Abstract
Notch is an integral membrane protein that functions as receptor for ligands such as jagged and delta that are associated with the surface of neighboring cells. Upon ligand binding, notch is proteolytically cleaved within its transmembrane domain by presenilin-1 (the enzymatic component of the γ-secretase complex) resulting in the release of a notch intracellular domain (NICD) which translocates to the nucleus where it regulates gene expression. Notch signaling plays multiple roles in the development of the central nervous system (CNS) including regulating neural stem cell (NSC) proliferation, survival, self-renewal and differentiation. Notch is also present in postmitotic neurons in the adult CNS wherein its activation influences structural and functional plasticity including processes involved in learning and memory. Recent findings suggest that notch signaling in neurons, glia and NSCs may be involved in pathological changes that occur in disorders such as stroke, Alzheimer’s disease and CNS tumors. Studies of animal models suggest the potential of agents that target notch signaling as therapeutic interventions for several different CNS disorders.
Keywords: Notch, Neural stem cells, Development, Synaptic plasticity, Brain Ischemia and Neurodegeneration
Notch proteins are cell surface transmembrane receptors that mediate multiple important cellular functions through cell-cell interactions. Four paralogs of the notch gene (notch 1–4) and five ligands (jagged 1 and 2; Delta 1, 2 and 3) have been identified in vertebrates (Ohishi et al., 2002). Notch-1 (commonly referred to as notch) has been widely investigated, while other notch’s have been studied minimally. Notch proteins contain extracellular EGF (epidermal growth factor) like repeats which interact with DSL domain of ligands. Activation of notch upon ligand binding is accompanied by proteolytic cleavage of notch by presenilin-1/γ-secretase which releases an intracellular domain of notch (NICD) from the membrane tether. Upon release, the NICD translocates to the nucleus where it associates with the CSL [CBF1/RBPJk/Su(H)/Lag1] family of DNA-binding proteins which represses or activates transcription via the recruitment of chromatin remodeling complexes containing histone deacetylase or histone acetylase proteins, respectively. NICD-CSL initiates transcriptional activation (Kramer, 2000; Kopan, 2002) through multiple mechanisms including: 1) recruitment of HATs (histone acetylases); 2) conversion of CSL from a transcriptional repressor to a transcriptional activator by interacting with SKIP (ski related protein) to dissociate SMRT (silencing mediator of retinoid and thyroid hormone receptor)/HDAC (histone deacetyalse); 3) recruitment of Mastermind/Lag3 to activate additional targets. Most of the notch target genes encode transcription regulators, many of which are critical in CNS development such as brain lipid binding protein (Anthony et al., 2005) and tissue specific basic helix-loop-helix gene families such as hairy enhancer of split (HES). Cell fate determination or other events influenced by notch signaling can also result from interaction with other molecular targets, such as NF-κB (Kramer, 2000; Liu et al., 2003) or the RNA-binding protein musashi (Imai et al., 2001). An overview of the notch signaling pathway is illustrated in Figure 1.
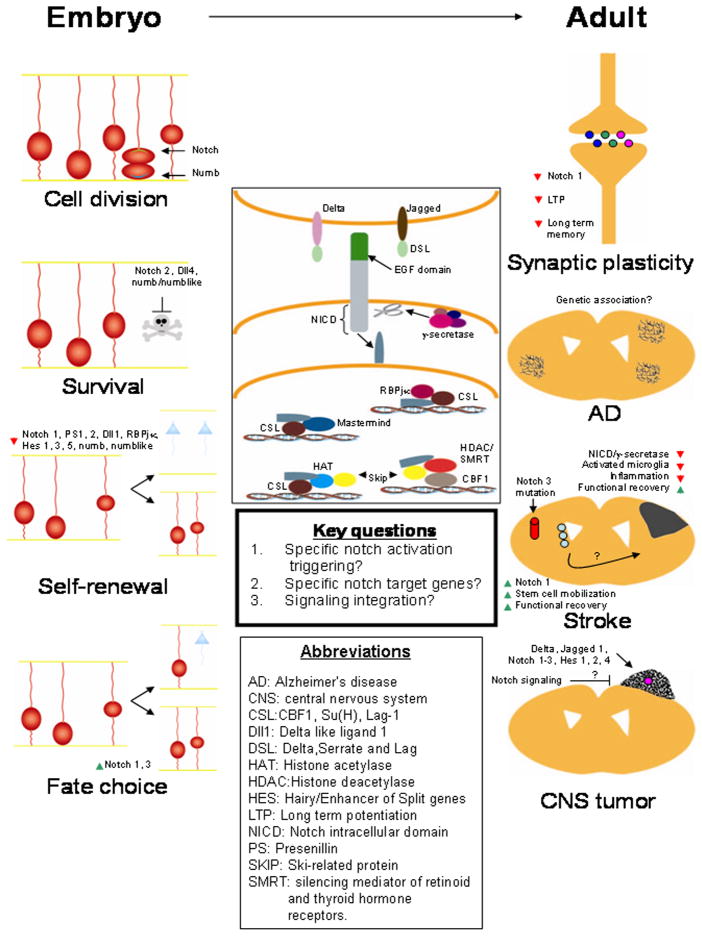
Figure 1.
Roles of notch signaling in development and neurological disorders.
The illustration depicts the canonical notch signaling pathway (center) and involvement in developmental processes (left) as well as adult neurological disorders (right). Neighboring cells possess the notch ligand (jagged or delta) and the target cell contains the notch receptor. Activation of notch upon ligand binding will release the notch intracellular domain (NICD) as a result of its cleavage by γ-secretase. NICD then translocates into the nucleus where it can interact with CSL and convert CSL from a transcriptional repressor to transcriptional activator. This conversion occurs by direct protein-protein interactions between NICD, SKIP and CSL, which leads to SMRT/HDAC dissociation. Notch/CSL recruit HATs to assist chromatin remodeling, and RBPJk and mastermind to activate specific targets. Notch signaling plays an important role during CNS development (left). During neural stem cell (NSC) divisions, numb is concentrated in the apical cell while notch is concentrated in the basal cell. NSC survival is promoted by notch-2, delta like ligand (Dll) 4, as well as numb and numblike. NSCs undergo precocious neuronal differentiation (blue cells) as a result of a compromised notch-1, presenilin (PS) 1 or 2, Dll1, RBPjκ, HES 1 or 2, numb, or numb like activity. In addition, a radial glia/NSC cell fate can be influenced by over-expression of either notch 1 or 3. Notch signaling has also been implicated in many adult neurological disorders (right). In synaptic plasticity, reduction in notch-1 signaling results in decreased long term potentiation (LTP) as well as reduction in learning and memory. Because mutations in presenilin-1/γ-secretase are linked to some inherited forms of Alzheimer’s disease, notch signaling has been proposed to play a role in this disease. In humans with mutations of notch-3, vascular development is impacted and leads to CADASIL a disorder characterized by cerebral vascular infarctions. In a mouse model of stoke, a reduction of notch 1 and/or γ-secretase leads to decreased activated microglia and inflammation, and improves functional outcome. Notch signaling in NSCs may enhance neurogenesis and functional recovery from a stroke. In CNS tumors, cancer stem cells express high levels of molecules in the notch signaling pathway (notch 1–3, delta, jagged 1, Hes 1, 2, 4), suggesting that modulation of notch signaling could be a potential therapeutic target to inhibit tumor progression.
The notch signaling pathway is an intercellular signaling mechanism critical for organogenesis, regulating an array of cellular processes including stem cell self-renewal, cell fate determination, cellular differentiation and death. In the CNS, notch protein and ligands are not only present in the embryonic stages, but also continuously present in the adult nervous system (Presente et al., 2001). During the entire lifetime, from birth to death, notch is actively involved in dynamic changes in the cellular architecture and function of the nervous system. It controls neurogenesis, the growth of axons and dendrites, synaptic plasticity and ultimately neuronal death. The present article summarizes the roles of notch signaling in the CNS from the embryo through to adulthood, and also considers the involvement of notch signaling in CNS damage and repair.
Notch signaling in NSCs during CNS development
During embryonic development, neurons and glia of the mammalian cerebral cortex are generated from proliferating neuroepithelial cells in the telencephalic ventricular zone (VZ), a zone surrounding the lateral ventricle (The Boulder Committee, 1970; Takahashi et al., 1995). In rodents, it occurs over a matter of a few days. Initially, there is a rapid expansion of NSCs within VZ by symmetric cell divisions, followed by asymmetric cell divisions sequentially generating neuronal and glial cells via differentiation (Temple, 2001). The identity of NSCs also changes during this process from neuroepithelial cells within the VZ to radial glia which span the length of the entire cortex as it thickens during neurogenesis (Fishell and Kriegstein, 2003; Gotz et al., 2005; Merkle et al., 2004). In developing nervous system the proliferation, differentiation and survival of NSC are the fundamental processes responsible for generating sufficient numbers of neurons required for the formation of neuronal circuits. The regulation of these developmental processes has a direct bearing on the cytoarchitecture, function and plasticity of the CNS. Within the embryonic VZ, there are a series of intrinsic and external regulators which are critical to ensure proper development of the telencephalon, and notch signaling represents one such mediator of cell to cell interactions. Although there is data demonstrating notch signaling in the adult NSC population, which persists in the subependymal zone (SEZ) of cerebral cortex and the subgranular zone (SGZ) of the hippocampal dentate gyrus, the majority of evidence for notch signaling in controlling NSC behavior comes from studies during embryonic neurogenesis.
The notch signaling pathway is a well known regulator of many cellular processes essential to development: self-renewal, differentiation, and apoptosis (Artavanis-Tsakonas et al., 1999; Bray, 2006). As the neighboring cell has the notch ligand and the target cell has the notch receptor, this signaling system could be used for lateral inhibition whereby notch signaling in one specific cell inhibits neighboring cells. While there are many examples of this in Drosophila, this mechanism has also been reported to regulate epidermal cells and their differentiated progeny in human skin (Lowell et al., 2000). In the developing CNS, notch signaling in NSC maintains those cells in a proliferating state (Zhong et al., 1997), whereas a protein called numb, which antagonizes notch, promotes cell cycle arrest and neuronal differentiation (Li et al., 2003). In this way, notch signaling controls NSC self-renewal, and cell fate specification. Accordingly, notch-deficient mice, and presenilin-1 knockout mice (in which notch signaling is prevented) exhibit profound abnormalities of brain development (Handler et al., 2000).
Notch, Numb, and NSC cell division
While the role of notch in regulating cell division is not well understood, numb, an antagonist of notch signaling (Spana et al., 1996; Guo et al., 1996; Frise et al., 1996) is a critical component of NSC division in a variety of species. First discovered to play a role in the Drosophila PNS (Uemura et al., 1989) with a similar function in the CNS (Spana et al.), numb preferentially segregates to one of the two spindle poles during cell division and ensures that the two daughter cells adopt different cell fates (Rhyu et al., 1994; Knoblich et al., 1995). The mammalian numb homologue has also been identified and is capable of rescuing the Drosophila mutation (Zhong et al., 1996). In the mammalian VZ, the orientation of the spindle may be indicative of cell fate (Chenn and McConnell, 1995; Haydar et al., 2003). While this model has recently been amended to include the inheritance of a small part of the plasma membrane, termed the apical membrane (Kosodo et al., 2004), a cell dividing with a cleavage plane parallel to the ventricular wall will generate an basal daughter cell which will migrate away from the VZ and an apical daughter cell which will remain a stem cell (Chenn and McConnell, 1995; Haydar et al., 2003). As first shown in the ferret (Chenn and McConnell, 1995; Chenn, 2005) cortex and later in the mouse (Zhong et al., 1996), numb localizes to the apical cell while notch localizes to the basal cell (Fig. 1), suggesting that numb inheritance is a requirement to remain a stem cell. Work in the mouse VZ was followed by a series of in vitro experiments analyzing NSC divisions using time lapse microscopy (Shen et al., 2002). In these experiments, consistent with the in vivo data, numb was asymmetrically distributed when the result of the NSC division was asymmetric and NSCs derived from numb KO mice underwent fewer asymmetric divisions (Shen et al., 2002). However, in NSCs derived from E13 mouse embryos (a period of embryonic neurogenesis in vivo), numb appeared to be asymmetrically distributed to the neuronal daughter cells and not to the NSC (Shen et al., 2002), as would be predicted from previous in vivo studies (Zhong et al., 1996; Chenn et al., 1995).
Notch and NSC behavior
Another role for notch signaling during CNS development is to promote NSC survival, self-renewal, and cell fate specification (neuronal or glial). Several studies have characterized members of the notch signaling pathway in the embryonic VZ. For example, in situ hybridization of the mouse neural tube showed high levels of notch1 and delta1 mRNA (Lindsell et al., 1996). Immunostaining of the VZ at E12 showed high levels of both notch-1 and notch-3 (Gainao et al., 2000; Dang et al., 2006). Using a LacZ transgenic reporter mouse, high expression of delta 1 in the VZ of the forebrain at E10 was also reported (Beckers et al., 2000). Complementary immunostaining of the embryonic human VZ also displayed expression of notch-1, and weak expression of notch-3 as well as strong expression of delta-1 and sporadic expression of jagged-1 (Kostyszyn et al., 2004).
Notch signaling has been shown to be a critical regulator of the behavior of the stem cell population within the VZ (Fig. 1) as demonstrated by several different approaches. For example, Dll4 (delta like ligand 4) administration to NSCs derived from E13.5 mice in vitro increases their survival (Androutsellis-Theotokis et al., 2006), and NSCs differentiated from ES cells lacking RBPJk display a failure of NSC self-renewal (Hitoshi et al., 2002). In addition, intraventricular injection of notch ligands into the brains of adult rats increases the numbers of newly-generated precursor cells (Androutsellis-Theotokis et al., 2006). Interestingly, a recent study utilizing a tamoxifen inducible numb/numblike deletion in the adult mouse SEZ demonstrated the role of notch signaling in ventricular wall integrity and neuroblast survival (Kuo et al., 2006). Activation of either notch-1 or notch-3 in the embryonic forebrain results in increased numbers of cells with a radial glial phenotype (as illustrated in Figure 1) (Gaiano et al., 2000; Dang et al., 2006). Recent re-characterization efforts of the VZ have shown 2 separate mitotic populations within the VZ in both mouse (Gal et al., 2006) and humans (Mo et al., 2007), and a new report has suggested that these two cell populations have a different response to the level of NICD/CBF1 signaling (Mizutani et al., 2005). Finally, although mice with mutations in different parts of the notch signaling pathway show some differences, the majority display a lack of NSC self-renewal and precocious neuronal differentiation (Yoon et al., 2005). While development of the embryonic CNS is a dynamic process, it is clear that notch signaling regulates fundamental processes involved in brain development including NSC self-renewal, survival and differentiation.
Role of notch signaling crosstalk in NSC behavior
The regulation of NSC behaviors involves multiple signaling pathways, some of which interact with the notch signaling pathway; examples include sonic hedgehog, JAK/STAT, RTK, TGF-β and Wnt (Hurlbut et al., 2007). There are several lines of evidence suggesting that notch can interact with growth factor and extracellular matrix (ECM) signals within the NSC niche to promote self-renewal or differentiation. For instance, notch signaling was upregulated in NSCs in vitro by the addition of either FGF1 or FGF2 (Faux et al., 2001), growth factors essential for self-renewal. The process of astrocyte differentiation from NSCs in vitro was promoted by notch cross talk with ciliary neurotrophic factor (CNTF) which activates the signal transducer and activator of transcription (STAT) pathway (Nagao et al., 2007). Crosstalk between notch and epidermal growth factor (EGF) signaling may also occur in NSCs because asymmetric cellular inheritance of notch and numb during asymmetric divisions is associated with asymmetric inheritance of the EGF receptor (Sun et al., 2005). More recently, crosstalk between notch, EGFR and β1 integrin in neural progenitor cells has been suggested by a physical interaction between NICD and the integrin cytoplasmic tail along with the presence of all three receptors within caveolin-containing lipid rafts (Campos et al., 2006). These studies provide evidence that during the complex process of embryonic neurogenesis, notch may not act alone in controlling NSC behavior and thus identification of additional members of the signaling nexus will be critical in elucidating the various roles of notch signaling in neurogenesis.
Notch in adult brain function
As described above, notch is a critical component of an evolutionarily conserved signaling mechanism that regulates many different stages of development. However, notch proteins and ligands are also expressed in cells of the adult nervous system (Presente et al., 2001), suggesting roles in CNS plasticity throughout life. The specific roles of notch in adult brain plasticity and neurological disorders have begun to be unraveled in recent years, and will be discussed in the following sections.
Neurite Remodeling
Axons and dendrites (neurites) are specialized and characteristic structures of a neuron that receive, integrate and transmit signals to other neurons. The complexity of connections between billions of neurons, and the intricate pattern of activity in the neuronal circuits, mediate all behaviors including learning and memory. As neurons differentiate from progenitor cells they rapidly grow neurites and form synapses with target neurons. A typical synapse consists of a presynaptic axon terminal specialized for the concentration and release of a neurotransmitter, and a postsynaptic dendritic spine which contains neurotransmitter receptors and downstream signaling molecules. Neurons in the mature nervous system are also capable of adaptive remodeling which can include neurite outgrowth or retraction, synapse formation or disassembly; and strengthening of existing synapses (Antonini and Stryker, 1993; Trachtenberg et al., 2002; Yuste and Bonhoeffer, 2001; Wong and Ghosh, 2002; Carlisle and Kennedy, 2005). Several studies found that notch signaling serves important functions in the regulation of neurite outgrowth and maintenance. Both Sestan et al., (1999) and Berezovska et al., (1999) found that notch activation inhibits neurite outgrowth or causes their retraction, whereas inhibition of notch-1 signaling promotes neurite extension. Redmond et al., (2000) found that NICD is translocated to the nucleus during neuronal differentiation where it may regulate the expression of genes that influence dendritic morphology. Increasing notch-1 signaling decreases average dendritic length, but increases dendritic branching. The effects of notch signaling on neurite plasticity may be relevant to the phenomenon of developmental pruning (Luo and O’Leary, 2005), which selectively eliminates unneeded synapses during critical periods of development so as to generate highly refined mature neuronal circuits (Fig. 1). Although the molecular mechanism by which notch regulates neurite remodeling is not totally clear, lateral inhibition is one possibility.
Synaptic plasticity
Modification of neurite morphology could provide a mechanism by which notch regulates synaptic plasticity (Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999; Matsuzaki et al., 2004). Indeed, Wang et al., (2004) provided direct evidence that notch signaling is involved in hippocampal synaptic plasticity. Long-term potentiation (LTP) is an experimentally-induced long-lasting increase in synaptic strength that is currently a prominent candidate mechanism of memory formation. Notch anti-sense transgenic (NAS) mice with an approximately 50% reduction of the notch level exhibited impaired LTP in response to high frequency stimulation, while maintaining normal basal synaptic transmission or paired-pulse facilitation (PPF). Long-term depression (LTD) was enhanced in NAS mice. Perfusion of hippocampal slices with a notch ligand (Jagged peptide) enhanced LTP without affecting PPF. Thus, notch signaling appears to enhance postsynaptic changes that mediate LTP.
Learning and memory
Synaptic plasticity is widely assumed to be the mechanism by which memory traces are encoded and stored in the CNS and thus is a neural basis of information storage. One link between notch signaling and learning and memory was suggested by the fact that notch is cleaved by presenilin-1, a protein associated with early-onset inherited forms of Alzheimer’s disease (AD) (De Strooper and Konig, 1999; Ray et al., 1999; Selkoe et al., 2003; Takasugi et al., 2003). Cleavage of notch by presenilin-1 in the γ– secretase enzyme complex is essential for NICD generation and downstream signaling (De Strooper and Konig, 1999; Takasugi et al., 2003; Ye et al., 1999; Struhl and Greenwald, 1999). The neurological phenotypes of presenilin-1 null mice and notch null mice are virtually indistinguishable with premature differentiation of neural progenitor cells and dysgenesis of the brain during embryonic development (Handler et al., 2000; De la Pompa et al., 1997).
Forebrain-specific conditional knockout of either notch or presenilin-1 results in learning and memory impairment in adult mice (Costa et al., 2003; Yu et al., 2001; Saura et al., 2004). The cognitive deficits in both presenilin-1 and notch null mice are restricted to both spatial learning and long term memory, but not other forms of learning and not short term memory. These results indicate that notch signaling is required for normal spatial learning and the formation of long term memories. Evidence for an important role for notch signaling in long-term memory were also obtained in studies of Drosophila with a temperature sensitive notch mutation or with RNA intereference-based knockdown of notch expression (Presente et al., 2004; Ge et al., 2004).
Notch in neurological disorders
In light of the importance of notch signaling in various types of developmental events and synaptic plasticity, and data from other cellular systems suggesting that notch can regulate cell survival (Mason et al., 2006; Purow et al., 2005), several laboratories have been investigating the possible involvement of notch signaling in neurological disorders and cellular responses to CNS injury. The findings reviewed below suggest that the roles of notch signaling in CNS injury and disease are complex, involving multiple cell types (neurons, glial cells, vascular cells and lymphocytes), and either detrimental or beneficial actions on the pathogenic process and functional outcome.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephaly (CADASIL)
Mutations in notch-3 cause an inherited disorder called CADASIL which is characterized by cerebral microvascular pathology associated with multiple infarcts and white matter damage (Louvi et al., 2006; Ringelstein and Nabavi, 2005; Ruchoux et al., 1995; Joutel et al., 2000). More than 130 different mutations in notch-3 have been identified with almost all of them being missense mutations that result in a single amino acid change within the extracellular EGF-repeat domain (Joutel et al., 1996). Notch-3 is primarily expressed in vascular smooth muscle cells (Joutel et al., 2000; Lindsell et al., 1996; Villa et al., 2001; Kitamoto et al., 2005; Williams et al., 1995; Prakash et al., 2002). Notch-3 gene was demonstrate to be important for generating functional arteries in mice by regulating arterial differentiation and maturation of vascular smooth muscle cells (Domenga et al.,). Therefore, notch-3 plays a critical role in controlling cerebral vascular development and homeostasis, and impairment of notch-3 function results in cerebral infarcts and associated functional deficits.
Brain ischemia-reperfusion (I/R) injuries
Ischemic neuronal death results from oxygen and glucose deprivation and is believed to be mediated by oxidative stress and overactivation of glutamate receptors leading to either necrosis (cell swelling and membrane rupture) or apoptosis (mitochondrial membrane permeabilization and release of cytochrome c, and activation of caspases) (Marzo et al., 1998; Mattson et al., 2003; Dirnagl et al., 1999; Mattson et al., 2003). While reperfusion of the ischemic brain is desirable, the return of the blood supply can paradoxically trigger an intense oxidative and inflammatory response and causes additional injury to adjacent brain tissue. Therefore, the pathological processes in I/R injuries are complex and involve blood brain barrier disruption, energy depletion, perturbed calcium homeostasis and excitotoxicity, oxidative stress, microglial activation and infiltration of proinflammatory leukocytes (Arumugam et al., 2005). A recent study (Arumugam et al., 2006) found that I/R can transiently activate γ-secretase which results in notch-1 cleavage and increased levels of NICD. The I/R-induced increase in NICD was significantly attenuated in NAS mice and in wild-type mice treated with γ-secretase inhibitors. Notch-1 antisense transgenic mice and normal mice treated with γ-secretase inhibitors exhibited less stroke-induced brain damage and improved functional outcome. Notch-1 signaling in neurons during ischemic conditions may enhance apoptotic signaling cascades in the neurons, while activation of notch-1 in microglial cells and leukocytes may exacerbate inflammatory processes that contribute to neuronal death (Fig. 1). Consistent with the latter findings are studies showing that notch-1 can activate T lymphocytes resulting cell proliferation and cytokine production (Eagar et al., 2004). While notch-1 activation in neurons and inflammatory cells may worsen the outcome of a stroke, notch-1 signaling in NSC and progenitor cells might promote recovery. Adult NSCs undergo the transition from proliferation to differentiation, and may provide a reservoir of cells capable of forming new neurons that may eventually integrate into neuronal circuits (Cage, 2000; Garcia-Verdugo et al., 1998). Accumulating evidence indicates that stroke induced by middle cerebral artery occlusion leads to increased NSC proliferation and differentiation into new neurons in the ipsilateral SVZ (Jin et al., 2001; Zhang et al., 2001; Arvidsson et al., 2002; Parent et al., 2002; Takasawa et al., 2002). These newly generated neurons are mobilized to the damaged striatal area (Arvidsson et al., 2002; Parent et al., 2002). Moreover, an array of factors including growth factors such as bFGF (Jin et al., 2002) and BDNF (Zigova et al., 1998; Pencea et al., 2001), glutamate (Bernabeu and sharp, 2000; Arvidsson et al., 2001) and erythropoietin (Shingo et al., 2001) may regulate neurogenesis following cerebral ischemia. A recent study (Androutsellis-theotokis et al., 2006) found that notch signaling is also important in regulating neurogenesis and the functional recovery in rodents with brain damage from stroke. In contrast to the effect of notch signaling in cortical neurons (Arumugam et al., 2006), activation of notch receptor by treatment with an exogenous notch ligand promotes NSC survival, while γ-secretase inhibitors antagonize the effects of the notch-1 ligand on NSC. Rapid activation of cytoplasmic signaling thorough PI3 kinase-Akt, the transcription factor STAT3, and mammalian target of rapamycin (mTOR) mediates the notch-1 activation signaling that promotes the survival of NSCs. Delayed treatment with agents that activate notch-1 may therefore promote recovery of function following a stroke. Clearly, a better understanding of notch signaling in the various cell types affected in a stroke will be required in order to develop therapeutic interventions that target notch signaling for the treatment of stroke.
Neurodegenerative disorders
The major age-related neurodegenerative disorders, AD and Parkinson’s disease (PD), are becoming more common as the average lifespan increases. Less common, but equally devastating to the patient and their loved ones are amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD). There is considerable reason to believe that notch-1 signaling influences the disease process in AD, but little is known concerning the possible involvement of notch-1 in PD, ALS and HD. AD is characterized by accumulation of the amyloid β-peptide (Aβ), synaptic dysfunction and degeneration, inflammation and death of neurons in brain regions involved in learning and memory processes (Mattson 2004). The fact that presenilin-1/γ-secretase is responsible for cleavage the amyloid precursor protein (APP) to generate Aβ, and also cleaves notch-1 to generate NICD has raised considerable interest in elucidating the roles of notch-1 signaling in AD.
Immunohistochemical analysis of postmortem hippocampal specimens suggest that levels of notch-1 are increased in this brain region in AD and Pick’s disease (Nagarsheth et al., 2006). Down syndrome patients exhibit mental retardation and develop AD-like pathology. Analysis of the expression of notch-1 target genes in postmortem brain tissue and fibroblasts from Down syndrome patients provided evidence for hyperactivation of the notch-1 signaling pathway in Down syndrome (Fischer et al., 2005). Data from the latter study revealed a similar increase in the expression of notch-1 and notch-1 target genes in brain tissue from AD patients and, moreover, suggested an interaction between notch-1 signaling and amyloidogenesis. Other studies have indeed demonstrated a direct interaction of APP with notch-1, and with notch-2 as well (Oh et al., 2005). The role of the APP – notch interaction in AD and related disorders is as yet unknown. Similar to the generation of Aβ upon cleavage of APP by γ-secretase, cleavage of Notch-1 by γ-secretase results in the release of a notch-1 Aβ-like peptide (Okochi et al., 2006). The amount of the notch-1 Aβ-like peptide produced is increased in cells expressing AD-linked presenilin-1 mutations.
Notch and CNS cancers
Considerable evidence suggests that notch signaling plays a critical role in tumor progression in a variety of organ systems throughout the body (Roy et al., 2007; Miele et al., 2006). There are several lines of evidence to suggest that notch signaling also plays a role in brain tumor progression. However, before discussing the critical studies of notch and CNS tumors, it is worth noting the view on brain tumors has gone through a renaissance over the past decade. The prevailing view is that a common type of brain cancer, glioma, is propagated by a small population of cancer stem cells which have the ability to self-renew and differentiate into neurons, astrocytes and oligodendrocytes (Dirks, 2006; Nakano et al., 2006). Interestingly, these cancer stem cells are responsive to factors such as bone morphogenic proteins (BMP), which are known to play key roles in the regulation of embryonic and adult NSC populations (Piccirillo et al., 2006). Given the importance of notch signaling in CNS development, it would be reasonable to predict that notch signaling also regulates cancer stem cells and brain tumor progression. This does, indeed, appear to be the case because analysis of a variety of human brain tumors has suggested that Dll3 is a good clinical marker of proneural gliomas (Phillips et al., 2006). In addition, human brain tumor-derived cancer cell clones express delta and jagged 1, while jagged 2 expression was decreased in comparison to clones derived from neurogenic regions (Ignatova et al., 2002). In a long term human glioma culture system, notch signaling (notch 1, 2, 3, hes 1, 2, 4) is also increased (Shiras et al., 2007).
Notch signaling has been suggested a therapeutic target for medulloblastomas because treatment with a γ-secretase inhibitor results in a decrease in the total number of cancer stem cells and inhibition of tumor progression (Fan et al., 2006). However, it is worth noting that the blockade of notch signaling has not been assessed on tumor progression or the cancer stem cell population in gliomas, although the use of γ-secretase inhibitors as a putative cancer therapy has been suggested (Shih et al., 2007). These studies confirm that notch signaling is involved in CNS tumors (Fig. 1) and suggest that by further elucidating these pathways, a better understanding of brain cancers and development of novel therapeutic agents that modify notch signaling are possible.
Perspectives
The elucidation of the role of notch signaling during CNS development, and the association of notch-related pathways with neurological disorders, has proven to be both scientifically interesting and of biomedical importance. Future studies in both physiological and pathological roles for notch signaling will benefit from more in-depth analysis of its molecular interactions in development and adult neuroplasticity. Among the questions that remain to be answered are: 1) what molecular mechanisms mediate activity-dependent notch activation?; 2) what gene targets of notch are responsible for the morphological and functional consequences of notch signaling in normal and disease states; 3) how does the notch signaling pathway interact with other signaling pathways in neurons and glial cells?
The mechanisms by which notch regulates synaptic plasticity are unknown. The transcription factor NF-κB may be one mediator of the effects of notch signaling on synaptic plasticity because NF-κB is reduced in NAS mice (Wang et al., 2004) and NF-κB can enhance LTP and is important for learning and memory (Mattson and Meffert, 2006). Ras signaling, which is critical for many morphological and functional responses of neurons to neurotrophic factors, has been shown to modulate and be modulated by notch signaling (Weijzen et al., 2002; Ordentlich et al., 1998; Berset et al., 2001). In addition, cAMP response element-binding protein (CREB) transcription is NMDA receptor-dependent and increases presenilin-1 expression, which could be important for γ-secretase cleavage of notch (Mitsuda et al., 2001). Understanding how notch signaling interacts with modulators of NF-κB, CREB, Ras and calcium signaling could be very important in understanding developmental and synaptic plasticity.
The roles of notch signaling in neurological disorders are complex, involving multiple cell types in ways that may either worsen or improve the outcome. Activation of notch in neurons increases their vulnerability to ischemic injury, and activation of notch in microglia and lymphocytes may promote damaging inflammatory processes (Arumugam et al., 2006). On the other hand, notch may play a pivotal role in injury-induced neurogenesis, thereby promoting recovery of function (Androutsellis-theotokis et al., 2006). Finally, notch signaling is emerging as an important factor in the development of CNS tumors. The identification of human CNS cancer stem cells has so far been based on their expression of prominin-1 (CD133) (Singh et al., 2003, 2004; Bao et al., 2006). Given the evidence that notch signaling is high in cancer stem cells, selection strategies based on notch expression, which has been used for embryonic CNS stem cells (Nagato et al., 2005; Basak and Taylor, 2007) could also be used alone or in combination with other markers to identify CNS cancer stem cells. It will also be important to establish which cells express notch and notch ligands within an intact CNS tumor. From a therapeutic perspective it may be possible to develop selective inhibitors or activators of notch signaling. The development of γ-secretase inhibitors with differential effects on APP and notch processing suggests the feasibility of developing notch-specific inhibitors.
Acknowledgments
We would like to apologize to all those whose work we could not include due to space limitations. We would like to thank K.C. Alexander (NIA) for assistance with the illustrations. This work was supported by the NIA Intramural Research Program. J.D.L. is supported by the NIH-Cambridge Graduate Partnership Program.
References
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 2005;19:1028–33. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–21. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7:229–42. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10–8. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- Beckers J, Caron A, Hrabe de Angelis M, Hans S, Campos-Ortega JA, Gossler A. Distinct regulatory elements direct delta1 expression in the nervous system and paraxial mesoderm of transgenic mice. Mech Dev. 2000;95:23–34. doi: 10.1016/s0925-4773(00)00322-1. [DOI] [PubMed] [Google Scholar]
- Berezovska O, McLean P, Knowles R, Frosh M, Lu FM, Lux SE, Hyman BT. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience. 1999;93:433–9. doi: 10.1016/s0306-4522(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Sharp FR. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 2000;20:1669–80. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Berset T, Hoier EF, Battu G, Canevascini S, Hajnal A. Notch inhibition of RAS signaling through MAP kinase phosphatase LIP-1 during C. elegans vulval development. Science. 2001;291:1055–1058. doi: 10.1126/science.1055642. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signaling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Cage F. Mammalian Neural Stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Campos LS, Decker L, Taylor V, Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem. 2006;281:5300–9. doi: 10.1074/jbc.M511886200. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–7. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Chenn A. The simple life (of cortical progenitors) Neuron. 2005;45(6):817–9. doi: 10.1016/j.neuron.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–41. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–54. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- Dang L, Yoon K, Wang M, Gaiano N. Notch3 signaling promotes radial glial/progenitor character in the mammalian telencephalon. Dev Neurosci. 2006;28:58–69. doi: 10.1159/000090753. [DOI] [PubMed] [Google Scholar]
- De la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–48. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Konig G. Alzheimer’s disease. A firm base for drug development. Nature. 1999;402:471–2. doi: 10.1038/44973. [DOI] [PubMed] [Google Scholar]
- Dirks PB. Cancer: stem cells and brain tumours. Nature. 2006;444:687–8. doi: 10.1038/444687a. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagar TN, Tang Q, Wolfe M, He Y, Pear WS, Bluestone JA. Notch 1 signaling regulates peripheral T cell activation. Immunity. 2004;20:407–15. doi: 10.1016/s1074-7613(04)00081-0. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- Faux CH, Turnley AM, Epa R, Cappai R, Bartlett PF. Interactions between fibroblast growth factors and Notch regulate neuronal differentiation. J Neurosci. 2001;21:5587–96. doi: 10.1523/JNEUROSCI.21-15-05587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DF, van Dijk R, Sluijs JA, Nair SM, Racchi M, Levelt CN, van Leeuwen FW, Hol EM. Activation of the Notch pathway in Down syndrome: cross-talk of Notch and APP. FASEB J. 2005;19:1451–1458. doi: 10.1096/fj.04-3395.com. [DOI] [PubMed] [Google Scholar]
- Frise E, Knoblich JA, Younger-Shepherd S, Jan LY, Jan YN. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc Natl Acad Sci U S A. 1996;93:11925–32. doi: 10.1073/pnas.93.21.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–56. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–48. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Ge X, Hannan F, Xie Z, Feng C, Tully T, Zhou H, Xie Z, Zhong Y. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci U S A. 2004;101:10172–6. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Barde YA. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46:369–72. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Ang E, Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci U S A. 2003;100:2890–5. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–58. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol. 2007;19:166–75. doi: 10.1016/j.ceb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–9. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Takahashi K, Takimoto H, Tomizuka K, Hayasaka M, Tabira T, Hanaoka K. Functional redundancy of the Notch gene family during mouse embryogenesis: analysis of Notch gene expression in Notch3-deficient mice. Biochem Biophys Res Commun. 2005;331:1154–62. doi: 10.1016/j.bbrc.2005.03.241. [DOI] [PubMed] [Google Scholar]
- Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–24. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyszyn B, Cowburn RF, Seiger A, Kjaeldgaard A, Sundstrom E. Distribution of presenilin 1 and 2 and their relation to Notch receptors and ligands in human embryonic/foetal central nervous system. Brain Res Dev Brain Res. 2004;151:75–86. doi: 10.1016/j.devbrainres.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377:624–7. doi: 10.1038/377624a0. [DOI] [PubMed] [Google Scholar]
- Kramer H. RIPping notch apart: a new role for endocytosis in signal transduction? Sci STKE. 2000;2000:1–3. doi: 10.1126/stke.2000.29.pe1. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–64. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Wang D, Shen Q, Schonemann MD, Gorski JA, Jones KR, Temple S, Jan LY, Jan YN. Inactivation of Numb and Numb like in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–18. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8:14–27. doi: 10.1006/mcne.1996.0040. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Arboleda-Velasquez JF, Artavanis-Tsakonas S. CADASIL: a critical look at a Notch disease. Dev Neurosci. 2006;28:5–12. doi: 10.1159/000090748. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–56. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–7. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. 2006;28:49–57. doi: 10.1159/000090752. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kB in nerve cell survival, plasticity and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006;6:905–18. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- Mitsuda N, Ohkubo N, Tamatani M, Lee YD, Taniguchi M, Namikawa K, Kiyama H, Yamaguchi A, Sato N, Sakata K, Ogihara T, Vitek MP, Tohyama M. Activated cAMP-response element-binding protein regulates neuronal expression of presenilin-1. J Biol Chem. 2001;276:9688–98. doi: 10.1074/jbc.M006153200. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signaling distinguishes neural stem cells and intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27:4132–45. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagato M, Heike T, Kato T, Yamanaka Y, Yoshimoto M, Shimazaki T, Okano H, Nakahata T. Prospective characterization of neural stem cells by flow cytometry analysis using a combination of surface markers. J Neurosci Res. 2005;80:456–466. doi: 10.1002/jnr.20442. [DOI] [PubMed] [Google Scholar]
- Nagao M, Sugimori M, Nakafuku M. Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27:3982–94. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarsheth MH, Viehman A, Lippa SM, Lippa CF. Notch-1 immunoexpression is increased in Alzheimer’s and Pick’s disease. J Neurol Sci. 2006;244:111–116. doi: 10.1016/j.jns.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Nakano I, Kornblum HI. Brain tumor stem cells. Pediatr Res. 2006;59:54R–8R. doi: 10.1203/01.pdr.0000203568.63482.f9. [DOI] [PubMed] [Google Scholar]
- Ohishi K, Varnum-Finney B, Flowers D, Anasetti C, Myerson D, Bernstein ID. Monocytes express high amounts of Notch and undergo cytokine specific apoptosis following interaction with the Notch ligand, Delta-1. Blood. 2002;95:2847–54. [PubMed] [Google Scholar]
- Oh SY, Ellenstein A, Chen CD, Hinman JD, Berg EA, Costello CE, Yamin R, Neve RL, Abraham CR. Amyloid precursor protein interacts with notch receptors. J Neurosci Res. 2005;82:32–42. doi: 10.1002/jnr.20625. [DOI] [PubMed] [Google Scholar]
- Okochi M, Fukumori A, Jiang J, Itoh N, Kimura R, Steiner H, Haass C, Tagami S, Takeda M. Secretion of the Notch-1 Abeta-like peptide during Notch signaling. J Biol Chem. 2006;281:7890–7898. doi: 10.1074/jbc.M513250200. [DOI] [PubMed] [Google Scholar]
- Ordentlich P, Lin A, Shen CP, Blaumueller C, Matsuno K, Artavanis-Tsakonas S, Kadesch T. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol Cell Biol. 1998;18:2230–9. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–17. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Prakash N, Hansson E, Betsholtz C, Mitsiadis T, Lendahl U. Mouse Notch 3 expression in the pre- and postnatal brain: relationship to the stroke and dementia syndrome CADASIL. Exp Cell Res. 2002;278:31–44. doi: 10.1006/excr.2002.5544. [DOI] [PubMed] [Google Scholar]
- Presente A, Andres A, Nye JS. Requirement of Notch in adulthood for neurological function and longevity. Neuroreport. 2001;12:3321–3325. doi: 10.1097/00001756-200110290-00035. [DOI] [PubMed] [Google Scholar]
- Presente A, Boyles RS, Serway CN, de Belle JS, Andres AJ. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci U S A. 2004;101:1764–8. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–63. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Yao M, Nowotny P, Mumm J, Zhang W, Wu JY, Kopan R, Goate AM. Evidence for a physical interaction between presenilin and Notch. Proc Natl Acad Sci U S A. 1999;96:3263–8. doi: 10.1073/pnas.96.6.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci. 2000;3:30–40. doi: 10.1038/71104. [DOI] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–91. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Nabavi DG. Cerebral small vessel diseases: cerebral microangiopathies. Curr Opin Neurol. 2005;18:179–188. doi: 10.1097/01.wco.0000162861.26971.03. [DOI] [PubMed] [Google Scholar]
- Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17:52–9. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol (Berl) 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–97. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–6. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- Shen QW, Zhong YN, Jan S. Temple: Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129(20):4843–53. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- Shih IeM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–43. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–89. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995;121:3187–95. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17:21–6. doi: 10.1016/s0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–5. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–86. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995;15:6058–68. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002;22:299–307. doi: 10.1097/00004647-200203000-00007. [DOI] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–41. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414(6859):112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- The Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–94. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58:349–60. doi: 10.1016/0092-8674(89)90849-0. [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–4. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–86. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- Williams R, Lendahl U, Lardelli M. Complementary and combinatorial patterns of Notch gene family expression during early mouse development. Mech Dev. 1995;53:357–68. doi: 10.1016/0925-4773(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–12. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Ye Y, Lukinova N, Fortini ME. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999;398:525–9. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–15. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, Younkin S, Kandel ER, Kirkwood A, Shen J. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–26. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–89. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Zhong W, Jiang MM, Weinmaster G, Jan LY, Jan YN. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development. 1997;124:1887–97. doi: 10.1242/dev.124.10.1887. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–45. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]