Abstract
We present a case of a 43-year-old woman who presented with a non-ST elevation myocardial infarction. During her first cardiac catheterisation, she was diagnosed with a chronic total occlusion of the right coronary artery and a flow limiting dissection of her middle left anterior descending artery. The dissection of the left anterior descending artery was stented with two overlapping everolimus-eluting stents. There were no complications from this percutaneous coronary intervention. On the following day, the patient continued to have persistent chest pain and returned to the catheterisation laboratory. It was then found that the patient had a total occlusion of the right coronary artery secondary to dissection. This was also stented with three everolimus-eluting stents with excellent clinical and angiographic results. It is important to consider spontaneous multivessel coronary dissections which can be treated successfully with percutaneous coronary intervention.
Background
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome with over 200 cases reported in the literature.1 Multivessel involvement is less common than single vessel disease and is found most commonly in the postpartum period. This case demonstrates an unusual presentation of multivessel SCAD in an otherwise healthy non-postpartum female.
Case presentation
A 43-year-old African American woman presented with a 1 h history of typical substernal chest pressure that occurred after she used the valsalva manoeuvre during a bowel movement. Her pain was sudden in onset and was associated with shortness of breath. While en route to the emergency room, she was given sublingual nitroglycerin and nitropaste which relieved her chest pain. Her medical history was remarkable for hypertension. She denied any previous history of cardiovascular disease. The patient had two successful pregnancies with children aged 13 and 24. She denied any history of tobacco, alcohol or illicit substances. Her family history was significant for her father suffering a nonfatal myocardial infarction at the age of 50. There was no history of familial or hereditary connective tissue disorders. She was not on oral contraceptives or estrogen hormone therapy. Her vital signs on presentation to the emergency room were 123/70 mm Hg, heart rate 95 beats/min, respiratory rate 20 breaths/min, temperature 36.8°C, and oxygen saturation of 98% on room air. Her physical examination was unremarkable without evidence of elevated jugular venous pressure, murmurs or crackles. Her electrocardiography (ECG) showed no ST changes or T-wave inversions.
Investigations
Her complete blood count was remarkable for a white blood count of 16 800/mm3, haemoglobin 12.5 g/dl, haematocrit 37.5% and normal platelet level. Her chemistries were remarkable for potassium of 2.8 mmol/l and normal renal function. Her coagulation laboratories were unremarkable and the first set of cardiac markers showed a troponin T 0.011 ng/ml (normal<0.01), a creatine kinase MB (CKMB) of 2.9 ng/ml and a creatine kinase (CK) of 116 IU/l. The presenting ECG was normal sinus rhythm without ischaemic changes.
From the emergency department, she was admitted to cardiology service for overnight observation and started on aspirin for anticoagulation. Her next set of cardiac markers 6 h later was significant for an elevation in a troponin T of 0.219 ng/ml, a CKMB of 10.6 ng/ml and a CK of 146 IU/l. The patient was started on lovenox 1 mg/kg subcutaneously twice a day. One hour later, the patient again experienced substernal chest pressure, this time with T-wave inversions in the anterior leads. A nitroglycerin drip was initiated with resolution of her chest pain. The following morning she underwent cardiac catheterisation.
Treatment
On the initial cardiac angiography the patient was found to have a dissection of the mid left anterior descending artery (LAD) (figure 1) confirmed by intravascular ultrasound (IVUS) and a chronic total occlusion (CTO) of her proximal right coronary artery (RCA). Her mid LAD was treated with two overlapping everolimus-eluting stents (Xience V, Abbott Vascular) with excellent results (figure 2) with a plan to possibly stage the RCA lesion at a later time as the LAD was thought to be the culprit lesion.
Figure 1.
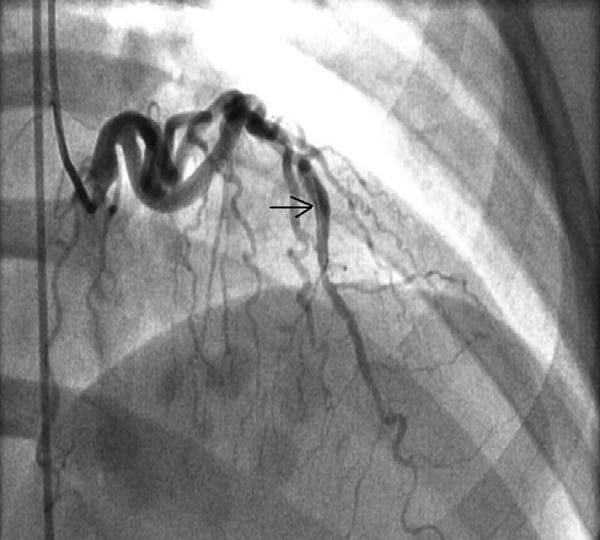
The left anterior descending artery (LAD) dissection, indicated by the black arrow, is visible above.
Figure 2.
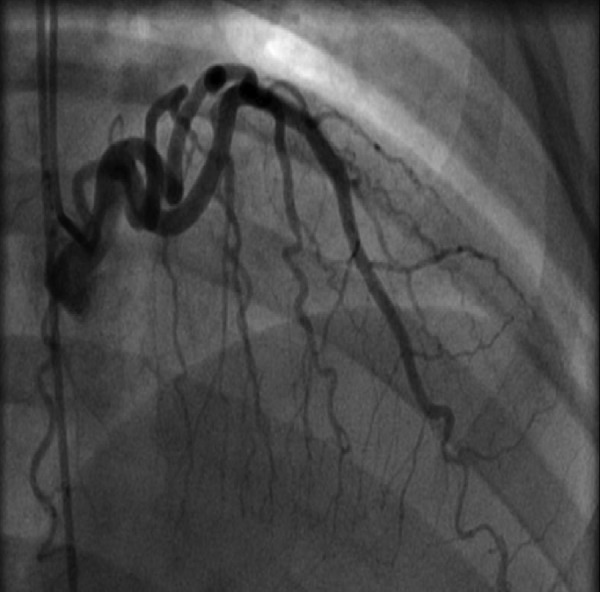
The left anterior descending artery (LAD)—region is shown after percutaneous coronary intervention, with resolution of the LAD lesion.
Twenty-four hours after initial catheterisation the patient continued to have recurrent substernal chest pain and a nitroglycerin drip was restarted. She was referred for repeat cardiac catheterisation and evaluation of the RCA lesion. What was originally noted to be a total occlusion of the RCA was a coronary dissection of the RCA (figure 3). The wire crossed the lesion easily and IVUS was used to confirm wire placement in the true lumen. The RCA dissection was treated with three Xience drug eluting stents with excellent angiographic results and complete resolution of her chest pain (figure 4).
Figure 3.
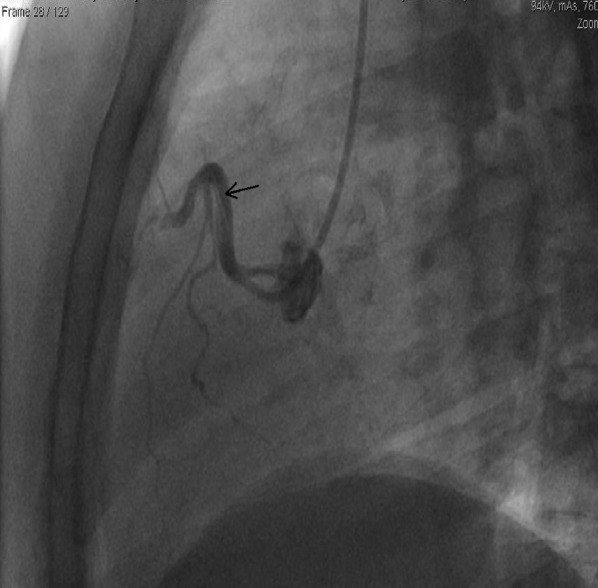
The patient was found to have a dissection in the right coronary artery (RCA), indicated by a black arrow.
Figure 4.
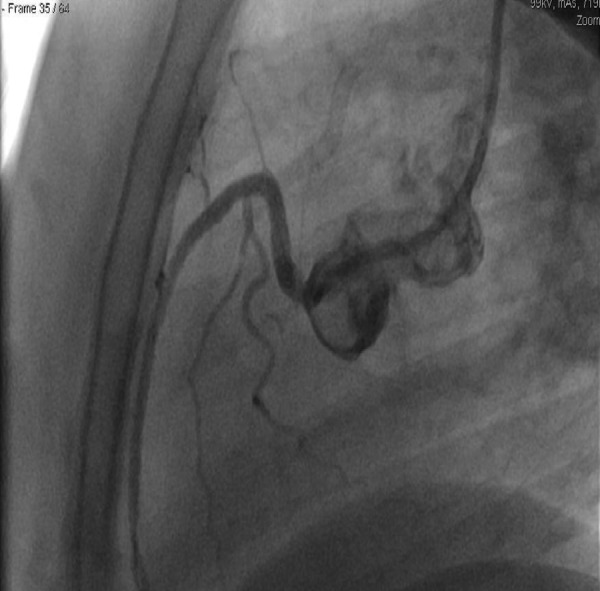
The right coronary artery (RCA)—the dissection plane is no longer visualised after percutaneous coronary intervention to the RCA.
For the remainder of hospitalisation, her hypertension proved resistant to medical therapy, eventually requiring four agents to obtain control. The elevated blood pressure was presumed to be secondary to hyperaldosteronism given her associated hypokalaemia. Unfortunately, she had already started on spirolactone before it was discovered that the laboratories for the aldosterone renin ratio were haemolysed. For the remainder of her 7 day hospital course she had no further episodes of chest pain and was discharged home on aspirin and clopidogrel.
Outcome and follow-up
The patient did very well after treatment of both her RCA and LAD lesions. After an extensive clinical and laboratory evaluation, to include a rheumatology consultation, there was no evidence of a connective tissue disorders. Her blood pressure proved challenging to control, and a CT scan of the abdomen demonstrated normal vasculature without signs of dissection or stenosis such as fibromuscular dysplasia in the renal arteries. At her 1 year follow-up appointment, the patient had a normal stress echocardiogram and a normal ejection fraction.
Discussion
SCAD is an uncommon cause of acute coronary syndrome. It is estimated that approximately 0.1–1.1% of acute coronary syndrome presentations are due to SCAD.2 Patients typically present with acute coronary syndrome, ventricular tachycardia, or sudden cardiac death.1 3–6 SCAD most commonly affects women and 80% are associated with the peripartum or postpartum period.6–10 There have also been cases associated with methamphetamine use,6 Ehlers-Danlos syndrome,11 Trousseau's syndrome, Marfan's syndrome, oral contraceptive use and severe hypertension.12
Isolated vessel involvement is much more common and involves the LAD in 75% of cases, right coronary artery in 20% and left circumflex in 4% with left main disease accounting for <1% of cases.13 Multiple vessel involvement is much less frequent and tends to involve the LAD and the RCA together as seen in our case.1 14 Most multivessel SCAD also occurs in the peripartum period.15
The mechanism of ischaemia is well described but the underlying pathophysiology that leads to dissection remains unclear. Initial presentations of SCAD were found on autopsy and it was hypothesised that expansion of the false lumen by bleeding or thrombosis compresses the true lumen which ultimately leads to ischaemia.16 Proposed mechanisms that lead to clot formation in the false lumen include an underlying inflammatory process,17 plaque rupture with disruption of the intima-media junction1 12 or hormonal changes leading to impaired collagen vascular synthesis.2
Treatment of SCAD varies largely based on the extent of dissection, presence of underlying coronary artery disease and the amount of myocardium affected. Management strategies include medical management with anticoagulants, β-blockers and dual antiplatelet therapy18; percutaneous intervention (PCI); and emergent coronary artery bypass surgery. There have been no studies comparing treatment options for multivessel SCAD; therefore, it is difficult to make clear recommendations on treatment. However, based on our review, it is typical for patients with multivessel disease to be treated with bypass surgery.1 6 10 19 We found one case of multivessel SCAD treated with stents however, in this case, the dissections did not occur simultaneously as the patient had three distinct dissections over a 2 week period.20
At the initial cardiac catheterisation our patient had a dissection in the LAD and a total occlusion of the RCA. During primary intervention it was felt that RCA occlusion was chronic and LAD dissection was the culprit lesion for her NSTEMI. The initial plan was to treat the culprit lesion and medically manage the CTO of the RCA. However, because of the patient's persistent symptoms, she was consequently taken back to the catheterisation laboratory the following day with successful intervention of her RCA dissection. In retrospect, the patient possibly had two spontaneous dissections, one in the LAD and one in the RCA which were both culprit lesions as she continued to have chest pain after successful intervention on the LAD lesion. Since there was a lack of ST elevation and symptom pattern on presentation, it was thought that the RCA lesion was a chronic total occlusion which contributed to focus our attention on the LAD. Once her symptoms failed to resolve and her cardiac enzymes continued to increase, the original diagnosis was questioned and repeat angiography confirmed that the RCA lesion was a dissection and not a chronic total occlusion. After treating the RCA, her cardiac enzymes continued to trend down. Spontaneous multivessel coronary artery dissection can occur simultaneously and PCI can be used to successfully treat multivessel SCAD.
Learning points.
Spontaneous coronary artery dissection (SCAD) should be considered in any patient presenting without cardiac risk factors with evidence of cardiac ischaemia.
Although multivessel SCAD is more common in the peripartum period it must still be considered in otherwise healthy individuals.
Percutaneous intervention is reasonable for patients with multivessel SCAD.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Karabag T, Dogan SM. A case of spontaneous multivessel coronary artery dissection presenting with acute myocardial infarction and ventricular tachycardia. Catheter Cardiovasc Interv 2012;79:113–16. [DOI] [PubMed] [Google Scholar]
- 2.Almeda FQ, Barkatullah S, Kavinsky CH. Spontaneous coronary artery dissection. Clin Cardiol 2004;27:377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCann AB, Whitbourn RJ. Spontaneous coronary artery dissection: a review of the etiology and available treatment options. Heart Vessels 2009;24:463–5. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a western Denmark Heart Registry Study. Catheter Cardiovasc Interv 2009;74:710–17. [DOI] [PubMed] [Google Scholar]
- 5.Dakik HA, Nader GA, Arja WA, et al. Asymptomatic spontaneous coronary artery dissection. Clin Cardiol 2010;33:E40–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwar M, Gill N. Spontaneous multivessel coronary artery dissection. J Invasive Cardiol 2010;22:E5–6. [PubMed] [Google Scholar]
- 7.Sanchez-Reclade A, Garcia S, Lopez T, et al. Multiple spontaneous coronary artery dissections postpartum: serial follow-up of intravascular ultrasound findings. Can J Cardiol 2010;26:E215–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder C, Stoler RC, Branning GB, et al. Postpartum multivessel spontaneous coronary artery dissection confirmed by coronary CT angiography. Proc (Bayl Univ Med Cent) 2006;19:338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzarelli S, Fiscella D, Amico F, et al. Multivessel spontaneous coronary artery dissection in a postpartum woman treated with multiple drug-eluting stents. J Cardiovasc Med 2009;10:340–3. [DOI] [PubMed] [Google Scholar]
- 10.Karadag B, Roffi M. Postpartal dissection of all coronary arteries in an In Vitro-Fertilized Postmenopausal Woman. Texas Heart J 2009;36:168–70. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura M, Yajima J, Oikawa Y, et al. Vascular Ehlers-Danlos syndrome—all three coronary artery spontaneous dissections. J of Cardiol 2009;53:458–62. [DOI] [PubMed] [Google Scholar]
- 12.Karabay CY, Biteker M, Zehir R, et al. Multiple spontaneous coronary artery dissection associated with Trousseau's syndrome. Cardiol J 2010;17:625–7.. [PubMed] [Google Scholar]
- 13.Thompson EA, Ferraris S, Gres T, et al. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J Invasive Cardiol 2005;17:59–61. [PubMed] [Google Scholar]
- 14.Cohen DE, Strimike CL. A case of multiple spontaneous coronary artery dissections. Catheter Cardiovasc Interv 2000;49:318–20. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Kim SH, Lim SY, et al. Simultaneous and spontaneous multivessel coronary artery dissection presenting as congestive heart failure. Heart Vessels 2010;26:338–41. [DOI] [PubMed] [Google Scholar]
- 16.Mulvany NJ, Ranson DL, Pilbeam MC. Isolated dissection of the coronary artery: a postmortem study of seven cases. Pathology 2001;33:307–11. [PubMed] [Google Scholar]
- 17.Robinowitz M, Virmani R, McAllister H. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med 1982;72:923–8. [DOI] [PubMed] [Google Scholar]
- 18.Hunt B, Chua R, Bett N. Conservative treatment of spontaneous dissection of multiple coronary arteries. Heart Lung Circ 2010;19:678–80. [DOI] [PubMed] [Google Scholar]
- 19.Konstantinov I, Saxena P, Shehatha J. Spontaneous multivessel coronary artery dissection: surgical management in a postmenopausal woman. Texas Heart Inst J 2009;36:360–1. [PMC free article] [PubMed] [Google Scholar]
- 20.Hardegree EL, Tweet MS, Hayes SN, et al. Multivessel spontaneous coronary artery dissection associated with hormonal infertility therapy in a 39-year-old female. J Cardiol Cases 2012;5:e69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


