Abstract
N-Acetylneuraminic acid (Neu5Ac or NANA) is the most predominant sialic acid in mammals. As a terminal component in many glycoproteins and glycolipids, sialic acid is believed to be an important biomarker related to various diseases. Its precursor, N-acetylmannosamine (ManNAc), is being investigated as a potential treatment for GNE myopathy. In this work, we developed two highly sensitive and selective liquid chromatography–tandem mass spectrometry (LC-MS/MS) methods for the quantitation of ManNAc and free Neu5Ac in human plasma. A fit-for-purpose approach was adopted during method validation and sample analysis. To measure the endogenous compounds and overcome the interference from plasma samples, a surrogate matrix that contained 5% bovine serum albumin (BSA) was used for the preparation of calibration standards and certain levels of quality control (QC) samples. QC samples at higher concentrations were prepared in the authentic matrix (human plasma) to best mimic incurred samples. For both methods, an Ostro 96-well phospholipid removal plate was used for sample extraction, which efficiently removed the phospholipids from the plasma samples prior to LC injection, eliminated matrix effect, and improved sensitivity. Chromatographic separation was achieved using hydrophilic interaction chromatography (HILIC) and gradient elution in order to retain the two polar compounds. The lower limit of quantitation (LLOQ) for ManNAc and Neu5Ac was 10.0 and 25.0 ng/mL, respectively. The overall accuracy of the two assays was no less than 91.7% based on three levels of QC samples. Inter- and intra-run precision (coefficient of variation [%CV]) across three analytical runs was less than 6.7% for ManNAc and less than 10.8% for Neu5Ac. These methods have been validated to support clinical studies.
Keywords: N-acetylmannosamine, N-acetylneuraminic acid, HILIC, LC-MS/MS, Phospholipid removal, Validation
1. Introduction
Sialic acids are the prominent members from neuraminic acid derivatives, which are structurally characterized by a 9-carbon chain. Serving as the major building bricks to glycoproteins and glycolipids, these saccharide ancients are often found in cellular secretions and on the surface of all mammalian cells, associated with many metabolic pathways [1]. Sialic acids have been recognized to be pivotal in biological processes such as cellular adhesion, cell-cell interactions, and signal transduction due to their negative charges and hydrophilicity [2,3]. Sialic acids can mediate or modulate a wide variety of physiological and pathological processes, and sialic acids have roles in both health and multiple disease areas, including immunology, cardiology, hematology, oncology, and others [2].
GNE myopathy is an autosomal recessive muscular disorder characterized by progressive muscle weakness and atrophy with onset in early adulthood [4,5]. GNE myopathy is caused by biallelic mutations in the GNE gene, which encodes the bifunctional enzyme uridine diphospho-N-acetylglucosamine 2-epimerase (UDP-GlcNAc epimerase) and N-acetylmannosamine kinase (ManNAc kinase). The enzyme catalyzes the first two committed steps in the biosynthesis of sialic acid [6] and mutations in GNE lead to decreased enzyme activity, decreased sialic acid biosynthesis and hyposialylation of muscle glycoproteins, resulting in muscle deterioration [7-9]. In mammals, the predominant sialic acid is N-acetylneuraminic acid (Neu5Ac). N-acetylmannosamine (ManNAc) is the precursor for the biosynthesis of Neu5Ac. Previous studies in mice showed that oral administration of ManNAc, Neu5Ac or sialyllactose ameliorated the muscle pathology of GNE myopathy, suggesting this disease could potentially be treated by extrinsic administration of sialic-acid–increasing compounds [10-12].
As the precursor of sialic acid, ManNAc is being developed by National Institutes of Health (NIH) and New Zealand Pharmaceuticals as a therapeutic agent for patients with GNE myopathy (ClinicalTrails.gov Identifiers NCT01634750 and NTC02346461). To assist in evaluating its safety, pharmacokinetics, and pharmacodynamics in the clinical studies, accurate analytical methods to determine the plasma concentrations of ManNAc and Neu5Ac were required. So far, several analytical methods have been reported to detect Neu5Ac in biological matrices including colorimetry [13,14], enzymology [15], 1H-NMR (proton nuclear magnetic resonance) spectroscopy [16], gas chromatography (GC) coupled with mass spectrometry (MS) [17], and high performance liquid chromatography (HPLC) coupled with ultraviolet (UV) detection [18,19], and fluorescent labeling [20]. These methods are limited by specificity and/or sensitivity, and they cannot be applied for definitive quantitation. Recently, additional methods for Neu5Ac were described based on liquid chromatography–tandem mass spectrometry (LC-MS/MS) [21,22]. These methods achieve the desired specificity. However, the reported lower limit of quantitation (LLOQ) is still in the micromolar (µM) range, which is too high for quantitation of the endogenous free Neu5Ac levels in plasma of GNE myopathy patients. Similarly, existing analytical methods for ManNAc determination requires derivatization with UV-absorbing or fluorophore groups [23], and they are limited by specificity and sensitivity for quantitation of endogenous ManNAc in human plasma.
In this work, we have developed and validated two bioanalytical methods for the quantitative analysis of ManNAc (method 1) and free Neu5Ac (method 2) in human plasma using LC-MS/MS. Due to the small molecular weight and the presence of multiple hydroxyl groups, chromatographic retention of the two compounds was challenging. Therefore, hydrophilic interaction chromatography (HILIC) was utilized to gain sufficient retention on the HPLC column. In addition, because ManNAc and Neu5Ac are endogenous compounds in plasma, calibration standards were prepared in a surrogate matrix for quantitation. The established LLOQ was 10 and 25 ng/mL for ManNAc and Neu5Ac, respectively, based on an extraction volume of 50 µL. These two methods demonstrated good sensitivity, selectivity, linearity, accuracy, and precision as well as assay ruggedness; they were validated using a “fit-for-purpose” approach. The validated methods were successfully used to support the GNE myopathy clinical trial.
2. Experimental details
2.1. Chemicals
Reference standards for ManNAc (purity: 92.6%) and Neu5Ac (purity: 97.3%) were obtained from New Zealand Pharmaceuticals. The stable isotopically labeled ManNAc-13C-d3 (isotopic purity: 99.9%) was purchased from Ricerca Biosciences, LLC (Concord, OH), and the stable isotopically labeled Neu5Ac-d3 (isotopic purity: 96.4%) was purchased from Medical Isotopes, Inc. (Pelham, NH). The chemical structures of all four compounds are shown in Fig. 1. HPLC-grade acetonitrile, methanol, and acetic acid (AA) were purchased from Fisher Scientific (Pittsburgh, PA). Trifluoracetic acid (TFA) was purchased from EMD Chemicals Inc. (Gibbstown, NJ). Deionized water was generated in-house using a Milli-Q Ultrapure water purification system from EMD Millipore (Billerica, MA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich Corporation (St Louis, MO), and human plasma (K2EDTA, storage condition: −20 °C) was obtained from Bioreclamation Inc. (Westbury, NY).
Fig. 1.
Chemical structures of N acetylmannosamine (ManNAc), ManNAc-13C-d3, N acetylneuraminic acid (Neu5Ac), and Neu5Ac-d3.gr1
2.2. LC-MS/MS system
HPLC separation was performed on a LC-20AD LC pump system (Shimadzu, Kyoto, Japan) coupled with a Sil-20AC HT autosampler (Shimadzu, Kyoto, Japan). An XBridge Amide column (3.5 µm, 100 × 2.1 mm) from Waters Corporation (Milford, MA) was used for chromatographic separation of ManNAc (method 1), and an Atlantis HILIC Silica column (5 µm, 50 × 3.0 mm) from Waters Corporation (Milford, MA) was used for chromatographic separation of Neu5Ac (method 2). The autosampler temperature was set at 6 °C, and the LC column was maintained at room temperature (20 ± 10 °C). MS analyses were conducted on a 4000 QTRAP LC-MS/MS System from AB Sciex (Foster City, CA) with a TurboIonSpray interface. The system was controlled by Analyst version 1.4.2 software.
2.3. LC-MS/MS conditions
The two mobile phases used were 0.2% AA and 0.05% TFA in water (mobile phase A) and 0.2% AA and 0.05% TFA in acetonitrile (mobile phase B) for both methods. The following gradient was applied at a flow rate of 0.8 mL/min for method 1 (ManNAc): linearly decreased from 97% to 65% of mobile phase B from 0 to 2.9 min, further decreased to 30% B from 2.9 to 3.0 min, held for 0.8 min, and increased back to 97% B in 0.1 min. The total run time was 4.8 min. For method 2 (Neu5Ac): the gradient was started from 96% of mobile phase B and linearly decreased to 70% B from 0.5 to 0.9 min, held for 0.9 min, dropped to 30% B from 1.8 to 2.3 min, held for 0.8 min, increased to 96% B in 0.1 min, and stopped at 4.1 min.
The analytes were monitored using selective reaction monitor (SRM) in positive-ion electrospray mode. For method 1 (ManNAc), the following optimized mass spectrometer conditions were used: turbo ion spray voltage was set at 5500 V, and the ion source temperature was set at 400 °C. Curtain gas, nebulizing gas, and auxiliary gas were set to 35, 50, and 50 psi, respectively. The declustering potential and collision energy were set at 35 V and 18 eV, respectively. The transitions monitored for ManNAc and ManNAc-13C-d3 were m/z 222→126 and 226→130, respectively. For method 2 (Neu5Ac), ion source temperature was set at 575 °C; collision energy was at 16 eV; and the transitions monitored for Neu5Ac was m/z 310→274 and for Neu5Ac-d3 was m/z 313→277. Other parameters were the same as for method 1.
3. Sample preparation
3.1. Stock solution, calibration standards and quality control
Separate stock solutions of ManNAc and Neu5Ac were prepared at a concentration of 1.00 mg/mL in 50:50 acetonitrile/water (v/v) for standard and quality control (QC) samples. The internal standard stock solutions of ManNAc-13C-d3 and Neu5Ac-d3 were prepared at 1.00 mg/mL in 50:50 acetonitrile/water (v/v). All prepared stock solutions were stored at 4 °C and protected from white light. Calibration standards were prepared in 5% BSA in water at range of 10.0 to 5000 ng/mL for ManNAc and 25.0 to 10,000 ng/mL for Neu5Ac. In addition, two lower levels of QC samples (LLOQ and low [LQC]) were prepared in 5% BSA at concentrations of 10.0 and 30.0 ng/mL for ManNAc and 25.0 and 75.0 ng/mL for Neu5Ac, respectively. The other three levels of QC samples (medium [MQC], high [HQC] and dilution [DQC]) were prepared in pooled blank human plasma with spiked concentrations of 200, 4000, and 25000 ng/mL for ManNAc and 200, 8000, and 50000 ng/mL for Neu5Ac, respectively. The endogenous concentrations of ManNAc and Neu5Ac in the human plasma were prequantified and added on top of the spiked concentrations, and these calculated totals were used as the nominal concentrations. All unknown samples, calibration standards, and QC samples were stored at −80 °C until analyzed.
3.2. Sample extraction
The same sample preparation procedure was used for ManNAc (method 1) and Neu5Ac (method 2). Aliquots of 50 µL of standards, QC samples, and study samples were pipetted into an Ostro 96-well sample preparation plate (Waters Corporation, Milford, MA) on top of a collection plate, and 25 µL of internal standard working solution (2000 ng/mL) was added to each sample followed by 200 µL of acetonitrile. The Ostro 96-well plate was capped and vortex mixed. A medium positive pressure was applied on the plate to let the solvent pass. Aliquots of 66 µL of the collected samples were transferred and further diluted with 200 µL of 70:30 acetonitrile/water (v/v).
4. Results and discussion
4.1. Bioanalysis strategy for endogenous compounds
As endogenous compounds, ManNAc and Neu5Ac are naturally present in human plasma. The lot-to-lot variable endogenous level makes it difficult to find a suitable matrix to prepare standards and QC samples for quantitation. This poses a challenge for developing bioanalytical assays and determining the sensitivity and selectivity, as well as evaluating recovery and matrix effect. There is no specific regulatory guidance for method validation of endogenous compounds as there is for validation of small-molecule pharmacokinetic studies. A “fit-for-purpose” validation approach is usually adopted for biomarker bioanalysis to support early phases of drug development [24-26]. The level of validation and acceptance criteria of the tests performed is determined based on the purpose of the study and further applications of the assay.
Different strategies [27,28] have been developed to overcome the challenges without compromising the quantitation results, including the two most adopted: (1) surrogate analyte in authentic matrix, and (2) authentic analyte in surrogate matrix. For the first approach, the surrogate analyte is typically a stable isotopically labeled analyte that does not preexist in the authentic matrix yet has the nearly identical physical and chemical properties as the analyte of interest. The labeled compound has the same LC behavior and MS ionization efficiency to mimic the authentic analyte but can be separately monitored in a different SRM transition, making it a perfect selection to establish the standard calibration curve. This calibration curve can be applied further to calculate the endogenous analyte concentrations based on the relative instrument responses of the unlabeled and labeled compounds. This approach provides the benefits of eliminating interferences introduced from the authentic matrix, lowering the detection limit, and preparing standards and QC samples in the authentic matrix to mimic real samples. However, a second labeled compound is often needed as an internal standard to improve assay ruggedness.
The second strategy, “authentic analyte in surrogate matrix”, utilizes a different matrix, either from a different species that is free of the endogenous compound, i.e., a “stripped” matrix that is pretreated to remove the endogenous compounds, or a synthetic matrix including neat buffers and protein solutions. During our method development and validation, only one stable isotopically labeled compound was available for ManNAc and Neu5Ac; therefore, we chose to proceed with the “surrogate matrix” approach. A protein solution containing 5% BSA was selected as the surrogate matrix for standards, LLOQ, and LQC sample preparation. Meanwhile, QC samples at high concentrations (MQC, HQC and DQC) were prepared in the human plasma to reflect the assay performance in the authentic matrix. The baseline levels of ManNAc and Neu5Ac in the human plasma pool used for QC sample preparation were prequantified. The measured concentrations were then calculated on top of the prepared concentrations to achieve the final nominal concentrations. In order to minimize the potential matrix effect and system bias introduced by the different types of matrices, stable isotopically labeled internal standards were used in both methods to compensate ionization efficiency.
4.2. HILIC chromatography development
Both ManNAc and Neu5Ac are highly hydrophilic with relatively low molecular weights (221 and 309 atomic mass units [amu], respectively). Chromatographic separation on a regular reverse-phase column is challenging. For Neu5Ac, although reverse-phase chromatography was reported in some methods, the poor retention could cause deteriorated peak shape, high matrix effect, and low sensitivity. A derivatization method was recently reported to increase hydrophobicity and achieve sufficient retention on a reverse-phase column [22], but this additional step lengthens the sample preparation time and increases assay complexity.
To overcome the retention challenge and be able to separate the analytes from other endogenous polar compounds (e.g., monosaccharides), we focused on two main objectives in the initial method development: retention and sensitivity. After exploring a variety of HPLC columns and chromatographic conditions, we developed two HILIC methods using an XBridge Amide column for method 1 (ManNAc) and an Atlantis HILIC Silica column for method 2 (Neu5Ac). HILIC-MS/MS assays are frequently used for polar analytes [29,30]. Addition of AA and TFA to the mobile phases was necessary to achieve the best peak shape and resolution. It is well known that TFA may cause some ion suppression. However, addition of AA to TFA-containing mobile phases could alleviate sensitivity loss [31]. In our newly developed methods, at a flow rate of 0.8 mL/min, ManNAc was efficiently retained with a retention time of 2.5 min (k′ = 8.5), and Neu5Ac at a retention time of 1.5 min (k′ = 4.5). This combination of the HPLC column and mobile phases provide satisfactory retention, good peak shape, and resolution to enable sensitive and selective measurements.
4.3. Matrix effect and ion suppression
Biological matrices such as plasma and serum are complex mediums that contain a variety of endogenous compounds. The abundant phospholipids present in plasma are considered a major concern for LC-MS based assays. The co-eluting compounds at high concentrations, such as phospholipids, could either enhance or suppress the ionization efficiency of the target analyte. This phenomenon is known as matrix effect [32,33], which can interfere with the analysis and can severely affect assay accuracy and precision. In some reported cases [34,35], the use of a stable isotopically labeled internal standard even failed to compensate for matrix effect, which resulted in assay failure. Therefore, it is important to recognize and overcome this challenge in an early stage of method development. Various approaches have been explored throughout the years for removal of phospholipids from biological matrixes [36]. Optimized sample cleanup procedures have been developed using protein precipitation [37], liquid-liquid extraction [38], and solid-phase extraction [39] by selecting different combinations solvents and pH conditions. Another efficient technique to remove phospholipids is chromatographic separation. New discoveries in chromatography have been described including HILIC [37], 2D LC [40], and even simply back-flushing the analytical column between injections [41].
During our method development, we first utilized a protein precipitation (PPT) extraction method by precipitating samples using acetonitrile. However, due to coextracted phospholipids, a severe matrix effect was observed for Neu5Ac, affecting the MS signal and assay ruggedness. Other extraction methods were then tested and the recovered analytes and phospholipids were carefully compared. We selected the most efficient method, an Ostro 96-well phospholipid removal plate, which provided about 70%–100% recovery for ManNAc and Neu5Ac, while it removed the phospholipid matrix effect by retaining the phospholipids on the extraction plate.
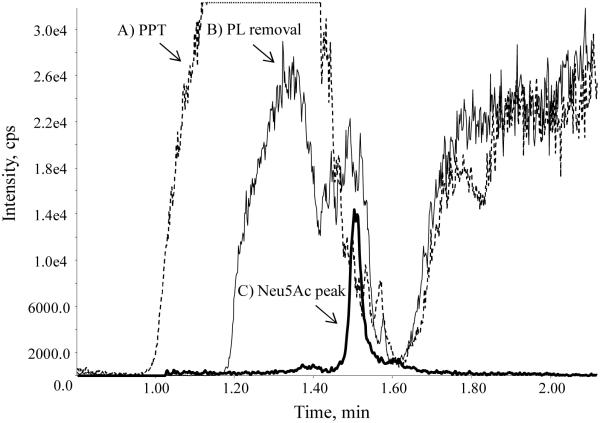
A post-column infusion test was performed by continuously delivering neat solution of Neu5Ac-d3 at a concentration of 2000 ng/mL directly to the MS ion source. A plasma sample extracted with PPT was injected followed by a sample extracted with the optimized extraction procedure. As shown in Fig. 2, when the PPT sample was injected, the MS response of Neu5Ac decreased sharply around its retention time of 1.5 min, leading to a weak and unstable signal. In comparison, the MS response remained at a higher level when our optimized phospholipid removal procedure was applied to the plasma sample. The post-column infusion test proved that this final method efficiently removed phospholipids from the samples prior to injection into the LC system, eliminated any matrix effect, and increased sensitivity.
Fig. 2.
Post-column infusion chromatograms of Neu5Ac-d3 after injection of (A) a plasma sample extracted with protein precipitation (PPT) and (B) a plasma sample extracted with phospholipid (PL) removal plate. A chromatogram of (C) Neu5Ac was overlaid to show the retention time.gr2
4.4 Calibration curve, sensitivity, and specificity
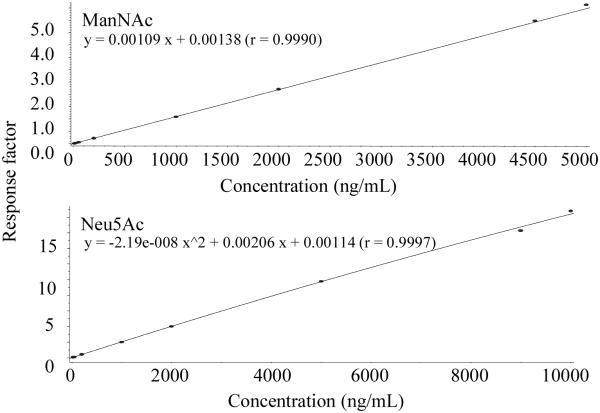
The analytical range for method 1 (ManNAc) was established from 10.0 to 5000 ng/mL and 25.0 to 10,000 ng/mL for method 2 (Neu5Ac). Both calibration curves were obtained from eight independent concentrations (Fig. 3). A linear regression with 1/x2 weighting was used for method 1, and a quadratic regression with 1/x2 weighting was used for method 2. For all the runs tested, the regression coefficiency was greater than 0.997 for ManNAc and greater than 0.998 for Neu5Ac.
Fig. 3.
Representative calibration curves of ManNAc and Neu5Ac in 5% BSA. A linear 1/x2 weighted regression model was used for ManNAc and a quadratic 1/x2 weighted regression model were used for Neu5Ac.gr3
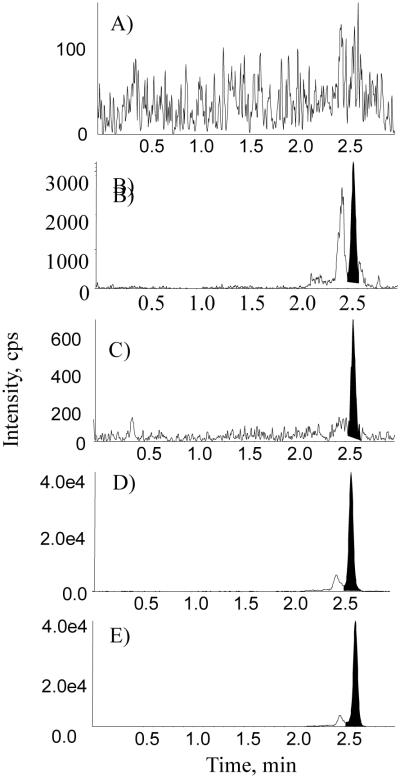
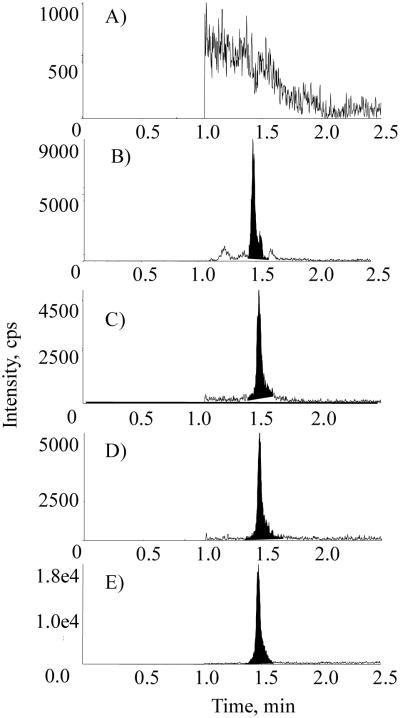
Representative chromatograms of a blank BSA sample, a blank human plasma sample, an LLOQ sample prepared in blank BSA, an incurred sample, and the internal standard in the incurred sample are presented in Figs. 4 and 5 for ManNAc and Neu5Ac, respectively. In the blank BSA sample, the absence of endogenous interference to ManNAc and Neu5Ac demonstrated assay specificity. In the blank human plasma sample, the endogenous levels of ManNAc and Neu5Ac were approximately 50 and 159 ng/mL, respectively (Figs. 4B and 5B). A signal-to-noise ratio of greater than 5 was achieved for the LLOQ sample with both methods. Assay specificity was also confirmed by comparing the retention time of the analytes between plasma samples and standards with reverse-phase chromatography [27].
Fig. 4.
Representative chromatograms of ManNAc in (A) blank 5% BSA, (B) blank human plasma, (C) an LLOQ sample in 5% BSA, (D) an incurred sample, and (E) internal standard in human plasma.gr4
Fig. 5.
Representative chromatograms of Neu5Ac in (A) blank 5% BSA, (B) blank human plasma, (C) an LLOQ in 5% BSA, (D) an incurred sample, and (E) internal standard in human plasma.gr5
4.5 Accuracy and precision
Intra- and inter-assay accuracy and precision (expressed as percent coefficient of variation [%CV]) were assessed over three separate days based on the maximum value from the LQC, MQC, and HQC samples. The assay accuracy was determined by calculating the ratios of the predicted concentrations to their nominal values, and the intra- and inter-assay precisions were determined using a one-way analysis of variance (ANOVA). For MQC and HQC that were prepared in human plasma, the nominal concentrations were calculated by combining the prequantified endogenous concentrations with the spiked concentrations. As presented in Table 1, the assay accuracy was within 100% ± 5.2% for ManNAc and 100% ± 8.3% for Neu5Ac. The intra-assay precision (%CV) was less than 6.7% for ManNAc and less than 10.8% for Neu5Ac, and the inter-assay precision (%CV) was less than 5.1% for ManNAc and less than 4.9% for Neu5Ac.
Table 1.
Assay accuracy and precision (%CV) for ManNAc and Neu5Ac in LQC, MQC, and HQC samples.
| ManNAc | LQC (30.0 ng/mL) |
MQC (251 ng/mL) |
HQC (4051 ng/mL) |
|---|---|---|---|
| Mean | 31.6 | 243 | 3922 |
| Accuracy (%) | 105.2 | 96.7 | 96.8 |
| Intra-assay precision (%CV) | <6.7 | <6.5 | <4.5 |
| Inter-assay precision (%CV) | 4.5 | 5.1 | 2.1 |
| Number of replicates (n) | 18 | 18 | 18 |
| Neu5Ac | LQC (75.0 ng/mL) | MQC (359 ng/mL) | HQC (8159 ng/mL) |
|---|---|---|---|
| Mean | 74.6 | 326 | 7326 |
| Accuracy (%) | 99.4 | 92.4 | 91.7 |
| Intra-assay precision (%CV) | <7.6 | <10.8 | <3.2 |
| Inter-assay precision (%CV) | 4.9 | 3.7 | 2 |
| Number of replicates (n) | 18 | 18 | 18 |
4.6 Matrix factor and extraction recovery
To evaluate matrix effect of the surrogate matrix, the extracts of the 5% BSA were reconstituted with a standard solution containing ManNAc or Neu5Ac and their respective internal standards at concentrations equivalent to the LQC level. In addition, three replicates of a neat solution containing the analyte and internal standard at the same concentrations were analyzed. The matrix factor was calculated as the ratio of the mean peak area of the spiked blank matrix extract samples and the mean peak area of the neat solutions and expressed as a percentage of the mean neat solution peak area. The matrix factor for ManNAc and ManNAc-13C-d3 in 5% BSA was 90.7% and 94.4%, respectively, and 101.6% and 99.2% for Neu5Ac and Neu5Ac-d3, respectively.
Recovery in 5% BSA and human plasma was determined by comparing the mean area of extracted LQC and HQC samples with the mean area of recovery samples with equivalent concentration levels. Three replicates of the recovery samples at each concentration level were prepared by adding the analyte and the internal standard to the extracts of blank matrix. The summary data containing recovery results for ManNAc and Neu5Ac in 5% BSA (from LQC samples) and human plasma (from HQC samples) are presented in Table 2. The recovery of Neu5Ac in human plasma was approximately 70%. All other recoveries were close to 100%.
Table 2.
Extraction recovery for ManNAc and Neu5Ac and their respective internal standards at a low concentration in 5% BSA and at a high concentration in human plasma.
| Recovery (%) | ||
|---|---|---|
| LQC in 5% BSA | HQC in Human Plasma | |
| ManNAc | 92.4 | 85.8 |
| ManNAc-13C-d3 | 112.8 | 113.7 |
| Neu5Ac | 113.6 | 68.2 |
| Neu5Ac-d3 | 121.3 | 69.5 |
4.7 Stability
The stability of ManNAc and Neu5Ac in both 5% BSA and human plasma was evaluated based on LQC and HQC samples using six replicates. As presented in Table 3, both compounds showed good stability in both authentic and surrogate matrices. ManNAc was stable in both matrices for at least 310 days when stored at −80 °C, for 4 freeze-thaw cycles, and for 16 h at room temperature. The autosampler reinjection stability and the processed sample stability for ManNAc in both 5% BSA and human plasma were established for 48 h at 2–8 °C. Similarly, the stability of Neu5Ac in both 5% BSA and human plasma was established for at least 309 days at –80 °C, for 4 freeze-thaw cycles, for 16 h at room temperature, for 96 h at 2–8 °C in the autosampler after extraction, and for 48 h at 2–8 °C comparing the processed QC samples against a freshly prepared curve.
Table 3.
Stability results for ManNAc and Neu5Ac in LQC (5% BSA) and in HQC (human plasma).
| ManNAc | LQC (30.0 ng/mL) | HQC (4051 ng/mL) |
|---|---|---|
| Deviation (%) | Deviation (%) | |
| Room temperature for 16 h | 8.4 | −7.8 |
| Freeze/thaw 4 cycles | 1.1 | 0.3 |
| Autosampler at 2–8 °C for 48 h | 8.5 | −4.7 |
| Processed sample at 2–8 °C for 48 h | 0.0 | −1.6 |
| Long-term at −80 °C for 310 days | 2.2 | 2.4 |
| Neu5Ac | LQC (75.0 ng/mL) | HQC (8159 ng/mL) |
|---|---|---|
| Deviation (%) | Deviation (%) | |
| Room temperature for 16 h | −0.6 | −6.2 |
| Freeze/thaw 4 cycles | 0.2 | −5.8 |
| Autosampler at 2–8 °C for 96 h | −1.6 | 1.8 |
| Processed sample at 2–8 °C for 48 h | 10.1 | −11.1 |
| Long-term −80 °C for 309 days | 3.6 | −5.8 |
4.8 Assay application and reproducibility
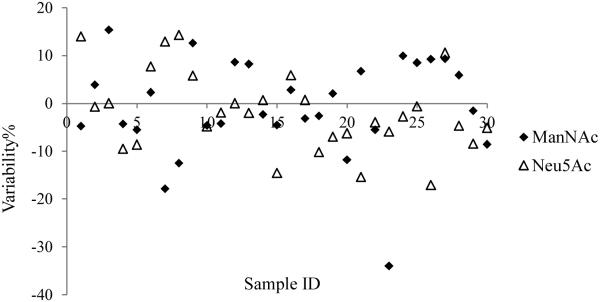
The validated methods were applied in support of a clinical study to measure ManNAc and Neu5Ac plasma concentrations in patients with GNE myopathy after oral administration of ManNAc. Plasma samples were collected from 0 h through 48 h postdose to assess the pharmacokinetics of both ManNAc and Neu5Ac. Representative chromatograms of ManNAc and Neu5Ac, and their corresponding internal standards, are presented in Figs. 4 and 5. To assess assay reproducibility, a total of 30 samples were reanalyzed for incurred sample reanalysis (ISR) test. As shown in Fig. 6, excellent data reproducibility was demonstrated with 96.7% (29 of 30) of the reassayed samples for ManNAc and 100% (30 of 30) of the reassayed samples for Neu5Ac meeting the acceptance criteria (the initial and repeated values within ± 20.0% of the mean values).
Fig. 6.
The incurred sample reanalysis results from the 30 samples tested demonstrated assay reproducibility (96.7% [29 of 30] for ManNAc and 100% [30 of 30] for Neu5Ac).gr6
5. Conclusions
In summary, we have developed LC-MS/MS methods to measure the concentrations of ManNAc and Neu5Ac in human plasma. A “fit-for-purpose” approach was applied for this project. The methods are rapid, specific, sensitive, and robust. Both compounds showed good stability in authentic and surrogate matrices. The validated assays were successfully implemented in the analyses of plasma samples from a clinical study of patients with GNE myopathy. The methodology described herein provides useful experimental approaches in quantifying analytes that are ubiquitous in biologic fluids.
Highlights.
HILIC LC-MS/MS methods for ManNAc and Neu5Ac in human plasma are described.
A fit-for-purpose approach was adopted during method validation and sample analysis.
A surrogate matrix, 5% BSA, was utilized for the preparation of calibration standards.
Extraction with a phospholipid removal plate eliminated matrix effect.
Acknowledgements
This project has been funded in whole or in part with federal funds from the Therapeutics for Rare and Neglected Diseases (TRND), National Center of Advancing Translational Sciences, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors would like to thank Tatiana K. Field at Alliance Pharma for her editorial and review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Boons G-J, Kamerling JP, Lee YC, Suzuki A, Taniguchi N, Voragen AGJ, editors. Comprehensive glycoscience: from chemistry to systems biology. first Elsevier Ltd.; Oxford, UK: 2007. [Google Scholar]
- [2].Varki A. Sialic acids in human health and disease. Trends Mol. Med. 2008;14:351. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009;19:507. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nishino I, Carrillo-Carrasco N, Argov Z. GNE myopathy: current update and future therapy. J. Neurol. Neurosurg. Psychiatry. 2015;86:385. doi: 10.1136/jnnp-2013-307051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huizing M, Krasnewich DM. Hereditary inclusion body myopathy: a decade of progress. Biochim. Biophys. Acta. 2009;1792:881. doi: 10.1016/j.bbadis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stasche R, Hinderlich S, Weise C, Effertz K, Lucka L, Moormann P, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Molecular cloning and functional expression of UDP-N-acetyl-glucosamine 2-epimerase/N-acetylmannosamine kinase. J. Biol. Chem. 1997;272:24319. doi: 10.1074/jbc.272.39.24319. [DOI] [PubMed] [Google Scholar]
- [7].Noguchi S, Keira Y, Murayama K, Ogawa M, Fujita M, Kawahara G, Oya Y, Imazawa M, Goto Y, Hayashi YK, Nonaka I, Nishino I. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J. Biol. Chem. 2004;279:11402. doi: 10.1074/jbc.M313171200. [DOI] [PubMed] [Google Scholar]
- [8].Gagiannis D, Orthmann A, Danssmann I, Schwarzkopf M, Weidemann W, Horstkorte R. Reduced sialylation status in UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (GNE)-deficient mice. Glycoconj. J. 2007;24:125. doi: 10.1007/s10719-006-9019-7. W. [DOI] [PubMed] [Google Scholar]
- [9].Nishino I, Malicdan MC, Murayama K, Nonaka I, Hayashi YK, Noguchi S. Molecular pathomechanism of distal myopathy with rimmed vacuoles. Acta Myol. 2005;24:80. [PubMed] [Google Scholar]
- [10].Niethamer TK, Yardeni T, Leoyklang P, Ciccone C, Astiz-Martinez A, Jacobs K, Dorward HM, Zerfas PM, Gahl WA, Huizing M. Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy. Mol. Genet. Metab. 2012;107:748. doi: 10.1016/j.ymgme.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yonekawa T, Malicdan MC, Cho A, Hayashi YK, Nonaka I, Mine T, Yamamoto T, Nishino I, Noguchi S. Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice. Brain. 2014;137:2670. doi: 10.1093/brain/awu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat. Med. 2009;15:690. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- [13].Crook M. The determination of plasma or serum sialic acid. Clin. Biochem. 1993;26:31. doi: 10.1016/0009-9120(93)90014-w. [DOI] [PubMed] [Google Scholar]
- [14].Matsuno K, Suzuki S. Simple fluorimetric method for quantification of sialic acids in glycoproteins. Anal. Biochem. 2008;375:53. doi: 10.1016/j.ab.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [15].Teshima S, Tamai K, Hayashi Y, Emi S. New enzymatic determination of sialic acid in serum. Clin. Chem. 1988;34:2291. [PubMed] [Google Scholar]
- [16].Haverkamp J, van Halbeek H, Dorland L, Vliegenthart JF, Pfeil R, Schauer R. High-resolution 1H-NMR spectroscopy of free and glycosidically linked O-acetylated sialic acids. Eur. J. Biochem. 1982;122:305. doi: 10.1111/j.1432-1033.1982.tb05881.x. [DOI] [PubMed] [Google Scholar]
- [17].Stankovics J, Molnar D, Burus I, Pinter Z. Infantile sialic acid storage disease diagnosed by gas chromatography-mass spectroscopy analyses of urine sample. J. Inherit. Metab. Dis. 1997;20:728. doi: 10.1023/a:1005359417508. [DOI] [PubMed] [Google Scholar]
- [18].Siskos PA, Spyridaki MH. Determination of sialic acids in biological fluids using reversed-phase ion-pair high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1999;724:205. doi: 10.1016/s0378-4347(98)00543-x. [DOI] [PubMed] [Google Scholar]
- [19].Makatsori E, Fermani K, Aletras A, Karamanos NK, Tsegenidis T. Screening of N-acylneuraminic acids in serum and tissue specimens of mouse C57BI with Lewis' lung cancer by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1998;712:23. doi: 10.1016/s0378-4347(98)00150-9. [DOI] [PubMed] [Google Scholar]
- [20].Galuska SP, Geyer H, Weinhold B, Kontou M, Röhrich RC, Bernard U, Gerardy-Schahn R, Reutter W, Münster-Kühnel A, Geyer R. Quantification of nucleotide-activated sialic acids by a combination of reduction and fluorescent labeling. Anal. Chem. 2010;82:4591. doi: 10.1021/ac100627e. [DOI] [PubMed] [Google Scholar]
- [21].van der Ham M, Prinsen BH, Huijmans JG, Abeling NG, Dorland B, Berger R, de Koning TJ, de Sain-van der Velden MG. Quantification of free and total sialic acid excretion by LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;848:251. doi: 10.1016/j.jchromb.2006.10.066. [DOI] [PubMed] [Google Scholar]
- [22].Tebani A, Schlemmer D, Imbard A, Rigal O, Porquet D, Benoist JF. Measurement of free and total sialic acid by isotopic dilution liquid chromatography tandem mass spectrometry method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:3694. doi: 10.1016/j.jchromb.2011.10.009. [DOI] [PubMed] [Google Scholar]
- [23].Yasuno S, Kokubo K, Kamei M. New method for determining the sugar composition of glycoproteins, glycolipids, and oligosaccharides by high-performance liquid chromatography. Biosci. Biotechnol. Biochem. 1999;63:1353. doi: 10.1271/bbb.63.1353. [DOI] [PubMed] [Google Scholar]
- [24].Cummings J, Ward TH, Dive C. Fit-for-purpose biomarker method validation in anticancer drug development. Drug Discov. Today. 2010;15:816. doi: 10.1016/j.drudis.2010.07.006. [DOI] [PubMed] [Google Scholar]
- [25].Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, Keller S, Weinryb I, Green M, Duan L, Rogers JA, Millham R, O'Brien PJ, Sailstad J, Khan M, Ray C, Wagner JA. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm. Res. 2006;23:312. doi: 10.1007/s11095-005-9045-3. [DOI] [PubMed] [Google Scholar]
- [26].Wang J, Lee J, Burns D, Doherty D, Brunner L, Peterson M, DeSilva B. “Fit-for-purpose” method validation and application of a biomarker (C-terminal telopeptides of type 1 collagen) in denosumab clinical studies. AAPS J. 2009;11:385. doi: 10.1208/s12248-009-9115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jian W, Edom RW, Weng N. Important considerations for quantitation of small-molecule biomarkers using LC-MS. Bioanalysis. 2012;4:2431. doi: 10.4155/bio.12.247. [DOI] [PubMed] [Google Scholar]
- [28].Houghton R, Horro Pita C, Ward I, Macarthur R. Generic approach to validation of small-molecule LC-MS/MS biomarker assays. Bioanalysis. 2009;1:1365. doi: 10.4155/bio.09.139. [DOI] [PubMed] [Google Scholar]
- [29].Jian W, Edom RW, Weng N. Recent advances in application of hydrophilic interaction chromatography for quantitative bioanalysis. J. Sep. Sci. 2010;33:681. doi: 10.1002/jssc.200900692. [DOI] [PubMed] [Google Scholar]
- [30].Jian W, Xu Y, Edom RW, Weng N. Analysis of polar metabolites by hydrophilic interaction chromatography-MS/MS. Bioanalysis. 2011;3:899. doi: 10.4155/bio.11.51. [DOI] [PubMed] [Google Scholar]
- [31].Shou WZ, Weng N. Simple means to alleviate sensitivity loss by trifluoroacetic acid (TFA) mobile phases in the hydrophilic interaction chromatography-electrospray tandem mass spectrometric (HILIC-ESI/MS/MS) bioanalysis of basic compounds. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;825:186. doi: 10.1016/j.jchromb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [32].Buhrman DL, Price PI, Rudewiczcor PJ. Quantitation of SR 27417 in human plasma using electrospray liquid chromatography-tandem mass spectrometry: A study of ion suppression. J. Am. Soc. Mass Spectrom. 1996;7:1099. doi: 10.1016/S1044-0305(96)00072-4. [DOI] [PubMed] [Google Scholar]
- [33].Jemal M, Xia YQ. LC-MS Development strategies for quantitative bioanalysis. Curr. Drug Metab. 2006;7:491. doi: 10.2174/138920006777697927. [DOI] [PubMed] [Google Scholar]
- [34].Liu G, Ji QC, Arnold ME. Identifying, evaluating, and controlling bioanalytical risks resulting from nonuniform matrix ion suppression/enhancement and nonlinear liquid chromatography-mass spectrometry assay response. Anal. Chem. 2010;82:9671. doi: 10.1021/ac1013018. [DOI] [PubMed] [Google Scholar]
- [35].Wang S, Cyronak M, Yang E. Does a stable isotopically labeled internal standard always correct analyte response? A matrix effect study on a LC/MS/MS method for the determination of carvedilol enantiomers in human plasma. J. Pharm. Biomed. Anal. 2007;43:701. doi: 10.1016/j.jpba.2006.08.010. [DOI] [PubMed] [Google Scholar]
- [36].Cote C, Bergeron A, Mess JN, Furtado M, Garofolo F. Matrix effect elimination during LC-MS/MS bioanalytical method development. Bioanalysis. 2009;1:1243. doi: 10.4155/bio.09.117. [DOI] [PubMed] [Google Scholar]
- [37].Chambers E, Wagrowski-Diehl DM, Lu Z, Mazzeo JR. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;852:22. doi: 10.1016/j.jchromb.2006.12.030. [DOI] [PubMed] [Google Scholar]
- [38].Liu G, Snapp HM, Ji QC, Arnold ME. Strategy of accelerated method development for high-throughput bioanalytical assays using ultra high-performance liquid chromatography coupled with mass spectrometry. Anal. Chem. 2009;81:9225. doi: 10.1021/ac901316w. [DOI] [PubMed] [Google Scholar]
- [39].Lahaie M, Mess JN, Furtado M, Garofolo F. Elimination of LC-MS/MS matrix effect due to phospholipids using specific solid-phase extraction elution conditions. Bioanalysis. 2010;2:1011. doi: 10.4155/bio.10.65. [DOI] [PubMed] [Google Scholar]
- [40].Sancho JV, Pozo OJ, López FJ, Hernández F. Different quantitation approaches for xenobiotics in human urine samples by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:639. doi: 10.1002/rcm.617. [DOI] [PubMed] [Google Scholar]
- [41].Ji QC, Rodila R, Gage EM, El-Shourbagy TA. A strategy of plasma protein quantitation by selective reaction monitoring of an intact protein. Anal. Chem. 2003;75:7008. doi: 10.1021/ac034930n. [DOI] [PubMed] [Google Scholar]