Abstract
Elimination rates and their corresponding half-lives are conceptually important and intuitively accessible pharmacokinetic measures of toxicant elimination, but regression-based estimates are biased proportional to the degree of continuing (background) exposure. We propose an alternative estimator, the censored normal regression model, which uses all observations, but treats individuals whose initial level failed to exceed their follow-up level as censored observations to weight the regression estimates from those that declined between blood draws. In this manner, we derive the intrinsic elimination rate, the elimination rate free from ongoing exposure, as a parameter in a regression with an unobserved, latent dependent variable. We utilize sequential measurements of persistent organic pollutants (POPs) levels from adolescence to adulthood, a period of intense change in size and body composition, to quantify individual-level change within a community exposed to significant quantities of contaminants over an extended period of time. Although much research has been conducted on effects of POPs, far less attention has been given to vectors of intake and changes in toxicant levels during the life course. We apply exploratory factor analysis (EFA) to types and timing of consumption, along with physical behavioral characteristics, to identify a structure of seven underlying factors. Although several variables show factorial complexity, the latent constructs included an age/maturation and period-related factor, a nutritional composite, consumption prior to pregnancy, fish and fowl consumed during pregnancy, factors distinguishing body mass and weight from height, and bottom-feeding fish consumption. Unadjusted and adjusted half-lives using the censored normal regression estimator, as well as estimated half-lives from conventional log concentration regressions, are reported for PCB groupings, specific congeners, p,p′-DDE, and HCB.
Keywords: Polychlorinated biphenyls; PCBs; persistent organic pollutants; POPs; hexachlorobenzene; HCB; p,p′-dichlorophenyldichloroethylene; DDT; Mohawk; Native American
1. Introduction
Persistent organic pollutants (POPs) are resistant to degradation, become widely distributed geographically, biomagnify in food chains, and bioaccumulate in the fatty tissue of non-humans and humans. Adverse effects to health, including the alteration of growth (Burns et al. 2011; Mendez et al. 2011), maturation and development (Denham et al. 2005; Dhooge et al. 2011; Dickerson et al. 2011; Ottinger et al. 2009), and cognitive function (Grandjean and Landrigan 2006; Newman et al. 2006; Newman et al. 2009; Stewart et al. 2000), have been reported. Polychlorinated biphenyls (PCBs) dichlorodiphenyldichloroethane (p,p′-DDE, a metabolite of DDT), and hexachlorobenzene (HCB), are widely recognized as persistent environmental pollutants. Although the use and emissions of these chemicals were severely restricted in the US in the mid-1970s, these toxicants are extremely difficult to destroy by either chemical, thermal, or biochemical processes due to their high thermodynamic stability, and destruction presents the risk of generating extremely toxic dibenzodioxins and dibenzofurans through partial oxidation (Hansen 1999; Shibamoto et al. 2007).
Humans continue to be exposed to different mixtures of POPs, especially PCBs, at background levels and via direct routes of intake (ingestion, inhalation, intrauterine transmission, etc.) (Grandjean et al. 2008; Knobeloch et al. 2009; Lignell et al. 2009; Matsumoto et al. 2009; World Health Organization 2003). Exposure beyond background levels continues primarily through the dietary intake of contaminated animal, fish, or fowl fats. Bottom feeding and long-lived predatory fish can accumulate high levels in their body fat and these residues can be passed on to humans and wildlife that consume them (Startin and Rose 2003). Exposures to PCBs, p,p′-DDE, and HCB have been linked to diabetes (Lee et al. 2010; Turyk et al. 2009), high blood pressure/hypertension (Goncharov et al. 2008; Langer 2010), thyroid hormone disruption (Chevrier et al. 2007; Langer et al. 2009; Schell et al. 2009; Schell and Gallo 2010), effects on sexual maturation and differentiation (Denham et al. 2005; Dhooge et al. 2011; Dickerson et al. 2011; Ottinger et al. 2009), and reproduction (Cohn et al. 2010; Cohn et al. 2011; Needham et al. 2011; Wigle et al. 2008). These endocrine disruption effects of POPs continue to be of concern, especially during critical times of growth and development such as adolescence and young adulthood, primarily due to their long half-lives (Stehr-Green 1989) and potentially adverse long-term health consequences.
Although much research has been conducted on health effects of POPs, far less attention has been given to vectors of intake and changes in toxicant levels during the life course. To the best of our knowledge, no other study has utilized sequential measurements of POP levels from adolescence to adulthood, a period of intense change in size and body composition to quantify individual-level change within a community exposed to significant quantities of contaminants over an extended period of time.
We begin by reporting serum levels of persistent organochlorines (PCBs, p,p′-DDE, HCB) within Akwesasne Mohawk adolescents (collected between 1995–2000) and again in young adulthood (collected between 2000–2005), with an average interval of four years between measurements. The Mohawk of Akwesasne have had significant changes in lifestyle following the discovery of contamination of the St. Lawrence River on which they depended for fish, hence reducing their reliance on the traditional fish-based diet to a less healthy one containing more fat and calories (Ravenscroft and Schell 2007). However, a substantial proportion of our sample evidence an absolute increase in POP levels at follow-up (young adulthood) over their baseline measure (adolescence).
Elimination half-life, the amount of time required to reduce a toxicant to one-half its level at initial measurement, is a conceptually important, and intuitively accessible, pharmacokinetic measure of toxicant elimination, but its conceptual utility is predicated on the assumption that differences in rates of elimination from the body depend primarily on compound-related pharmacokinetics and host-related factors that affect individuals’ metabolism rates (Lotti 2003; Matthews and Dedrick 1984). Continuing exposure between initial and follow-up measurement violates this assumption and confounds estimation of elimination kinetics. Most of the literature on elimination half-lives is now dismissed as having reported “apparent” elimination half-lives (Shirai and Kissel 1996; Milbrath et al., 2009; see discussion of apparent versus intrinsic half-lives in Ritter, 2011). The concern over bias introduced by ongoing exposure is so severe that it has led some to conclude that it is impossible to offer reliable half-life figures from longitudinal data with ongoing environmental exposure (Lotti 2003; Yakushiji et al. 1984). At a minimum, the concern has produced a preference for the development of methods for half-life estimation from cross-sectional data (Ritter et al. 2011; Wong et al. 2013) or regression of log serum concentrations on time after primary exposure ceases for longitudinal data (cf. Bartell 2012). Neither of these approaches is likely to have strong causal inference after extrapolation to the real world (Manski 1995) or adequately respond to bias introduced by ongoing exposure and host-related factors on elimination (Milbrath et al. 2009).
Accordingly, this analysis has two objectives. First, we determine the factor structure underlying fifteen items representing dietary intake, measures of body burden/storage reservoirs, and other known correlates of toxicant exposure. Second, we estimate intrinsic elimination rates using an exponential decay model and a regression method ideally suited to a population under continuing exposure, with and without adjustment for within-individual effects of the (seven) identified constructs and other host-related covariates. We report the corresponding intrinsic elimination half-lives for PCBs, p,p′-DDE, and HCB.
2. Materials and Methods
2.1 Sample and site characteristics
The Akwesasne Mohawk Nation (AMN) is a sovereign nation situated on the St. Lawrence River with territory bordering New York State, Ontario and Quebec, Canada. The Akwesasne community is one of several communities comprising the Kahniakehaka/Mohawk nation, and is traditionally known as the keeper of the Eastern Door of the Iroquois Confederacy (the Haudenosaunee Confederacy) with a population approximating 12,000 – 13,000 people (Akwesasne Task Force On The Environment 1997; Fitzgerald et al. 1998; George-Kanentiio 1995).
Industrial development along the St. Lawrence River began in the 1950s, and major industrial facilities located around Cornwall, Ontario, and Massena, New York, discharged significant quantities of contaminants, including PCBs, p,p′-DDE, HCB and mirex, into the St. Lawrence River and its three tributaries (Sloan and Jock 1990). Contamination of the local waters entered the local food chain, and some local species of fish, birds, amphibians and mammals were found to have levels exceeding the US Food and Drug administration’s tolerance limits for human consumption (Forti et al. 1995; Sloan and Jock 1990), leading to the issuance of fish and game advisories in the late 1980s and early 1990s (Fitzgerald et al. 1995; Fitzgerald et al. 1998).
2.2 Data collection
The University at Albany, State University of New York’s Institutional Review Board approved all study protocols and informed consent procedures. Additionally, assent from minors was obtained from all participants. For both projects, all data collection was performed by project staff, all members of the Akwesasne community, and data were collected without prior knowledge of participants’ exposure status. Study protocols and methods have previously been described in detail (Gallo et al. 2011; Newman et al. 2006; Newman et al. 2009; Schell et al. 2003), and are briefly reviewed here.
Data were collected for the Mohawk Adolescent Well-Being Study (MAWBs) between 1995 and 2000. In brief, 294 mother/youth pairs were recruited, and due to attrition resulted in a final sample size of 271 participants (131 males and 140 females) between 10 and 16.99 years of age (for more detail on recruitment and sampling see Schell et al. 2002, 2003; Gallo et al. 2005, 2007).
In MAWBs, the youth’s mother completed interviews and questionnaires to obtain information about the youth’s family background including sociodemographic status and sources of exposure such as diet (from Food Frequency Questionnaire (FFQ)), breastfeeding history and duration, as well as the mother’s consumption of locally caught fish, fowl and game before and during the pregnancy with the child participant (from a modified dietary questionnaire). Two overall questions were asked about locally-caught fish consumption: 1) whether the mother consumed any locally caught fish (yes/no); and 2) how many meals she consumed before (12 mos) and during her pregnancy (9 mos). This information was then characterized further into types of fish consumed (bass, trout, sturgeon, etc.) and number of meals eaten of each type of fish. Since consumption of bottom-feeding fish would result in higher toxicant levels given the toxicants’ predilection to bind to sediment (Startin and Rose 2003), chemical viscosity and density (Kuzyk et al. 2005a; Kuzyk et al. 2005b; Wirgin et al. 2011), fish consumption-based measures were also differentiated between bottom-feeding (catfish, bullhead, eel and sturgeon) and top-feeding fish (bass, perch, walleye/pickerel, pike, and trout).
The Young Adult Well-Being study (YAWBs) was conducted from 2000 to 2005. Young adults were eligible if they had participated in MAWBs, and were now between 17 and 20 years of age (Gallo et al. 2011). The YAWBs sample consisted of 154 participants; however two persons were omitted because reported organochlorine levels from our earlier study, MAWBs, were not available and one person was excluded because serum POP levels were not available from the follow-up study, leaving a final sample size of 151 individuals.
In YAWBs, each participant completed interviews and sociodemographic questionnaires providing information about life style factors, recreational and traditional activities, current cigarette and alcohol use, breastfeeding history and duration (in consultation with their mother and checked against MAWBs data), prescription and over-the-counter medicine use, diet, sex (males =0, females =1), age (when blood was drawn), educational status (as the highest year of education completed), body mass index (calculated), weight (kg; self-report), height (cm; self-report), and, as a proxy for socioeconomic status, education, current and past employment and living environment. (For more detail see Gallo et al. 2011).
2.3 Laboratory analysis of toxicants and lipid measurements
For both MAWBs and YAWBs, blood specimens were collected by trained Mohawk staff. The sample was collected within a five-hour window to minimize the effects of diurnal variation (particularly with regard to endocrine assessment). Approximately 95% of the participants had their blood drawn at first rising. Participants were asked to not eat any locally caught, trapped, or grown food for three days prior to the collection and to not eat or drink anything after 10 p.m. the previous evening.
In MAWBs, assessment of cholesterol, triglycerides, and glucose was performed at the Clinical Chemistry and Hematology Laboratory, Wadsworth Center for Laboratories and Research, New York State Department of Health, a CLIA-approved member of the CDC reference laboratory network for lipid measurements (Myers et al. 2000). In YAWBs, clinical chemistries were performed at the clinical laboratories of the Albany Medical Center in Albany, NY, a New York State and CLIA accredited laboratory meeting all proficiency requirements.
Organochlorine pesticide and PCB analyses for both studies were conducted at the University at Albany’s Exposure Assessment Laboratory. The laboratory is accredited by the NYSDOH Clinical Laboratory Evaluation Program and participated in the Arctic Monitoring and Assessment Programme (AMAP) Ring Test for Persistent Organic Pollutants in Human Serum. The same laboratory analysis protocol was used for both studies (for more detail see Schell et al. 2003 and Gallo et al. 2011). Analysis of low and high level QC performance samples indicated accuracy and precision of ±15% of nominal and ≤15% RSD, respectively. Complete details of the laboratory protocol for PCB analysis have been published (DeCaprio et al. 2000, 2005). In brief, high resolution, ultratrace, congener-specific analysis was performed by parallel dual-column (splitless injection) gas chromatography (GC) with electron capture detection (ECD) (Agilent Technologies, Inc., Santa Clara, CA). This method quantitates up to 83 individual PCB congeners and 18 PCB congeners as pairs or triplets, as well as p,p′-DDE, HCB, and mirex (a total of 94 analytical peaks). An instrument change (i.e., from an Agilent model 5890 GC with standard ECDs to a model 6890 with micro-ECDs) allowed for a lowering of detection limits for most individual congeners in YAWBs as compared to MAWBS. LODs (MDLs) for detected congeners ranged from 2 to 24 ppt (pg/g serum) for a 2.5 g serum specimen and are listed in a previous publication (Gallo et al. 2011). The analytes include all of the major Aroclor-derived congeners typically present in human samples plus a number of sporadic or rare congeners. Individual chlorinated biphenyl (CB) congeners are identified according to the IUPAC numbering system (Ballschmiter and Zell 1980; Guitart et al. 1993). Data were expressed on a whole-weight basis without lipid adjustment to maintain consistency with the earlier data analyses.
2.4 Congener Groupings
Due to improvements in technology, limits of detection (LOD) for PCBs were marginally lower in the second project. Of the 16 PCB congeners detected in 50% or more of the sample, the LOD of nine PCB congeners and p,p′-DDE decreased by less than 0.01 ppb and by less than 0.02 ppb for the remaining seven PCB congeners and HCB. According to most common practice and to maintain consistency with previous study data, results below the LOD were imputed as ½ the LOD prior to log transformation. Analysis of the data with an input of zero for data below the LOD resulted in no significant differences in geometric mean levels from that using ½ LOD imputation. To insure comparability of our data with the majority of previous work, we chose to use mass-based concentrations (i.e., ng/g serum; ppb) for statistical analyses. However, some workers have also employed MW-based concentration data (i.e., pmol/g serum or lipid) for such analyses (e.g., Fängström et al. 2005). Repeat analyses in the present study using the pmol/g metric demonstrated only minimal differences in the results compared to those using the ng/g metric (data not shown).
Both the degree of chlorination and chlorination pattern of individual PCB congeners were considered in the composition of the PCB groupings. Environmental persistence, bioaccumulation in food chains, distribution in human tissue, and the toxicologic action (i.e., dioxin-like vs. Ah receptor-independent effects) of PCBs depend on the chemical structure of individual congeners (Laden et al. 1999; McFarland and Clarke 1989). Therefore, we grouped individual PCB congeners with >50% detection rate according to structure and mechanism in both studies.
To compare levels between the follow-up study (YAWBs) and those in the previous one (MAWBs), we constructed three groups of PCB congeners using the same commonly detected PCBs congeners groupings that were used in MAWBs (for more detail see Schell et al. 2003), i.e., Σ16PCB50%, the sum of all congeners found in 50% or more of the MAWBs sample (CBs 52, 70, 74, 84, 87, 95, 99, 101[+90], 105, 110, 118, 138[+163+164], 149[+123], 153, 180, 187); Σ8PerPCB: the sum of 8 persistent PCBs, again found in 50% or more of the MAWBs sample (CBs 74, 99, 105, 118, 138[+163+164], 153, 180, 187); and Σ6NonPerPCB: those congeners generally considered to be non-persistent (CBs 52, 84, 95, 101[+90], 110, and 149[+123]), also detected in 50% or more of the MAWBs sample. While there is debate over some classification schemes that include CBs 87 and 70 as moderately persistent (Hansen 2001), other data suggest that they should be fairly readily metabolized in humans and should be considered non-persistent (Brown 1994). Because of this uncertainty, they were excluded from both the persistent and non-persistent congener variables. PCB congeners for which all reported values were below the laboratory LOD in MAWBS included CBs 1, 3, 6, 63, 67 and 185 and in YAWBs included CBs 3, 6, 63, and 67; these were not included in any calculations (for more detail see Schell et al. 2003; Gallo et al. 2011). Only negligible levels of mirex were found in YAWBs, therefore mirex was not considered in this analysis (Gallo et al. 2011).
2.5 Statistical methodologies
Statistical analyses were conducted with SPSS v.20 (IBM 2012) and Stata/IC v. 12.1 for Windows (StataCorp 2011). While our focal product is unbiased elimination half-lives, we initially report and interpret descriptive measures of percent annual change in PCBs (Σ16PCB50%, Σ8PerPCBs, Σ6NonPerPCBs, and CBs 105, 118, 138[+163+164], and 153) and toxicants (p,p′-DDE and HCB) between the two studies, scaling the anticipated decline in a toxicant from its initial level in adolescence to its follow-up level in young adulthood as an expected positive value.
While description based on observed annual percent change is interesting and important, toxicokinetic fate modeling in which half-life calculations are derived from elimination rates requires a higher standard of assessment. It is well known that half-life estimates will be artificially high if they fail to account for continuing exposure and the effect of host-related factors on elimination (Shirai and Kissell 1996; Milbrath et al. 2009). Therefore, common practice in prior research utilizing longitudinal data estimates the elimination rate constant, under an exponential decay model, from the derivative (slope) in a linear regression of first-difference in log serum concentration between initial and follow-up on the first-difference in time between follow-up and initial measurement, adjusted for change in relevant host-related factors such as mass of fat or BMI (cf. Yakushiji et al. 1984; Grandjean et al. 2008; Milbrath et al. 2009). This form of regression is the well-known “fixed effects” model. It has desirable properties for causal inference, based in large part on its exclusive use of within-individual variation, but it is still highly susceptible to inconsistent estimates of elimination due to continuing exposure, including prediction of negative values of the rate of change, heteroscedastic errors, and influential outliers. These violations follow from the assumption that the data generating process is an exponential decay model for each individual:
| (1) |
where Yt is the toxicant concentration at time t, Y0 is the initial concentration, and εt is a normally distributed error term for the ith individual (Grandjean et al. 2008). This function cannot apply if Yt > Y0.
We propose that a censored normal regression model to account for ongoing exposure can offer a conceptual match to the problem of estimating intrinsic elimination rates using serum concentrations thought to follow an exponential decay by treating individuals with non-positive difference in log-transformed concentrations between initial and follow-up individuals as censored observations. In the absence of ongoing exposure, non-positive change in log concentration is a logical impossibility and half-life calculation would be undefined. In a population under any background exposure some proportion of cases, typically observations near the lower limit of y at Y0, will show non-positive change. We make use of this condition to approximate the proportion of ln(Y0 )−ln(Yt) that is free from ongoing exposure by the estimated probability of being uncensored. The decomposition of the censored normal regression model in the next section will show that the estimated probability accounts for ongoing exposure by weighting the elimination rate parameter.
2.6 The Tobit model
The structural equation in the Tobit model is
| (2) |
where ui is an independently distributed error term assumed to be normal with zero mean and constant variance σ2 and is a latent variable that is observed for values greater than τ, where τ is the point, or limit, of censoring (Tobin 1958; McDonald and Moffitt 1980). In our application is a positive-valued difference in log-transformed concentrations at initial and follow-up measurements, Xi is the duration between measurements, and βx is the elimination rate parameter.
The observed y is defined in the measurement equation:
| (3) |
We assume that τ = 0, that is, that the data are censored at 0, thus increasing levels between initial (MAWBs) and follow-up (YAWBs) measurement are precluded from directly entering our calculations of the unbiased elimination rate and half-life. Our recognition that yi cannot include non-positive values of , even though non-positive difference in log-transformed concentrations at initial and follow-up measurements are measured, is an unusual operationalization of the latent variable in the censored normal regression model. Typically censoring means that values at the limit are unmeasured. Our definition of is based on elimination kinetics, not on the presence or absence of non-missing values.
It is important to recognize that Tobin’s model uses all observations, both those that are at the limit (censored) and those above it, to estimate a regression line, by assuming that there is an underlying, stochastic index equal to (Xi β + ui) which is observed only when it is positive (i.e., above the limiting value). There are actually three expected values that can be derived from the Tobit model: the expected value of the latent variable, E [y*], the expected value of the uncensored observations, E [ y | y > τ], and the expected value of y. The expected value of y in the model is
| (4) |
where z = Xβ/σ, f (z) is the unit normal density, and F(z) is the cumulative normal distribution function. This is the probability of being uncensored multiplied by the expected value of y given y is uncensored. In addition we can show that the marginal effect on the expected value of y (censored and uncensored) is:
| (5) |
McDonald and Moffitt argue that in most applications the decomposition is necessary to locate the effect of interest. The first form of the decomposition in eq. 5, with X defined as the duration between blood draws, shows that the estimated probability of observing an uncensored (positive-valued) observation at each value (duration) of X is a factor that scales the parameter (βx) that alters the latent dependent variable. The second form of the decomposition in eq. 5 allows us to see that a change in X affects the conditional mean of the latent dependent variable, , in the positive part of the distribution and it affects the probability that the observation will fall in that part of the distribution. The corresponding estimator of intrinsic elimination half-life follows accordingly:
| (6) |
We report the associated half-lives, estimated with and without statistical controls for sources of exposure and host-related characteristics. We also provide estimated half-lives derived from linear regressions with statistical controls to facilitate comparison against previous attempts to account for continuing exposure by including covariates for sources of intake and from a subsample that excludes individuals whose levels increased or were unchanged over the follow up period (cf. Grandjean et al. 2008; Knobeloch et al. 2008). We use non-lipid adjusted measures of PCBs, CBs, and toxicants because statistical controls include lipid-based or related covariates (as described in the following section).
2.7 Exploratory factor analysis
Exploratory factor analysis (EFA) is a popular and effective means of identifying the number and nature of the underlying factors responsible for covariation in a set of data (Hatcher 1994; Fabrigar et al. 1999). We applied the principal factor method with promax rotation to 15 measures of consumption and a variety of other physical and behavioral characteristics using squared multiple correlations as prior commonality estimates. Items entered into the factor analysis included years between blood draws between projects, whether the participant was born in 1985 or later vs. 1984 (i.e., before or after fish consumption advisories were issued by the New York State Department of Health), maternal fish and fowl consumption before and during pregnancy (number of reported meals and number of reported meals divided into top- and bottom-feeding fish), dietary intake (total caloric, fat and protein intake), age (in years), sexual maturation stage as determined by Tanner (Tanner 1990), weight (kg), height (cm), BMI (kg/m2), each measured at the initial wave (MAWBs). These covariates were selected based on literature review (Arisawa et al. 2011; Blanck et al. 2000; Burns et al. 2011; Dhooge et al. 2010; Fitzgerald et al. 2010; Leijs et al. 2009; Patel et al. 2010), on our earlier findings (Denham et al. 2005; Gallo et al. 2002, 2005, 2011; Schell et al. 2009), and on correlations with toxicant levels at p≤0.20. Preliminary analyses found that two measures of breast feeding (duration of breast feeding and a simple indicator of whether or not the respondent had ever breast fed), maternal smoking during pregnancy, gravidity (Gallo et al. 2011), levels of three clinical analytes (triglycerides, cholesterol, glucose), and a proxy for socioeconomic status (SES),1 did not load on any factor. These items were removed from the EFA, but were reintroduced, along with sex of the respondent, as individual covariates to adjust our regression estimates of elimination rates.
3. Results
3.1 Description of MAWBs and YAWBs samples
Basic descriptive statistics from the initial wave of this project (MAWBs) and at follow-up (YAWBs) for all items entered into the EFA and other candidate characteristics are shown in Table 1.
Table 1.
Characteristics of the sample during the first and second study (n=151).
| Characteristic | MAWBs (1995–2000) | YAWBs (2000–2005) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | % yes | Mean | SD | Max | % yes | |
| Youth/Young adult | ||||||||
|
| ||||||||
| Age (yrs) | 13.5 | 1.74 | 16.9 | - | 18.1 | 1.09 | 21.4 | |
| Born before issuance of fish advisories | 60% | 60% | ||||||
| Breast fed (Y/N) | - | - | - | 48% | - | - | - | 49% |
| Breast feeding duration (mos) | 3.1 | 5.01 | 30.0 | - | - | - | - | - |
| BMI (kg/m2) | 23.9 | 5.18 | 43.5 | - | 25.7 | 4.86 | 45.8 | - |
| Height (cm) | 157.7 | 10.53 | 185.1 | - | 167.6 | 9.04 | 190.5 | - |
| Weight (kg) | 60.4 | 16.92 | 116.1 | - | 72.8 | 17.47 | 128.8 | - |
| Tanner Stage | 3.3 | 1.05 | 5.0 | - | - | - | - | - |
| Cholesterol (mg/dL) | 157.1 | 27.53 | 243.0 | - | - | - | - | - |
| Triglycerides (mg/dL) | 86.5 | 39.93 | 207.0 | - | - | - | - | - |
| Total caloric intake (kcal) | 2669.7 | 1030.64 | 6620.8 | - | 2058.7 | 904.10 | 6413.7 | - |
| Total fat intake (g/day) | 119.4 | 55.60 | 402.3 | - | 87.9 | 42.66 | 300.7 | - |
| Total protein intake (g/day) | 92.5 | 37.54 | 240.2 | - | 76.5 | 35.53 | 271.8 | - |
| Birth order position | 2.4 | 1.53 | 8.0 | - | - | - | - | - |
| 1st born | 36% | 37% | ||||||
| Current cigarette use (Y/N) | - | - | - | 17% | - | - | - | 49% |
| Number of years between blood draw | - | - | - | - | 4.1 | 1.35 | 7.1 | - |
|
| ||||||||
| Maternal | ||||||||
|
| ||||||||
| Maternal cigarette use during pregnancy (Y/N) | - | - | - | 42% | - | - | - | - |
| Socioeconomic index | 24.4 | 5.61 | 37.0 | - | - | - | - | |
| - | - | - | - | |||||
| Maternal fish and fowl consumption before pregnancy (Y/N) | - | - | - | 73% | - | - | - | - |
| Sum of reported fish and fowl meals consumed before pregnancy (#/12 mo) | 28.3 | 56.54 | 359.5 | - | - | - | - | |
| - | - | - | - | |||||
| Maternal fish and fowl consumption during pregnancy (Y/N) | - | - | - | 56% | - | - | - | - |
| Sum of reported fish and fowl meals consumed during pregnancy (#/9 mo) | 8.7 | 23.34 | 191.0 | - | - | - | - | |
The median age of the 151 individuals included in this analysis during the MAWBs wave was 13.5 years. The average body mass index (BMI) was 23.9 kg/m2 and was significantly higher in males (25.4 kg/m2; p≤0.001) than females (23.1 kg/m2). Approximately half of respondents were breastfed, with an average duration of just over three months, and about 40% of respondents’ mothers smoked during their pregnancy.
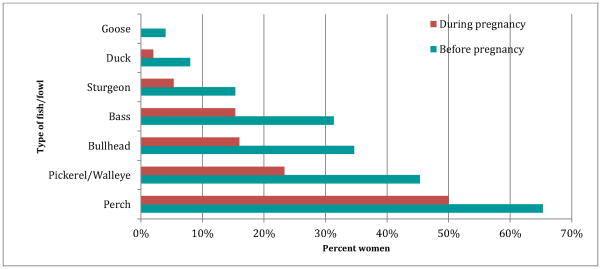
The top three locally caught types of fish most often consumed by Akwesasne women were perch, walleye, and bullhead (see Figure 1). Less than 6% ate catfish, eel, or trout either before or during pregnancy. Waterfowl consumption was negligible with only a few women consuming any wild duck or goose (8% and 4% respectively) before pregnancy, and during pregnancy not one woman ate goose, and only 2% consumed any duck. Average maternal fish consumption before pregnancy was significantly greater than consumption during pregnancy (p=0.001). Average number of locally-caught fish meals ranged from 9 to over 190 meals over the course of a pregnancy, a significant decrease from 28 to 358 meals per year before pregnancy.
Figure 1.
Percent of women who consumed any locally caught fish or fowl before or during pregnancy.
At follow-up, the mean age in the YAWBs wave was 18.1 years. Nearly 50% of the young adults reported that they currently smoked cigarettes, and 95% consumed alcohol in the past year. Males had a significantly higher BMI (27.6 kg/m2; p≤ 0.001) than females. For a more comprehensive description of YAWBs characteristics, see Gallo et al. 2011.
3.2 Initial and follow-up toxicant levels and change
Levels of PCB groupings, p,p′-DDE and HCB, and individual PCB congeners of the participants as youth and again as young adults are shown in Table 2. CBs 118, 138[+163+164] and 153 were detected in nearly all participants in YAWBs (GM: 0.05 ppb, 0.07 ppb, 0.08 ppb, respectively), while CBs 105, 180, and 70 had the highest rate of detection in the earlier study (GM: 0.03 ppb, 0.03 ppb, and 0.02 ppb respectively; results not shown).
Table 2.
Summary and specific PCB congener levels within the Akwesasne Mohawk adolescent population (in ppb; n=151).
| MAWBs | YAWBs | p-value | % with increased levels (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| ROD* | Mean | GM | SD | Max | ROD* | Mean | GM | SD | Max | |||
| Σ16PCB50%a,b | - | 0.77 | 0.70 | 0.371 | 2.71 | - | 0.50 | 0.46 | 0.287 | 2.66 | ||
| Σ of 8 Persistent PCBsa,c | - | 0.46 | 0.41 | 0.276 | 2.45 | - | 0.35 | 0.31 | 0.228 | 1.89 | 0.001 | 25(37) |
| Σ of 6 Non-persistent PCBsa,d | - | 0.24 | 0.21 | 0.124 | 0.72 | - | 0.12 | 0.11 | 0.082 | 0.74 | 0.001 | 9 (14) |
| p,p′-DDE (ppb)a | 100 % | 0.45 | 0.38 | 0.384 | 3.08 | 99% | 0.39 | 0.33 | 0.237 | 1.61 | 0.003 | 34(52) |
| HCB (ppb)a | 99% | 0.04 | 0.03 | 0.019 | 0.19 | 100% | 0.04 | 0.03 | 0.015 | 0.11 | 0.37 | 44(67) |
|
| ||||||||||||
| PCB IUPAC#a
| ||||||||||||
| 105 | 67% | 0.03 | 0.03 | 0.015 | 0.10 | 86% | 0.02 | 0.01 | 0.021 | 0.10 | 0.010 | 32(49) |
| 118 | 99% | 0.08 | 0.07 | 0.040 | 0.28 | 100% | 0.05 | 0.04 | 0.042 | 0.42 | 0.001 | 23(34) |
| 138 [+163+164]e | 89% | 0.08 | 0.07 | 0.058 | 0.47 | 100% | 0.07 | 0.06 | 0.050 | 0.41 | 0.010 | 36(54) |
| 153 | 97% | 0.11 | 0.09 | 0.101 | 0.98 | 98% | 0.08 | 0.07 | 0.056 | 0.45 | 0.001 | 30(45) |
ROD: Rates of detection as described in Schell et al. 2003, and Gallo et al. 2011
All values below the YAWBs mdl was replaced by the YAWBs mdl and all values below the MAWBs mdl were replaced by the MAWBs mdl
Congeners with ≥ 50% detection rate; Sum of IUPAC#s 52, 70, 74, 84, 87, 95, 99, 101[+90], 105, 110, 118, 138[+163+164], 149[+123], 153, 180, 187
Sum of IUPAC#s: 74, 99, 105, 118, 138[+163+164], 153, 180, 187
Sum of IUPAC#s: 52, 84, 95, 101[+90], 110, and 149[+123]
Bracket indicates ‘minor’ congener based on Aroclor concentration (Hansen, 1999).
Levels of PCBs in all groups and p,p′-DDE decreased from 15 to more than 40 percent. Levels of the non-persistent congeners had the sharpest decline (median 0.11 ppb; 48% decrease). In contrast, levels of HCB were unchanged from MAWBs to YAWBs.
In both studies, HCB and p,p′-DDE were found in nearly 100% of the sample; only HCB was significantly higher in males (p=0.05) in the YAWBs wave data. In general, levels of all PCB groups and other toxicants did not differ significantly by sex. In YAWBs, breastfed individuals (n=73) had significantly higher levels of Σ16PCB50%, and Σ8PerPCBs, CBs# 52, 74, 138, 153, and 187 (p≤0.05). In MAWBs, significantly higher levels of Σ16PCB50%, Σ8PerPCBs, p,p′-DDE, HCB, and CBs# 52, 74, 118, 153, 180, and 187 were found in breastfed youth (p≤0.03). Levels of p,p′-DDE were significantly reduced in those who had been breastfed as infants (p≤0.001). Further statistical testing determined that levels of p,p′-DDE and the three PCB groupings were reduced significantly less in proportion to the length of time the individual was breastfed (p=0.04). HCB was unrelated to breastfeeding duration. Mean time elapsed between blood draws in each study was 4.1 years, and there were slight, non-significant differences in mean interval by sex (males=3.9 years, females = 4.1 years), and breast feeding status (BF= 4.3 years, NBF = 3.9 years).
A substantial percentage of respondents had higher absolute levels at follow-up (YAWBs) than in the initial (MAWBs) wave (Table 2). Forty-four percent of individuals increased their levels of HCB and 34% increased their p,p′-DDE level. Nine percent (n=14) of the sample had increased levels of Σ6NonPCBs. Most of these subjects demonstrated 25 – 35% higher absolute levels in young adulthood than in adolescence. Given the large percentage of respondents with an absolute increase, Table 3 supplements the information given in Table 2 by disaggregating annual percent change for each PCB grouping, congener, and other toxicants for the subsample of individuals whose levels decreased from their initial blood draw versus the full sample. Table 3 underscores the impact of ongoing exposure in our sample.
Table 3.
Annual percent changea of PCB grouping and specific congeners, p,p′-DDE, and HCB between studies.
| All individuals | Individuals with decreased levels only | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| n | Mean | Median | SD | Min | Max | n | Mean | Median | SD | Min | Max | |
| Annual percent change | ||||||||||||
|
| ||||||||||||
| Σ16PCB50%b | 151 | 6.8 | 7.8 | 13.24 | −84.3 | 51.7 | 128 | 10.3 | 9.4 | 7.28 | 0.1 | 51.7 |
| Σ8PerPCBsc | 151 | 3.4 | 5.8 | 15.02 | −105.5 | 49.0 | 114 | 8.9 | 8.3 | 6.74 | 0.3 | 49.0 |
| Σ6NONPerPCBsd | 151 | 10.5 | 11.1 | 14.91 | −81.4 | 55.3 | 137 | 13.5 | 12.6 | 8.90 | 0.3 | 55.3 |
| p,p′-DDE | 151 | 1.0 | 3.2 | 10.96 | −35.6 | 24.2 | 99 | 7.0 | 5.7 | 5.26 | 0.4 | 24.2 |
| HCB | 151 | −1.7 | 2.0 | 14.68 | −90.4 | 21.8 | 84 | 7.1 | 6.4 | 4.56 | 0.5 | 21.8 |
|
| ||||||||||||
| PCB IUPAC# | ||||||||||||
|
| ||||||||||||
| 105 | 151 | 1.1 | 8.9 | 30.40 | −187.1 | 60.9 | 102 | 16.3 | 14.6 | 10.90 | 0.7 | 60.9 |
| 118 | 151 | 5.2 | 9.3 | 22.70 | −195.4 | 54.4 | 117 | 12.5 | 12.2 | 7.91 | 1.2 | 54.4 |
| 138 [+163+164]e | 151 | −3.6 | 3.8 | 26.67 | −145.3 | 47.4 | 97 | 9.5 | 9.2 | 7.04 | 0.4 | 47.4 |
| 153 | 151 | 0.9 | 5.9 | 21.10 | −151.3 | 48.9 | 106 | 9.8 | 9.4 | 6.73 | 0.3 | 48.9 |
Congeners with ≥ 50% detection rate in MAWBs;IUPAC #s: 52, 70, 74, 84, 87, 95, 99, 101[+90], 105, 110, 118, 149[+123], 138[+163+164], 153, 180, 187.
Sum of IUPAC#s: 74, 99, 105, 118, 138[+163+164], 153, 180, 187
Sum of IUPAC#s: 52,84,95,101,110,123[+149].
Bracket indicates ‘minor’ congener based on aroclor concentration (Hansen, 1999).
3.3. The number and nature of host-related characteristics and vectors of dietary intake
To understand the factor structure underlying our data, we employed exploratory factor analysis (EFA). We used several criteria to determine that seven factors would be retained for rotation to a final solution, including the Kaiser criterion; the scree test; proportion of common variance accounted for; and interpretability, including conceptual clarity of the factor structure. Four of the 15 items were found to load on the first factor (a variable was said to load on a given factor if the loading was 0.4 or greater), accounting for approximately 30% of the common variance among the 15 items. This is an age/maturation and period-related factor, consisting of an indicator of whether the adolescent was born before 1985 or after (the year fish advisories were issued), adolescents’ age in years, the years between the first and second blood draws, and adolescents’ Tanner Stage (an earlier Tanner stage in an adolescent indicate more maturation occurred between studies).
The second factor is a nutritional composite, indicating the adolescent’s total caloric, protein and fat intake as measured by the NCI-FFQ. This factor accounts for approximately 25% of the common variance. The third factor is a “consumption prior to pregnancy” factor (annualized sum of fish and fowl meals consumed before pregnancy, annualized number of top-feeding fish meals consumed before pregnancy, and annualized number of bottom-feeding fish meals consumed before pregnancy). The third factor accounts for about 18% of the common variance. Factors four and five each explain about 10% of common variance. Factor four identifies fish and fowl consumption during pregnancy (same measurement, though during rather than before pregnancy, as in the third factor, but without bottom-feeding fish meals). Factors five and six describe body dimensions. Factor 5 consists of BMI and weight, while the sixth factor isolates height. The final factor, accounting for about three percent of common variance, is another fish consumption factor, but consists solely in the consumption of bottom-feeding fish before and during pregnancy.
A different perspective on how the variables are related to the factors is provided through an examination of the factor structure, the correlations of the 15 individual items with the seven factors. These correlations, along with the other covariates that were initially considered in the EFA and to be used as controls in our multiple regression estimates of intrinsic elimination rates, are reported in Table 4.
Table 4.
Correlations between PCA factors and characteristics of the sample measured at time of initial blood draw
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 7 | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
|
Pearson correlation
|
|||||||
| Age (yrs) | 0.94** | 0.09 | 0.02 | 0.12 | 0.30** | 0.57** | 0.11 |
| Sex | −0.05 | −0.15 | 0.04 | 0.00 | −0.27** | −0.30** | −0.10 |
| Born before issuance of fish advisories | −0.80** | −0.05 | 0.01 | −0.03 | −0.10 | −0.40** | −0.08 |
| Breast fed (y/n) | −0.12 | 0.06 | 0.06 | −0.15 | −0.10 | 0.05 | 0.13 |
| Breast feeding duration (mos) | −0.14 | −0.03 | 0.20* | −0.14 | −0.11 | 0.00 | 0.21** |
| BMI (kg/m2) | 0.17* | 0.10 | −0.05 | 0.00 | 0.98** | 0.25** | 0.07 |
| Height (cm) | 0.73** | 0.15 | −0.04 | 0.07 | 0.42** | 0.97** | 0.01 |
| Weight (kg) | 0.45** | 0.13 | −0.05 | 0.03 | 0.95** | 0.66** | 0.06 |
| Tanner Stage | 0.81** | 0.06 | 0.02 | 0.11 | 0.22** | 0.50** | −0.04 |
| Cholesterol | −0.23** | −0.08 | 0.04 | 0.01 | −0.07 | −0.16* | 0.12 |
| Triglycerides (mg/dL) | −0.08 | 0.06 | −0.07 | −0.03 | 0.24** | 0.00 | 0.17* |
| Total caloric intake | 0.12 | 0.99** | 0.11 | 0.19* | 0.12 | 0.17* | 0.17* |
| Total fat intake | 0.05 | 0.96** | 0.05 | 0.15 | 0.13 | 0.09 | 0.18* |
| Total protein intake | 0.13 | 0.94** | 0.09 | 0.23** | 0.07 | 0.11 | 0.08 |
| Gravidity | −0.05 | −0.02 | 0.22** | −0.02 | 0.09 | 0.04 | 0.29** |
| Number of years between blood draw | −0.79** | −0.11 | 0.11 | −0.09 | −0.22** | −0.32** | 0.02 |
| Maternal cigarette use during pregnancy (y/n) | 0.09 | 0.11 | −0.05 | −0.07 | 0.05 | 0.00 | 0.00 |
| Socioeconomic index | 0.00 | −0.12 | −0.07 | −0.12 | −0.07 | 0.05 | 0.11 |
| Fish consumption before pregnancy | |||||||
| Sum of reported bottom-feeding fish meals consumed before pregnancy (#/12 mo) | −0.06 | 0.04 | 0.65** | −0.12 | −0.11 | 0.03 | 0.73** |
| Sum of reported top-feeding fish meals consumed before pregnancy (#/12 mo) | 0.01 | 0.11 | 0.94** | 0.51** | −0.02 | −0.02 | −0.11 |
| Sum of reported fish and fowl meals consumed before pregnancy (#/12 mo) | −0.03 | 0.11 | 0.99** | 0.40** | −0.05 | 0.00 | 0.15 |
| Fish consumption during pregnancy | |||||||
| Sum of reported bottom-feeding fish meals consumed during pregnancy (#/9 mo) | 0.09 | 0.17* | 0.08 | 0.18* | 0.11 | 0.05 | 0.44** |
| Sum of reported top-feeding fish meals consumed during pregnancy (#/9 mo) | 0.14 | 0.21* | 0.38** | 0.98** | 0.01 | 0.01 | −0.21* |
| Sum of reported fish and fowl meals consumed during pregnancy (#/9 mo) | 0.10 | 0.22** | 0.37** | 0.98** | 0.03 | 0.02 | −0.08 |
Factor 1: Age and maturation - age (yrs), Tanner Stage, years between blood draws, Born before or after fish advisory
Factor 2: Nutrition - total protein, fat and caloric intake
Factor 3: Maternal consumption of local fish or fowl before pregnancy
Factor 4: Maternal consumption of local fish or fowl during pregnancy
Factor 5: Weight and BMI
Factor 6: Height
Factor 7: Maternal consumption of bottom feeding fish both before and during pregnancy
Examination of the factor structure helps to explain aspects of the rotated pattern matrix. The factor structure generally evidences a “simple structure” wherein most of the variables have relatively high factor loadings on only one factor and most of the factors have relatively high factor loadings for some variables, the “body dimension” variables. However, BMI, weight, and height drive several notable exceptions, as they evidence factorial complexity. Here we see that the first, age/period-related, factor is also substantially correlated with height and weight (but not BMI). Our fifth factor, that identified BMI and weight, also reveals substantial correlation with height, while our “height” (sixth) factor has moderate to strong correlations with weight, age, and Tanner Stage. Factors two, three, four, and seven, maintain their conceptual distinctiveness.
3.4 Tobit regression estimation of intrinsic elimination rates and half-lives
We estimate elimination rates, and their associated half-lives, by fitting Tobit regression models. Log-transformed change between studies in levels of four individual PCB congeners (CBs 105, 118, 138, and 153), three PCB groupings (Σ16PCB50%, Σ8PerPCBs, Σ6NonPerPCBs), and HCB and p,p′-DDE, with τ set to the maximum yi that fails to exceed an exponential decay function of time between initial and follow-up blood draws, is regressed on duration of exposure between initial and follow-up blood draws, with and without adjustment for the seven latent constructs that underlie measures of consumption, sex of the respondent, and the other covariates described previously (see section 2.7 on exploratory factor analysis). We interpret the estimated regression slope, after applying the McDonald-Moffitt decomposition, on the duration between blood draws as the intrinsic elimination rate. We report the unadjusted (Model 1) and adjusted (Model 2) half-lives in Table 5. The regression analyses also allow us to assess the contribution of the latent constructs and other covariates to the adjustment that gives the intrinsic half-lives. We also estimate half-lives using the conventional linear regression method, adjusting for the seven factors, sex, and other covariates, using all respondents (Model 3) and only respondents whose initial toxicant level exceeded their follow-up level (Model 4). These estimated half-lives provide a benchmark by which illustrate the impact of our censored normal regression method by comparison to previously used methods for dealing with ongoing exposure.
Table 5.
Estimate of half-lives (in years) of PCB grouping and specific congeners, p,p′-DDE, and HCB.
| Model 1* Unadjusted Half-life | Model 2** Adjusted Half-life | Model 3*** Adjusted Half-life LR Method (all cases) | Model 4**** Adjusted Half-life LR Method (no increase cases) | |
|---|---|---|---|---|
|
| ||||
| Mean | Mean | Mean | Mean | |
|
| ||||
| Σ16PCB50%a | 8.4 | 7.91 | 6.64 | 5.15 |
| Σ8PerPCBsb | 12.54 | 13.19 | 10.35 | 6.18 |
| Σ6NONPerPCBsc | 5.46 | 4.5 | 4.08 | 3.5 |
| p,p′-DDE | 35.62 | 55.78 | 20.9 | 7.2 |
| HCB | 69.52 | 84.74 | 73.45 | 8.67 |
|
| ||||
| PCB IUPAC#a
| ||||
| 105 | 8.43 | 7.19 | 4.47 | 2.36 |
| 118 | 7.68 | 6.49 | 5.4 | 3.71 |
| 138 [+163+164]d | 22.32 | 24.67 | 27.75 | 5.82 |
| 153 | 14.13 | 16.32 | 13.18 | 5.55 |
Half-life estimated with Tobit regression without adjusting for any covariates
Half-life estimated with Tobit regression adjusted for vectors of intake and controls
Half-life estimated with linear regression (LR) using all respondents (adjusted for vectors of intake and controls)
Half-life estimated with linear regression (LR) eliminating respondents with increased POP level (adjusted for vectors of intake and controls)
Congeners with ≥ 50% detection rate in MAWBs: IUPAC #s: 52, 70, 74, 84, 87, 95, 99, 101[+90], 105, 110, 118, 149[+123], 138[+163+164], 153, 180, 187
Sum of IUPAC#s: 74, 99, 105, 118, 138[+163+164], 153, 180, 187
Sum of IUPAC#s: 52,84,95,101,110,123[+149]
Bracket indicates ‘minor’ congener based on aroclor concentration (Hansen, 1999)
Comparison of the unadjusted (Model 1) Tobit regression with first-order rate constant only to adjusted (Model 2) Tobit regression with factors and other covariates in addition to first-order rate constant estimated half-lives showed mixed results. Estimated half-lives ranged from just five years (Σ6NonPerPCBs) to over 35 years (p,p′-DDE). The adjusted estimates produced a decrease in half-life for Σ16PCB50%, Σ6NonPerPCBs, and CBs 105 and 118, but increased half-life for Σ8PerPCBs, p,p′-DDE, HCB, and CBs 138 and 153. In some instances, the adjustments had trivial impact (e.g., estimated half-life of Σ16PCB50% decreased by five months, while Σ8PerPCBs’ half-life increased by about seven months). The impact on HCB and p,p′-DDE half-lives was far more consequential and likely results not only from the effectiveness of our regression covariates, but also from selectivity in our study population to background concentration. HCB increased from about 67 years to over 78 years and p,p′-DDE half-life more than doubled. Ongoing exposure to HCB, p,p′-DDE, and some PCB congeners in our population is very high; recall that Table 2 shows that 44 and 34 percent of individuals had an absolute increase in HCB and p,p′-DDE, respectively, between blood draws.
The decompositions shown in section 2.6 should be clear that OLS regression on the full sample will produce inconsistent estimates because E [ y] is a non-linear function of X, β, and σ; and OLS assumes linearity. In addition, OLS regression on the uncensored sample (Model 4) will also produce inconsistent estimates because it omits relevant parts of the estimator of β. Regardless, a comparison of half-life estimates reported under Models 3 and 4 help reinforce the value of our method. Failure to account for ongoing exposure in the half-lives reported under Model 3 produces estimates that are all shorter than those in Model 2, except CB 138, which is three years longer. Estimated half-lives based on exclusion of individuals with non-positive values (Model 4) are far worse. Half-lives reported in Model 4 are all substantially less than those in Models 2 and 3; and the difference is clearly related to the proportion of cases with non-positive values (again refer to Table 2).
Of the seven factors identified in the EFA, only the “nutritional factor” (factor 2: caloric, protein, and fat intake) and fish/fowl consumption during pregnancy (factor 4) were not significantly related to any of the elimination rates. None of the factors or other covariates was significantly related to CB 138 half-life, although consumption before pregnancy (factor 3) was marginally significant (p=0.073). Consumption before pregnancy (factor 3) was significantly and negatively related to Σ16PCB50%, Σ8PerPCBs, and positively related to DDE. The factor that describes age and maturation (factor 1) was positively associated with Σ6NonPerPCBs and CB 118, suggesting the older the participant the greater the decline in these two POP levels from initial measurement to follow-up. Body mass index and weight (factor 5) was found to have a negative adjustment on HCB and CB 153; bottom fish consumption (factor 7) was negatively associated with p,p′-DDE and CB 105. Height (factor 6) also showed a significant and negative effect on CB 105. An indicator of whether the respondent had been breastfed, gravidity, and triglycerides were the only controls found to significantly add to half-life prediction.
4. Discussion
While there are a number of cross sectional investigations that have identified factors associated with current organochlorine body burden, there is a paucity of reports on serial PCB data among humans, especially within the age bracket that we have considered here. To the best of our knowledge, this is the first report of serial congener-specific PCB data from adolescence to young adulthood. Thus, the current study provides an exceptional opportunity to examine changes in serum organochlorine levels of young Akwesasne men and women relative to late childhood and adolescent levels.
Akwesasne young adult toxicant levels were found to have decreased overall in the past four years, yet continue to be higher in the breastfed individuals than in the non-breastfed, with the exceptions of Σ6NONPerPCBs, HCB and CB 105. This is consistent with our earlier report on breastfed adolescents (Schell et al. 2003). This overall decrease suggests that there is less consumption of locally caught fish, fowl or wildlife, that body burden of these PCBs has been reduced via metabolism and excretion, and potentially lower levels of airborne congeners now than four years earlier.
Nearly 44%, 32%, and 36% of the young adults had increased HCB, CBs 105 and 138[+163+164] levels respectively, which would suggest continued exposure. This continued post-natal exposure would obfuscate any effect by breast-feeding. The Akwesasne community continues to live surrounded by industrial facilities, some recently closed, that have not been remediated and continue to leak residual toxicants into the air, soil and water.
While we concur with Megson et al. (2013) that accounting for the source and contaminant pathway is important when attempting to age date exposure, our investigation of the number and nature of vectors of dietary intake suggests that source and pathway also bear consequences within a narrow age range and historical period. We identified seven underlying factors for the type and timing of consumption and a set of physical and behavioral characteristics. The latent constructs included an age/maturation and period-related factor, a nutritional composite, consumption prior to pregnancy, fish and fowl consumed during pregnancy, factors distinguishing body mass and weight from height, and bottom-feeding fish consumption (see Table 4). Elimination rates and their half-lives are often adjusted for the body burden – age relationship (cf. (Quinn and Wania 2012), but our EFA has shown that distinct dietary routines are also underlying factors that should be part of the regression model specification.
A variety of potential solutions for bias in half-life estimates have been proposed for populations with continuing (background) exposure (Bartell 2012; Wong et al. 2013). We proposed and applied an alternative estimator, the Tobit model, to calculate unbiased half-life expectation at mean levels of model covariates using all available information by separating cases whose change between initial measurement and follow-up exceeded change anticipated by the first-order rate constant. We also compared unadjusted against the adjusted estimated half-lives (see Table 5). In some instances, the adjustments had trivial impact, while in others the adjustment increased half-life by as much as a decade. In addition, the adjusted estimates produced a decrease in half-life for Σ16PCB50%, Σ6NonPerPCB, and CBs 105 and 118, but increased half-life for Σ8PerPCB, p,p′-DDE, HCB, and CBs 138 and 153. Of the seven factors identified in the EFA, only two were not significantly related to any of the elimination half-lives; and although fish/fowl consumption during pregnancy was not associated with half-lives, the other two dietary intake factors showed associations with several PCB groupings, CBs, and other POPs.
Estimated half-lives ranged from approximately five years (Σ6NonPerPCBs) to over 84 years (HCB). The level of the organochlorine HCB, a contaminant in several pesticides, remained virtually unchanged between studies, yet two factors, BMI/weight (factor 5) and, to a less extent, consumption prior to pregnancy (factor 3; p=0.079) were predictive of half-life. HCB is highly persistent due to its chemical stability and resistance to biodegradation, with an estimated half-life of over two years in the atmosphere, and over four years in soil. The average time between analyses of the participant’s blood is slightly over four years, hence perhaps not long enough to find a substantial change in levels between these two projects.
5. Conclusion
This study utilized sequential measurements of persistent organic pollutants levels from adolescence to adulthood, a period of intense change in size and body composition to quantify individual-level change within a community exposed to environmental toxicants. We estimated unbiased elimination rates, or half-lives, ranging from five to over 84 years. While toxicant levels were found to have decreased overall in the past four years with the exception of HCB, many continue to be higher in the breastfed individuals than in the non-breastfed. The overall decrease suggests that body burden of these PCBs has been reduced via metabolism and excretion. Other possible contributors may be a reduction in the levels of airborne congeners from four years earlier, and the community’s decreased consumption of locally caught fish, fowl or wildlife. Examination of the factors behind the continued increase in levels of some of these toxicants is warranted.
Highlights.
We utilized sequential measurements of POPs to quantify individual-level change.
Unadjusted and adjusted half-lives are reported for PCBs, p,p′-DDE, and HCB.
Ongoing exposure was considered in estimation of POPs intrinsic elimination rate.
EFA identified individual-level host characteristics related to change in POP levels.
Change in toxicant levels was related to change in maturation and year of birth.
Acknowledgments
Funding: This work was supported by grants from the National Institute of Environmental Health Sciences (NIEHS-ESO4913; ES10904), and the National Center on Minority Health and Health Disparities (NCMHD-1P20MD003373).
We thank the Akwesasne Mohawk community for their cooperation and participation in this research and a special thanks to David Carpenter, Agnes “Sweets’ Jacobs, Maxine Cole, Priscilla Worswick, Dawn David, Alice Tarbell, and Julia Ravenscroft. This work was supported by grants from the National Institute of Environmental Health Sciences (NIEHS-ESO4913; ES10904), and the National Center on Minority Health and Health Disparities (NCMHD-1P20MD003373).
Abbreviations
- AMN
Akwesasne Mohawk Nation
- ΣPCB50%
Sum of IUPAC#s 52, 70, 74, 84, 87, 95, 99, 101[+90], 105, 110, 118, 138[+163+164], 149[+123], 153, 180, 187
- ΣPERPCB8
Sum of IUPAC#s 74, 99, 105, 118, 138[+163+164], 153, 180, 187
- ΣNONPER6
Sum of IUPAC#s 52, 84, 95, 101[+90], 110, and 149[+123], HCB, Hexachlorobenzene
- MAWBs
Mohawk Adolescent Well Being study
- p
p′-DDE, p,p′-dichlorophenyldichloroethylene
- PCBs
Polychlorinated Biphenyls
- POPs
Persistent organic pollutants
- ppb
Parts per billion
- ppt
Parts per trillion
Footnotes
We acknowledge that estimating SES is difficult, especially in a Native American population. Nevertheless, we calculated a weighted variable, which included maternal education, maternal employment and marital status, and size of residence.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akwesasne Task Force On The Environment. Superfund clean-up at Akwesasne: a case study in environmental justice. Int J Contemp Sociol. 1997;34(2):267–290. [Google Scholar]
- Arisawa K, Uemura H, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Nagai M. Dietary patterns and blood levels of PCDDs, PCDFs, and dioxin-like PCBs in 1656 Japanese individuals. Chemosphere. 2011;82(5):656–662. doi: 10.1016/j.chemosphere.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Ballschmiter K, Zell M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Z Anal Chem. 1980;302:20–31. [Google Scholar]
- Bartell SM. Bias in half-life estimates using log concentration regression in the presence of background exposures, and potential solutions. Journal of Exposure Science & Environmental Epidemiology. 2012;22:299–303. doi: 10.1038/jes.2012.2. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11(6):641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Brown JF. Determination of PCB Metabolic, Excretion, and Accumulation Rates for Use as Indicators of Biological Response and Relative Risk. Environ Sci Technol. 1994;28(13):2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Sergeyev O, Korrick S, Lee MM, Revich B, Hauser R. Serum dioxins and polychlorinated biphenyls are associated with growth among Russian boys. Pediatr. 2011;127(1):e59–68. doi: 10.1542/peds.2009-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Eskenazi B, Bradman A, Fenster L, Barr DB. Associations between Prenatal Exposure to Polychlorinated Biphenyls and Neonatal Thyroid-Stimulating Hormone Levels in a Mexican-American Population, Salinas Valley, California. Environ Health Perspect. 2007;115(10):1490–1496. doi: 10.1289/ehp.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health. 2010;65(3):127–134. doi: 10.1080/19338241003730887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Sholtz RI, Ferrara A, Park JS, Schwingl PJ. Polychlorinated biphenyl (PCB) exposure in mothers and time to pregnancy in daughters. Reprod Toxicol. 2011;31(3):290–296. doi: 10.1016/j.reprotox.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ Akwesasne Task Force on the Environment. PCB exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environmental Research. 2005;98(3):284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP, Tarbell AM, Bott A, Wagemaker DL, Williams RL, O’Hehir CM. Routine analysis of 101 polychlorinated biphenyl congeners in human serum by parallel dual-column gas chromatography with electron capture detection. J Anal Toxicol. 2000;24(6):403–420. doi: 10.1093/jat/24.6.403. [DOI] [PubMed] [Google Scholar]
- Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP Akwesasne Task Force on the Environment. Relationship of Lead, Mercury, Mirex, Dichlorodiphenyldichloroethylene, Hexachlorobenzene, and Polychlorinated Biphenyls to Timing of Menarche Among Akwesasne Mohawk Girls. Pediatr. 2005;115(2):e127–e134. doi: 10.1542/peds.2004-1161. [DOI] [PubMed] [Google Scholar]
- Dhooge W, Den Hond E, Bruckers L, Schoeters G, Nelen V, van de Mieroop E, van Larebeke N. Internal exposure to pollutants and sex hormone levels in Flemish male adolescents in a cross-sectional study: associations and dose-response relationships. J Expo Sci Environ Epidemiol. 2011;21(3):224–233. doi: 10.1038/jes.2010.2. [DOI] [PubMed] [Google Scholar]
- Dhooge W, Den Hond E, Koppen G, Bruckers L, Nelen V, Van De Mieroop E, Van Larebeke N. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int. 2010;36(4):330–337. doi: 10.1016/j.envint.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152(2):581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the Use of Exploratory Factor Analysis in Psychological Research. Psychological Methods. 1999;4(3):272–299. [Google Scholar]
- Fängström B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, Weihe P, Bergman Å. Concentrations of polybrominated diphenyl ethers, polychlorinated biphenyls, and polychlorobiphenylols in serum from pregnant Faroese women and their children 7 years later. Environmental Science & Technology. 2005;39(24):9457–9463. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Fletcher BA, Belanger E, Tao L, Kannan K, Hwang SA. Fish consumption and concentrations of polybrominated diphenyl ethers (PBDEs) in the serum of older residents of upper Hudson River communities. Arch Environ Occup Health. 2010;65(4):183–190. doi: 10.1080/19338241003730929. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang SA, Brix KA, Bush B, Cook K, Worswick P. Fish PCB concentrations and consumption patterns among Mohawk women at Akwesasne. J Expo Anal Environ Epidemiol. 1995;5(1):1–19. [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang SA, Bush B, Cook K, Worswick P. Fish consumption and breast milk PCB concentrations among Mohawk women at Akwesasne. Am J Epidemiol. 1998;148(2):164–172. doi: 10.1093/oxfordjournals.aje.a009620. [DOI] [PubMed] [Google Scholar]
- Forti A, Bogdan KG, Horn E. Health Risk Assessment for the Akwesasne Mohawk Population from Exposure to Chemical Contaminants in Fish and Wildlife. Albany, NY: New York State Department of Health; 1995. [Google Scholar]
- Gallo MV, Ravenscroft J, Schell LM, DiCaprio A . Akwesasne Task Force On The Environment. Environmental contaminants and growth of Mohawk adolescents at Akwesasne. In: Gilli G, Schell LM, Benso L, editors. Human Growth from Conception to Maturity. London: Smith-Gordon; 2002. pp. 279–287. [Google Scholar]
- Gallo MV, Schell LM Akwesasne Task Force On The Environment. Height, weight and body mass index among Akwesasne Mohawk youth. Am J Hum Biol. 2005;17:269–279. doi: 10.1002/ajhb.20316. [DOI] [PubMed] [Google Scholar]
- Gallo MV, Schell LM Akwesasne Task Force on the Environment. Selected anthropometric measurements of Akwesasne Mohawk youth: skinfolds, circumferences, and breadths. Am J Hum Biol. 2007;19(4):525–536. doi: 10.1002/ajhb.20612. [DOI] [PubMed] [Google Scholar]
- Gallo MV, Schell LM, DeCaprio AP, Jacobs A. Levels of persistent organic pollutant and their predictors among young adults. Chemosphere. 2011;83(10):1374–1382. doi: 10.1016/j.chemosphere.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George-Kanentiio D. Iroquois population in 1995. Akwesasne Notes. 1995;1(3–4):61. [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, McCaffrey RJ, Carpenter DO Akwesasne Task Force on the Environment. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106(2):226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, Barr DB, Needham LL, Weihe P, Heinzow B. Elimination half-lives of polychlorinated biphenyl congeners in children. Environ Sci Technol. 2008;42(18):6991–6996. doi: 10.1021/es800778q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Guitart R, Puig P, Gomez-Catalan J. Requirement for a standardized nomenclature criterium for PCBs: Computer-assisted assignment of correct congener denomination and numbering. Chemosphere. 1993;27:1451–1459. [Google Scholar]
- Hansen LG. The ortho side of PCBs: Occurrence and Disposition. Norwell, MA: Kluwer Academic Publishers; 1999. [Google Scholar]
- Hansen LG. Identification of steady state and episodic PCB congeners from multiple pathway exposures. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. pp. 48–56. [Google Scholar]
- Hatcher L. A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling. Cary, NC: SAS Institute Inc; 1994. [Google Scholar]
- IBM. Statistical Package for the Social Sciences (Version 20) New York: IBM Corporation; 2012. [Google Scholar]
- Knobeloch L, Turyk M, Imm P, Schrank C, Anderson H. Temporal changes in PCB and DDE levels among a cohort of frequent and infrequent consumers of Great Lakes sportfish. Environ Res. 2009;109(1):66–72. doi: 10.1016/j.envres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Kuzyk ZA, Hodson PV, Solomon SM, Reimer KJ. Biological responses to PCB exposure in shorthorn sculpin from Saglek Bay, Labrador. The Science of the Total Environment. 2005;351–352:285–300. doi: 10.1016/j.scitotenv.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kuzyk ZA, Stow JP, Burgess NM, Solomon SM, Reimer KJ. PCBs in sediments and the coastal food web near a local contaminant source in Saglek Bay, Labrador. The Science of the Total Environment. 2005;351–352:264–284. doi: 10.1016/j.scitotenv.2005.04.050. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, Hunter DJ. Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environ Health Perspect. 1999;107(1):75–81. doi: 10.1289/ehp.9910775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front Neuroendocrinol. 2010;31(4):497–518. doi: 10.1016/j.yfrne.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Susienkova K, Radikova Z, Koska J, Klimes I. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin) Thyroid Res. 2009;2(1):3. doi: 10.1186/1756-6614-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118(9):1235–1242. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, Koppe JG, Olie K, van Aalderen WM, de VP, ten Tusscher GW. Effects of dioxins, PCBs, and PBDEs on immunology and hematology in adolescents. Environmental Science and Technology. 2009;43(20):7946–7951. doi: 10.1021/es901480f. [DOI] [PubMed] [Google Scholar]
- Lignell S, Aune M, Darnerud PO, Cnattingius S, Glynn A. Persistent organochlorine and organobromine compounds in mother’s milk from Sweden 1996–2006: compound-specific temporal trends. Environ Res. 2009;109(6):760–767. doi: 10.1016/j.envres.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lotti M. Pharmacokinetics and blood levels of polychlorinated biphenyls. Toxicol Rev. 2003;22:203–215. doi: 10.2165/00139709-200322040-00003. [DOI] [PubMed] [Google Scholar]
- Manski CF. Identification Problems in the Social Sciences. Cambridge, MA: Harvard University Press; 1995. [Google Scholar]
- Matsumoto S, Akahane M, Kanagawa Y, Koike S, Yoshimura T, Mitoma C, Imamura T. Variation in half-life of penta-chlorodibenzofuran (PeCDF) blood level among Yusho patients. Chemosphere. 2009;77(5):658–662. doi: 10.1016/j.chemosphere.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Dedrick RL. Pharmacokinetics of PCBs. Annual Review of Pharmacological Toxicology. 1984;24:85–103. doi: 10.1146/annurev.pa.24.040184.000505. [DOI] [PubMed] [Google Scholar]
- McDonald JF, Moffitt RA. The Uses of Tobit Analysis. The Review of Economics and Statistics. 1980;62(2):318–321. [Google Scholar]
- McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: Considerations for a congener- specific analysis. Environ Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megson D, O’Sullivan G, Comber S, Worsfold PJ, Lohan MC, Edwards MR, Shields WJ, Sandau CD, Patterson DG. Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci Total Environ. 2013;461:99–107. doi: 10.1016/j.scitotenv.2013.04.082. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Garcia-Esteban R, Guxens M, Vrijheid M, Kogevinas M, Goni F, Sunyer J. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ Health Perspect. 2011;119(2):272–278. doi: 10.1289/ehp.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrath MD, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46(11):1762–1772. [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Neilsen F, Patterson DG, Jr, Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Aucompaugh AG, Schell LM, Denham M, DeCaprio AP, Gallo MV Akwesasne Task Force on the Environment. PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicol Teratol. 2006;28(4):439–445. doi: 10.1016/j.ntt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD Akwesasne Task Force on Environment. Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotoxicology. 2009;30(4):686–696. doi: 10.1016/j.neuro.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger MA, Lavoie ET, Abdelnabi M, Quinn MJ, Jr, Marcell A, Dean K. An overview of dioxin-like compounds, PCB, and pesticide exposures associated with sexual differentiation of neuroendocrine systems, fluctuating asymmetry, and behavioral effects in birds. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(4):286–300. doi: 10.1080/10590500903310229. [DOI] [PubMed] [Google Scholar]
- Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association study (EWAS) on type 2 diabetes mellitus. PLoS One. 2010;5(5):e10746. doi: 10.1371/journal.pone.0010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CL, Wania F. Understanding differences in the body burden-age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environmental Health Perspectives. 2012;120:554–559. doi: 10.1289/ehp.1104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenscroft J, Schell LM. Dietary patterns and exposure to environmental contamination at Akwesasne. Paper presented at the 32nd Annual Meeting of the Human Biology Association; Philadelphia, PA.. 2007. [Google Scholar]
- Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, Hungerbuhler K. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the united kingdom. Environmental Health Perspectives. 2011;119:225–231. doi: 10.1289/ehp.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, DeCaprio AP, Gallo MV, Hubicki L. Polychlorinated biphenyls and thyroid function in adolescents of the Mohawk Nation at Akwesasne. In: Gilli G, Schell LM, Benso L, editors. Human Growth from Conception to Maturity. London: Smith-Gordon; 2002. pp. 289–296. [Google Scholar]
- Schell LM, Gallo MV. Relationships of putative endocrine disruptors to human sexual maturation and thyroid activity in youth. Physiol Behav. 2010;99(2):246–253. doi: 10.1016/j.physbeh.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Ravenscroft J, DeCaprio AP. Persistent organic pollutants and anti-thyroid peroxidase levels in Akwesasne Mohawk young adults. Environ Res. 2009;109(1):86–92. doi: 10.1016/j.envres.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Hubicki LA, DeCaprio AP, Gallo MV, Ravenscroft J, Tarbell A Akwesasne Task Force on the Environment. Organochlorines, lead, and mercury in Akwesasne Mohawk youth. Environmental Health Perspectives. 2003;111(7):954–961. doi: 10.1289/ehp.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto T, Yasuhara A, Katami T. Dioxin formation from waste incineration. Rev Environ Contam Toxicol. 2007;190:1–41. doi: 10.1007/978-0-387-36903-7_1. [DOI] [PubMed] [Google Scholar]
- Shirai JH, Kissel JC. Uncertainty in estimated half-lives of PCBs in humans: Impact on exposure assessment. The Science of the Total Environment. 1996;187:199–210. doi: 10.1016/0048-9697(96)05142-x. [DOI] [PubMed] [Google Scholar]
- Sloan RJ, Jock K. C New York State Department of Environmental, editor. Chemical contaminants in fish from the St Lawrence River drainage on lands of the Mohawk Nation at Akwesasne and near the General Motors Corporation/Central Foundry Division, Massena, NY Plant. New York: New York State Department of Environmental Conservation; 1990. [Google Scholar]
- Startin JR, Rose MD. Dioxins and Dioxinlike PCBs in Food Dioxins and Health. John Wiley & Sons, Inc; 2003. pp. 89–136. (Reprinted from: NOT IN FILE) [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- Stehr-Green PA. Demographic and seasonal influences on human serum pesticide residue levels. J Toxicol Environ Health. 1989;27(4):405–421. doi: 10.1080/15287398909531312. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The Relationship between Prenatal PCB Exposure and Intelligence (IQ) in 9-Year-Old Children. Environ Health Perspect. 2000;116(10):1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM. Foetus into man: physical growth from conception to maturity. Cambridge, Mass: Harvard University Press; 1990. Rev. and enl. [Google Scholar]
- Tobin J. Estimation of Relationships for Limited Dependent Variables. Econometrica. 1958;26:24–36. [Google Scholar]
- Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p′-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009;75(5):674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn ME. Mechanistic basis of resistance to PCBs in Atlantic tomcod from the Hudson River. Science. 2011;331(6022):1322–1325. doi: 10.1126/science.1197296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Cousins IT, MacLeod M. Bounding uncertainties in intrinsic human elimination half-lives and intake of polybrominated diphenyl ethers in the North American population. Environ Inter. 2013;59:168–174. doi: 10.1016/j.envint.2013.05.004. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Concise International Chemical Assessment Document. Vol. 55. Geneva: Inter-Organization Programme for the Sound Management of Chemicals; 2003. Polychlorinated biphenyls: Human health aspects. [Google Scholar]
- Yakushiji T, Watanabe I, Kuwabara K, Tanaka R, Kashimoto T, Kunita N, et al. Rate of decrease and half-life of polychlorinated biphenyls (PCBs) in the blood of mothers and their children occupationally exposed to PCBs. Archives of Environmental Contamination and Toxicology. 1984;13:341–345. doi: 10.1007/BF01055285. [DOI] [PubMed] [Google Scholar]