Abstract
Basophils have recently been identified as antigen-presenting cells that are required for optimal antibody responses. New findings now show that activation of these cells can amplify autoimmune responses in systemic lupus erythematosus (SLE).
Since the first clinical trials of tumor necrosis factor antagonists for the treatment of rheumatoid arthritis were initiated almost two decades ago, individuals with SLE have lived with the hope that an effective treatment for them is just around the corner. Many clinical trials have recently been performed in subjects with lupus using various strategies, including conventional immunosuppressant agents, cell depletion approaches, antigen-specific immunomodulation and targeting of antigen–nonspecific, immune-activating molecules.
Despite a marked improvement in disease activity and longevity in lupus-prone mice, the effects of these agents have been much less encouraging in humans. Patients with lupus continue to suffer from treatment-induced toxicities and early cardiovascular death and continue to progress to end-stage renal disease. What are we missing in our understanding of this disease?
In this issue of Nature Medicine, Charles et al.1 help fill in the gaps. The researchers report that basophils—rare granulocytes that protect from parasites and contribute to allergic reactions—also help drive spontaneous SLE nephritis in a mouse model. When activated by immune complexes containing IgE autoantibodies, basophils increased their surface expression of major histocompatibility complex (MHC) class II and B cell–activating factor belonging to the TNF family (BAFF)—a molecule that supports B cell differentiation and survival. Basophils also migrated to secondary lymphoid organs, where they secreted the cytokine interleukin-4 (IL-4), which helps drive a T helper type 2 (TH2)-mediated response, in which the activated T cells also make IL-4 and antibody production is skewed toward the IgG1 and IgE isotypes. The researchers also showed that people with SLE have both serum IgE autoantibodies and activated circulating basophils that can migrate to the spleens and lymph nodes1.
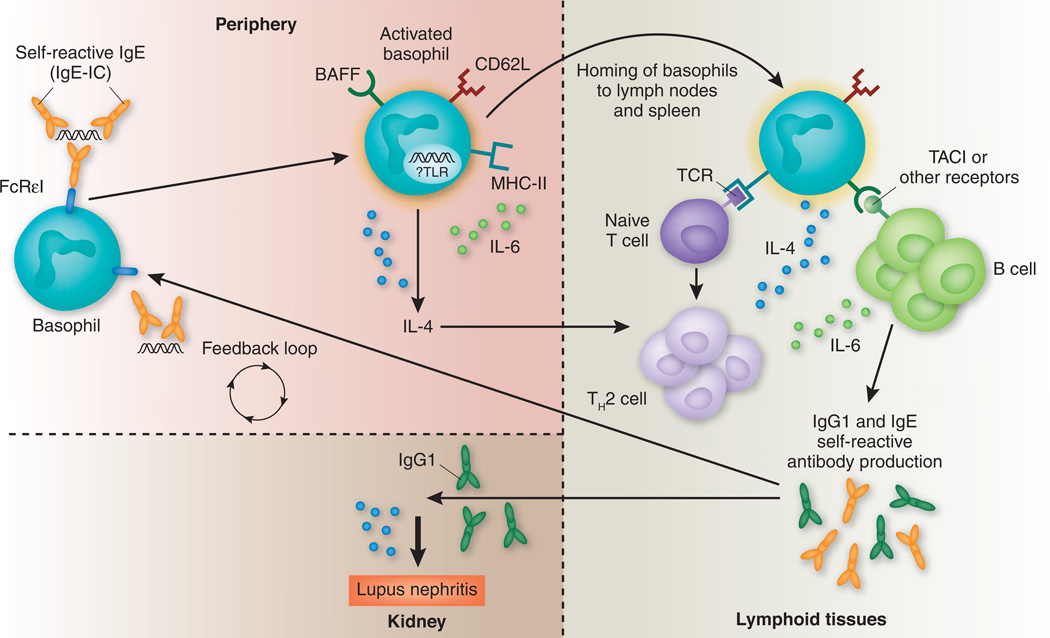
Because BAFF and IL-4 help induce class switching to IgE, this study suggest that exacerbation of SLE disease activity by autoreactive IgE is a new disease amplification loop, with basophils as a central component (Fig. 1).
Figure 1.
Basophil activation by autoreactive IgE. Peripheral blood basophils are activated by IgE-containing immune complexes (IgE-IC) to upregulate CD62L that allows them to migrate into lymphoid organs. Upregulation of MHC class II (MHC-II) confers antigen-presenting properties, and upregulation of IL-4 promulgates a TH2 (IL-4–producing) T cell phenotype. IL-4, together with increased basophil cell surface expression of BAFF and increased production of IL-6 from multiple cell types (including basophils), enhances autoantibody class switching to IgG1 that can deposit in the kidneys and IgE that further perpetuates basophil activation. TACI, transmembrane activator and calcium modulator ligand interactor.
Several groups have recently identified a subset of basophils capable of presenting antigen and activating TH2 cells in the absence of dendritic cells. These basophils express MHC class II and CD62 ligand (CD62L), a receptor for homing of leukocytes to secondary lymphoid tissues. They also produce IL-4, a cytokine that induces TH2 responses but blocks TH1 immune responses. The basophils also secrete IL-6, which enhances T cell–mediated B cell proliferation and immunoglobulin production2–5.
How basophils capture antigen is still a matter of debate. Some studies have suggested that they directly endocytose soluble antigens4 or, alternatively, that they ingest IgE-antigen complexes through the high-affinity IgE receptor FcεRI (ref. 5). Basophils constitutively express TLR9 (ref. 6), but whether nucleic acid–containing IgE immune complexes found in individuals with SLE activate basophils more effectively than complexes containing conventional antigens is still unknown. It is also not clear whether the upregulation of BAFF on basophils reported by Charles et al.1 is simply a consequence of FcεRI engagement or requires concomitant TLR9 engagement, and whether BAFF is responsible for basophil-mediated class switching and immunoglobulin production3. Activated basophils migrate to secondary lymphoid organs, but it is still unknown how and where they interact with T cells, B cells or both.
To examine the role of basophils in lupus, Charles et al.1 used mice deficient in the Src family tyrosine kinase Lyn, a mouse model of SLE. The B cells of such mice are defective in phosphorylation of inhibitory receptors, resulting in B cell hyperactivation, production of antinuclear antibodies and a mild form of glomerulonephritis that develops at a late age. Disease in these mice is dependent on both IL-6 and myeloid differentiation factor-88 (refs. 7,8).
Charles et al.1 found that depletion of basophils ameliorated glomerulonephritis in this mouse SLE model. The researchers then tested mice deficient in IgE and IL-4 and found a similar effect1. The findings, however, need to be interpreted carefully, as the role of IL-4 in SLE can be quite variable. IL-4 antagonism ameliorates nephritis in one inbred lupus strain that, like Lyn-deficient mice, has high serum amounts of IL-4 and IgE autoantibodies9, but administration of IL-4 also protects from nephritis in other lupus-prone strains by decreasing autoantibody titers10. These differences illustrate the complexity of the immunological defects that may be found in SLE.
SLE is characterized by perturbations in the function of diverse cell types, including B cells, T cells, monocytes, plasmacytoid dendritic cells, granulocytes and resident cells in target organs. Autoantibodies of multiple isotypes deposit in sites of inflammation, and increased concentrations of cytokines and chemokines are present in the circulation of individuals with SLE. CD4+ T cells are crucial components of protective immune responses and of immune responses in SLE—upon differentiation CD4+ T cells exert diverse effector functions that correlate with the profile of cytokines that they secrete.
Exaggerated TH111, TH29, TH1712 and follicular T helper cells13 responses—which together span almost the entire range of T cell functional profiles—have all been associated with autoantibody production in various mouse lupus models or in humans with SLE, and their effector cytokines may also mediate different types of inflammation in target organs. The nature of the cytokine response may reflect the pathway that is first activated, which could be dictated by genetic factors that skew toward a particular type of effector T cell response or could reflect the nature of the particular environmental trigger that breaks tolerance, such as helminths or viruses.
Which of the T helper–mediated responses found in the various mouse models is most likely to represent human disease? A number of studies suggest that diffuse proliferative glomerulonephritis in humans is characterized by renal TH1 polarization11, but, as new T cell subsets continue to be discovered, and it is increasingly recognized that T helper lineage commitment may be flexible14, this issue is far from settled. Because interferon-γ, the primary TH1 cytokine, can negatively regulate both basophils and IL-4 production, it remains to be determined whether the 30% of individuals with SLE with high concentrations of IgE antinuclear antibodies15 will be the ones to respond to therapeutic basophil modulation, as suggested by this study of the Lyn-deficient model.
These considerations leave us with a therapeutic conundrum. Autoantibodies are central to SLE disease, because they can directly activate cells expressing intracellular nucleic acid sensors and can deposit in multiple organs, leading to systemic inflammation, tissue injury and amplification of pathways for perpetuating both autoimmunity and tissue damage. However, we still do not know how to choose crucial pharmacologic targets from among those predicted by mouse models, especially because some of the activation pathways identified are opposing. Furthermore, once amplification pathways are established, we might not effectively treat disease by targeting a single cell type or a single activation pathway.
Although the ultimate goal is to personalize treatment for individual patients and for each disease stage, we need to be careful not to initiate potentially toxic interventions too early or to block pathways that may be protective in some patients.
Work such as that presented by Charles et al.1 continues to reveal pathogenetic mechanisms of SLE and to suggest that we still have more therapeutic strategies to consider. Whether basophils will turn out to be central players in nephritis in some individuals with lupus is now an important question that can only be addressed by studies blocking basophil activation in humans, including, perhaps, a clinical trial in appropriately selected subjects.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Charles N, et al. Nat. Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denzel A, et al. Nat. Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, et al. Nat. Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol CL, et al. Nat. Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimoto T, et al. Nat. Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 6.Komiya A, et al. Int. Arch. Allergy Immunol. 2006;140(Suppl 1):23–27. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 7.Silver KL, et al. Eur. J. Immunol. 2007;37:2734–2743. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 8.Tsantikos E, et al. J. Immunol. 2010;184:1348–1360. doi: 10.4049/jimmunol.0901878. [DOI] [PubMed] [Google Scholar]
- 9.Singh RR, et al. J. Immunol. 2003;170:4818–4825. doi: 10.4049/jimmunol.170.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi T, et al. Nephrol. Dial. Transplant. 2007;22:3131–3138. doi: 10.1093/ndt/gfm465. [DOI] [PubMed] [Google Scholar]
- 11.Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F. Clin. Exp. Immunol. 2008;154:247–254. doi: 10.1111/j.1365-2249.2008.03758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu HC, et al. Nat. Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 13.Simpson N, et al. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 14.O’Shea JJ, Paul WE. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atta AM, Santiago MB, Guerra FG, Pereira MM, Sousa Atta ML. Int. Arch. Allergy Immunol. 2010;152:401–406. doi: 10.1159/000288293. [DOI] [PubMed] [Google Scholar]