366. doi: 10.1016/j.jemermed.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos D, Singh S, Thornton G, et al. Synthetic cannabinoid use: a case series of adolescents. J Adolesc Health. 2011;49:347–349. doi: 10.1016/j.jadohealth.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011;117:152–157. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Simmons J, Cookman L, Kang C, et al. Three cases of "spice" exposure. Clin Toxicol (Phila) 2011;49:431–433. doi: 10.3109/15563650.2011.584316. [DOI] [PubMed] [Google Scholar]
- 11.Simmons JR, Skinner CG, Williams J, et al. Intoxication from smoking "spice". Ann Emerg Med. 2011;57:187–188. doi: 10.1016/j.annemergmed.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Government. Synthetic Drug Abuse Prevention Act. 2012 1152b,S.31871-139.
- 13.European Monitoring Centre for Drugs, Drug Addiction (EMCDDA) Understanding the 'Spice' phenomenon. Lisbon: EMCDDA; 2009. Available at: http://www.emcdda.europa.eu/html.cfm/index90917EN.html. [Google Scholar]
- 14.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Drug Use: 2012 Overview Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- 15.Hu X, Primack BA, Barnett TE, et al. College students and use of K2: an emerging drug of abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16. doi: 10.1186/1747-597X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlfarth A, Scheidweiler KB, Chen X, et al. Qualitative confirmation of 9 synthetic cannabinoids and 20 metabolites in human urine using LC-MS/MS and library search. Anal Chem. 2013;85:3730–3738. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grigoryev A, Melnik A, Savchuk S, et al. Gas and liquid chromatography-mass spectrometry studies on the metabolism of the synthetic phenylacetylindole cannabimimetic JWH-250, the psychoactive component of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2519–2526. doi: 10.1016/j.jchromb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Grigoryev A, Savchuk S, Melnik A, et al. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1126–1136. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Hutter M, Broecker S, Kneisel S, et al. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in 'herbal mixtures' using LC-MS/MS techniques. J Mass Spectrom. 2012;47:54–65. doi: 10.1002/jms.2026. [DOI] [PubMed] [Google Scholar]
- 21.Scheidweiler KB, Jarvis MJ, Huestis MA. Nontargeted SWATH acquisition for identifying 47 synthetic cannabinoid metabolites in human urine by liquid chromatography-high-resolution tandem mass spectrometry. Anal Bioanal Chem. 2014 doi: 10.1007/s00216-014-8118-8. [DOI] [PubMed] [Google Scholar]
- 22.Moran CL, Le VH, Chimalakonda KC, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jager AD, Warner JV, Henman M, et al. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine--an Australian perspective. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;897:22–31. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Yanes EG, Lovett DP. High-throughput bioanalytical method for analysis of synthetic cannabinoid metabolites in urine using salting-out sample preparation and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;909:42–50. doi: 10.1016/j.jchromb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Chimalakonda KC, Moran CL, Kennedy PD, et al. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011;83:6381–6388. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheidweiler KB, Huestis MA. Simultaneous quantification of 20 synthetic cannabinoids and 21 metabolites, and semi-quantification of 12 alkyl hydroxy metabolites in human urine by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2014;1327:105–117. doi: 10.1016/j.chroma.2013.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffiths P, Sedefov R, Gallegos A, et al. How globalization and market innovation challenge how we think about and respond to drug use: 'Spice' a case study. Addiction. 2010;105:951–953. doi: 10.1111/j.1360-0443.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- 28.Barnes AJ, Young S, Spinelli E, et al. Evaluation of a homogenous enzyme immunoassay for the detection of synthetic cannabinoids in urine. Forensic Sci Int. 2014;241:27–34. doi: 10.1016/j.forsciint.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinelli E, Barnes AJ, Young S, et al. Performance characteristics of an ELISA screening assay for urinary synthetic cannabinoids. Drug Test Anal. 2014 doi: 10.1002/dta.1702. [DOI] [PubMed] [Google Scholar]
- 30.Arntson A, Ofsa B, Lancaster D, et al. Validation of a novel immunoassay for the detection of synthetic cannabinoids and metabolites in urine specimens. J Anal Toxicol. 2013;37:284–290. doi: 10.1093/jat/bkt024. [DOI] [PubMed] [Google Scholar]