Abstract
Background
Population-level control of modifiable cardiovascular disease (CVD) risk factors is suboptimal.
Objectives
1) To demonstrate the use of electronically downloaded electronic health record (EHR) data to assess guideline concordance in a large cohort of primary care patients, 2) To provide a contemporary assessment of BP and LDL non-control in primary care, and 3) To demonstrate the effect of risk adjustment of rates of non-control of BP and LDL for differences in patient mix on these clinic-level performance measures.
Design
Observational comparative effectiveness.
Patients
All 232,172 adult patients ≥age 18 with ≥1 visit within two years in 33 primary care clinics with electronic health records (EHR).
Interventions
None.
Main Measures
Rates of BP and LDL non-control, based on current guidelines, were calculated from electronically downloaded EHR data. Non-control rates were risk-adjusted using multi-variable models of patient-level variables.
Key Results
Overall, 16.0% of the 227,122 patients with known BP and 14.9% of the 136,771 patients with known LDL were uncontrolled. Clinic-level risk-adjusted BP non-control ranged from 7.7% to 26.5%, while that for LDL ranged from 5.8% to 23.6%. Non-control rates exceeded an achievable benchmark for 85% (28/33) and 79% (26/33) of clinics for BP and LDL, respectively. Risk-adjustment significantly influences clinic rank order for rate of non-control.
Conclusions
We have demonstrated that the use of electronic collection of data on a large number of patients from fee-for-services, primary care clinics required for the audit and feedback of BP and LDL non-control is feasible. Non-control rates for most clinics are substantially higher than that achievable. Risk-adjustment of non-control rates results in very different clinic rank-order of clinics from non-adjusted data.
Keywords: hypertension, hyperlipidemia, clinical practice guideline, risk adjustment, guideline adherence
INTRODUCTION
Background/rationale
More than one-third of American adults have one or more of the following cardiovascular diseases (CVD): hypertension, coronary heart disease (CHD), stroke, or heart failure. In 2008 CVD was the primary cause of 32.8% of all U.S. deaths. Similarly, CVD is the most common reason for hospitalization, accounting for 18% of the total of 34,369,000 hospitalizations and one fourth of the total cost of inpatient hospital care in the United States1. Between 2010 and 2030, total direct medical costs of CVD (in real 2008$) are projected to triple, from $273 billion to $818 billion2.
Modifiable risk factors account for most CVD. The Atherosclerosis Risk in Communities Study (ARRIC) followed 14,162 middle-aged adults, who were free of recognized CVD at entry, for a mean of 13.1 years3. The vast majority (86.2%) of the 1,492 CVD events occurred in the 66.5% of the population with ≥1 risk factor. The population-attributable fraction suggested that having at least 1 elevated risk factor accounted for 70.2% of CVD events.
Despite effective antihypertensive and antihyperlipidemic medications shown to reduce major adverse cardiovascular events (MACE) in large-scale randomized trials4;5, the control of BP and cholesterol in the U.S. remains suboptimal. National Health and Nutrition Examination Survey (NHANES) data from 2005 to 2006 showed that 20.3% of US adults had uncontrolled blood pressure (BP) defined as ≥140/906. For each 10 mm Hg decrease in systolic BP, the average risk of heart disease and stroke mortality decreases by 30% and 40%, respectively7. An estimated 33,500,000 adults ≥20 years of age have total cholesterol levels ≥240 mg/dL for a prevalence of 16.2%1;8. Cohort studies, based on half a million men and 18,000 ischemic heart disease events, estimate that a 10% long-term reduction in serum cholesterol would lower the risk of ischemic heart disease by 50% at age 409.
Objectives
The objectives of this report are: 1) To demonstrate the use of electronically downloaded electronic health record (EHR) data to assess guideline concordance in a large cohort of primary care patients, 2) To provide a contemporary assessment of BP and LDL non-control in primary care, and 3) To demonstrate the effect of risk adjustment of rates of non-control of BP and LDL for differences in patient mix on these clinic-level performance measures.
METHODS
Study design
This an observational study comparing evidence-based, risk-adjusted non-control rates for BP and LDL across 33 clinics.
Setting
This study is being conducted in the DARTNet Collaborative, a group of practice-based research networks that are working to build a national collection of EHR data10–12. DARTNet, in collaboration with QED Clinical, Inc., d/b/as CINA (http://www.cina-us.com/), has developed data extraction, transformation, and loading (ETL) processes, which allow aggregation of data from disparate EHRs into a harmonized database. The Cardiovascular Risk Reduction Learning Community (CRRLC) includes 33 primary care clinics from 10 private, fee-for-services, health care delivery organizations participating in DARTNet. Two organizations were affiliated with a academic medical center; one provided sites for a community residency program affiliated with an academic medical center; while the remainder were not academically affiliated.
Participants
The study population consisted of all 232,172 patients who met our overall criteria of age ≥18 years and ≥1 clinic appointment within the preceding two years.
Guideline translation to calculate BP and LDL non-control rates
We relied extensively on the guidelines developed under of the auspices of the NHLBI for control of BP (JNC7)13 and low density lipoprotein cholesterol (LDL) (NCEP)14. As have others15, we found translating guidelines from their published, largely-text format into algorithms suitable for electronic data analysis a challenging task, requiring multiple revisions. Our CRRLC Steering Committee, composed of four primary care physicians recruited from participating clinics and two university-based subject matter experts (see acknowledgement at the conclusion of this report), were central in resolving questions in this process.
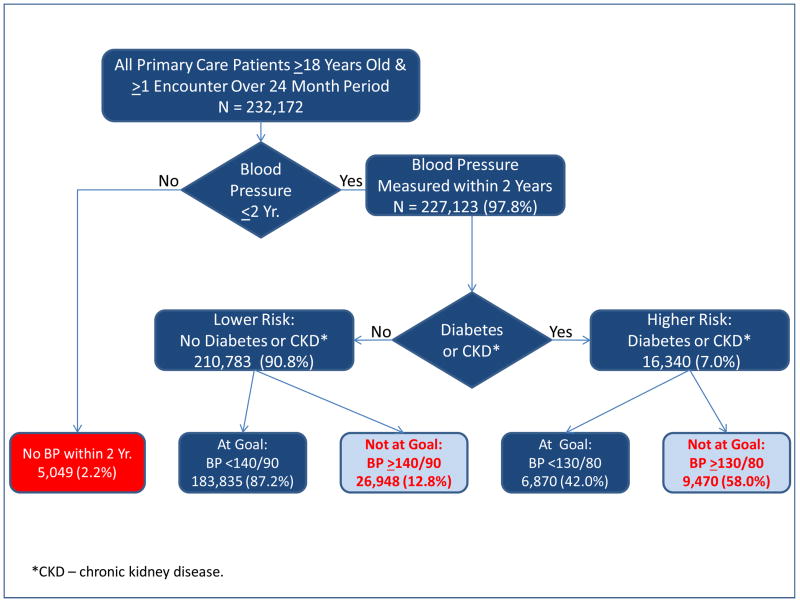
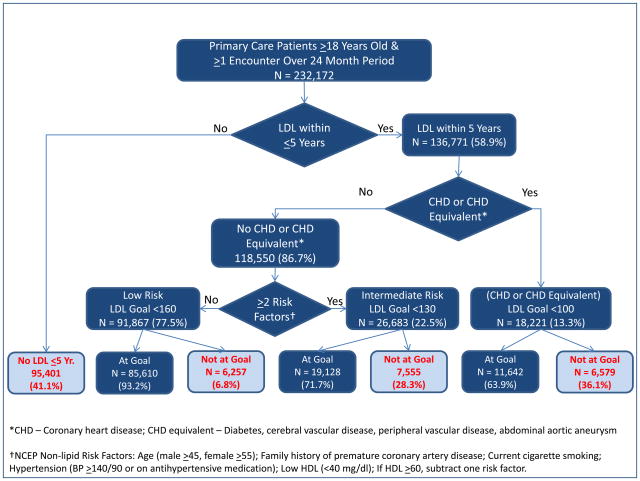
Calculation of non-control rates in both the JNC7 and NCEP guidelines requires categorizing patients according to their risk for MACE using CVD risk factors and presence or absence of coronary heart disease. The results of the translation of JNC7 and NCEP guidelines into hierarchical flow diagrams, on which construction of electronic algorithms to calculate non-control rates were based, are shown in Figures 1 and 2, respectively; additional details are provided in Appendix Tables 1 and 2.
Figure 1.
Flow Diagram Illustrating Both JNC7 Criteria for BP Control and Number and Percent of Patients at Each Step.
Figure 2.
Flow Diagram Illustrating NCEP Criteria for LDL Control and Number and Percent of Patients at Each Step.
Appendix Table 1.
ICD-9 Code Criteria for Comorbidity and Coronary Heart Disease Risk Factors Used to Construct JNC7 Guideline Concordance Algorithm (Figure 1)
|
Source Material:
Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; Roccella, E.J. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42(6):1205–1252. or http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.htm 2009 ICD-9-CM. http://icd9cm.chrisendres.com/
Appendix Table 2.
ICD-9 Code Criteria for Comorbidity and Coronary Heart Disease Risk Factors Used to Construct the NCEP Guideline Concordance Algorithm (Figure 3).
|
- HDL-cholesterol level is ≥60 mg/dL (−1)
- Age (men ≥45 years; women ≥55 years) (1)
- Cigarette smoking (1)
- Hypertension (BP ≥140/90 mmHg (average of two most recent measurements) or on antihypertensive medication) (1)
- Low HDL cholesterol (<40 mg/dL) (1)
- High HDL cholesterol (≥60 mg/dl (− 1)
- Family history of premature CHD (CHD in male first-degree relative <55 years; CHD in female firstdegree relative <65 years) (not consistently available in DARTNet) (1)
Source Material:
Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–3421 or http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm.
2009 ICD-9-CM. http://icd9cm.chrisendres.com/
Data sources/management
The data reported here are drawn from clinic visit EHR data, including problem lists, patient demographics, BP measurements, and laboratory data between 1/1/2006 and 12/31/2010. For both the JNC7 and NCEP guidelines, the data required to assess and risk-adjust guideline non-control include BP and LDL measurements, concomitant comorbidity, and other risk factors for MACE (e.g., age, cigarette smoking, HDL-cholesterol, etc.). Additionally, we collected data on clinic appointments and encounters, antihypertensive and antihyperlipidemic medications prescribed, height, weight, year of birth, and tobacco use/abuse. When two or more BP measurements were available, we used the average of the two most recent. While age, BP, LDL, and HDL are relatively easily retrievable from the EHR of CRRLC organizations, the definition of comorbidities using ICD-9 codes requires grouping into clusters, which are not provided in either the complete JNC713 or NCEP14 documents. We used the clustering of ICD-9 codes into comorbidities developed for ambulatory care by Schneeweiss16 and subsequently modified by Pace17. This grouping did not contain ICD-9 code clusters for chronic kidney disease and abdominal aortic aneurysm, which we created using our clinical judgment (Appendix Table 3).
Appendix Table 3.
ICD-9 Diagnostic Code Clusters for Morbidity Assessment in Ambulatory Care.
| # | DIAGNOSTIC/PROCESS CLUSTER | CODES TO INCLUDE | |
|---|---|---|---|
| From | To | ||
| 1 | Hernia (external abdominal) | 550 | |
| 551.0 | 551.2 | ||
| 552.0 | 552.2 | ||
| 553.0 | 553.2 | ||
| 2 | Abdominal pain | 789 | |
| 3 | Acne, diseases of sweat and sebaceous glands | 695.3 | |
| 705 | 705.9 | ||
| 706.0 | 706.9 | ||
| 4 | Intestinal infectious diseases/Acute gastroenteritis | 001 | 005.9 |
| 006.0 | 006.2 | ||
| 007 | 009 | ||
| 558.9 | |||
| 5 | Acute sprains, strains | 840 | 848.9 |
| 6 | Adverse effects of medicinal agents | 960 | 979.9 |
| 995 | 995.2 | ||
| 995.4 | |||
| 7 | Alcohol & drug abuse | 291 | 292.9 |
| 303 | 305.8 | ||
| 571.0 | 571.3 | ||
| 648.3 | |||
| 8 | Allergic reaction | 995.3 | |
| 9 | Allergy treatment/desensitization | V07.1 | |
| V72.7 | |||
| 10 | Iron deficiency and other deficiency anemias | 280 | 281.9 |
| 11 | Arrhythmia | 427 | 427.9 |
| 785.0 | |||
| 12 | Asthma | 493 | 493.9 |
| 13 | Breast lump | 611.72 | |
| 14 | Burns | 940 | 949.9 |
| 15 | Bursitis, Synovitis, tenosynovitis | 726 | |
| 727.00 | 727.01 | ||
| 727.04 | 727.9 | ||
| 727.2 | 727.3 | ||
| 16 | Cataract, aphakia | 366 | 366.9 |
| 379.3x | |||
| 743.3x | |||
| 998.82 | |||
| V45.61 | |||
| 17 | Cerebral vascular disease/CVA | 430 | 438.9 |
| 18 | Chest pain | 786.5x | |
| 19 | Heart failure | 428 | 428.9x |
| 20 | Conjunctivitis, keratitis | 053.21 | |
| 054.42 | 054.43 | ||
| 077 | 077.9x | ||
| 130.1 | |||
| 370 | 370.9 | ||
| 372 | 372.3x | ||
| 21 | Contraception | v25.0 | v25.9 |
| 22 | COPD/chronic bronchitis | 491 | 492.9 |
| 494 | 494.9 | ||
| 496 | 496.9 | ||
| 23 | Deafness | 387 | 387.9 |
| 388.2 | |||
| 389 | 389.9 | ||
| 24 | Degenerative joint disease | 715 | 717.x |
| 25 | Depression, anxiety, neuroses (nonpsychotic) | 300.0 | |
| 300.4 | |||
| 300.5 | |||
| 306 | |||
| 308 | 309 | ||
| 311 | |||
| 313 | |||
| 799.2 | |||
| 26 | Dermatitis & eczema | 690 | 693.9 |
| 698.2 | 698.4 | ||
| 706.3 | |||
| 27 | Dermatophytosis | 110 | 111.9 |
| 28 | Diabetes mellitus | 250 | |
| 648.0 | |||
| 29 | Diaphragmatic hernia | 551.3 | |
| 552.3 | |||
| 553.3 | |||
| 30 | Disease of hair and hair follicles | 704 | 704.9 |
| 31 | Diverticular disease | 562 | |
| 32 | Thrombophlebitis, pulmonary embolism | 415.1 | |
| 451 | |||
| 453 | |||
| 673 | |||
| V12.51 | V12.52 | ||
| 33 | Impacted cerumen (wax in ear) | 380.4 | |
| 34 | Enlarged tonsils | 474 | |
| 35 | Fibrocystic breast disease | 610 | |
| 36 | Fibrositis & myalgia | 719.4 | 719.5 |
| 729.0 | 729.1 | ||
| 729.4 | 729.5 | ||
| 37 | Foreign body in eye | 930 | 930.9 |
| 360.5x | 360.6 | ||
| 38 | Fractures & dislocations | 800 | 839.9 |
| 39 | Ganglion | 727.4x | |
| 40 | Gall bladder and biliary tract diseases | 574 | 576.9 |
| 41 | Glaucoma | 365 | |
| 42 | Gout | 274 | |
| 43 | Headache | 339 | |
| 346 | |||
| 784 | |||
| 307.81 | |||
| 44 | Hematuria | 599.7x | |
| 45 | Helminthiasis, scabies, lice | 120 | 129.9 |
| 132 | 133.9 | ||
| 46 | Hemorrhoids/perirectal disease | 455 | 455.9 |
| 565 | 566.9 | ||
| 569 | 569.4 | ||
| 47 | Hepatitis/mononucleosis | 070 | |
| 075 | |||
| 573.3 | |||
| 48 | Hyperlipidemia | 272 | 272.4 |
| 49 | Hypertension | 401 | 405.9 |
| 437.2 | |||
| 796.2 | |||
| 50 | Infections of eyelid | 373 | 373.2 |
| 373.4 | 373.6 | ||
| 51 | Infertility | 606 | |
| 628 | |||
| v26.0 | v26.2 | ||
| v26.8 | v26.9 | ||
| 52 | Irritable bowel syndrome | 564.1 | |
| 564.5 | |||
| 53 | Ischemic heart disease | 410 | 414.9 |
| 429.7 | |||
| V45.81 | |||
| V45.82 | |||
| 54 | Keratoses | 702.0 | 702.1 |
| Lacerations/contusions | 530.7 | ||
| 618.7 | |||
| 620.6 | |||
| 622.3 | |||
| 623.4 | |||
| 624.4 | |||
| 664 | |||
| 665.3x | 665.4 | ||
| 800.1x | |||
| 800.6x | |||
| 801.1x | |||
| 801.6x | |||
| 803.1x | |||
| 803.6x | |||
| 804.1x | |||
| 804.6x | |||
| 851 | |||
| 861 | |||
| 865 | 866 | ||
| 870 | 887.x | ||
| 890 | |||
| 891 | 897.x | ||
| 900 | 904.x | ||
| 910 | 929.x | ||
| 950 | 957.x | ||
| 959.x | |||
| 998.2 | |||
| 56 | Low back pain | ||
| 720 | |||
| 721.3 | |||
| 721.42 | |||
| 722.10 | |||
| 722.52 | |||
| 724.02 | |||
| 724.2 | 724.3 | ||
| 724.6 | 724.7 | ||
| 57 | Lymphadenopathy | 785.6 | |
| 58 | Medical & surgical aftercare | V51.0 | V55 |
| V58.7 | V58.9 | ||
| V67.0 | V67.9 | ||
| 59 | Menopausal symptoms | 256.3x | |
| 627.2 | |||
| 627.4 | 627.9 | ||
| 60 | Menstrual disorders | 625.3 | 625.4 |
| 626 | 627.1 | ||
| 61 | Neoplasm, malignant, involving skin | 172 | 173.9 |
| 232 | 232.9 | ||
| 62 | Neoplasm, malignant, not involving skin | 140 | 165.9 |
| 170 | 171.9 | ||
| 174 | 176.x9 | ||
| 179 | 209.x | ||
| 230 | 231.9 | ||
| 233 | 234.9 | ||
| 63 | Neoplasm, benign | 210 | 229.9 |
| 235 | 239.9 | ||
| 64 | Non-fungal skin infections | 607.1 | 607.2 |
| 680 | 686.9 | ||
| 65 | Obesity | 278 | |
| 66 | Otitis externa | 380.1 | 380.2 |
| 67 | Otitis media | 381 | 381.4 |
| 382 | 382.9 | ||
| 384 | 384.1 | ||
| 388.7 | |||
| 385.1 | |||
| 68 | Parkinson’s disease | 332.x | |
| 69 | Peptic diseases | 530.1 | 530.2 |
| 531 | 535.9 | ||
| 530.81 | |||
| 70 | Peripheral neuropathy | 354 | 355.9 |
| 356.1 | 356.4 | ||
| 357 | 357.9 | ||
| 71 | Peripheral vascular disease | 440.2 | 440.4 |
| 443.x | |||
| 72 | Personality disorders | 301 | 301.9 |
| 73 | Pregnancy and abortion | 630.x | 633x |
| 634.x | 639.9 | ||
| 640 | 646.4 | ||
| 646.7 | 646.9 | ||
| 650 | 6666.x | ||
| 670 | 677.x | ||
| v22.0 | v24.9 | ||
| 74 | Prostatitis & benign prostatic hypertrophy | 600.0 | 601.9 |
| 75 | Psoriasis/pityriasis | 696 | 696.9 |
| 76 | Psychosocial problem | v60.0 | v62.9 |
| 77 | Refractive errors | 367.0 | 367.9 |
| 78 | Renal calculi | 592.0 | 592.9 |
| 79 | Respiratory tract infection, acute upper | 032.0 | 034.9 |
| 460 | 460.9 | ||
| 462 | 465.9 | ||
| 475 | 475.9 | ||
| 487.1 | 487.9 | ||
| 519.8 | |||
| 80 | Respiratory tract infection, acute lower | 466 | 466.9 |
| 480 | 488 | ||
| 490 | 490.9 | ||
| 81 | Rheumatoid diseases | 714 | 714.9 |
| 82 | Rhinitis, chronic | 472.0 | |
| 472.2 | |||
| 477 | 477.9 | ||
| 83 | Routine health maintenance | V01.0 | V07.0 |
| V07.2 | V07.9 | ||
| V20.0 | V21.9 | ||
| V28.0 | V28.9 | ||
| V30.0 | V37.9 | ||
| V39.0 | V39.9 | ||
| V65.5 | |||
| V70.0 | V72.6 | ||
| V72.8 | V82.9 | ||
| 84 | Schizophrenia & affective psychosis | 295 | 298.9 |
| 85 | Scoliosis/kyphosis | 737 | 737.9 |
| 86 | Seizure disorder | 345 | 345.9 |
| 780.3 | |||
| 779.0 | |||
| 87 | Sexually transmitted diseases | 054.1 | |
| 090 | 99.9 | ||
| 112.1 | 112.2 | ||
| 608 | |||
| 614 | 614.9.9 | ||
| 616.x | |||
| 88 | Sinusitis | 461 | 461.9 |
| 473 | 473.9 | ||
| 89 | Skin ulcer | 707 | 707.9 |
| 90 | Strabismus | 378 | 378.9 |
| 91 | Thyroid disease | 240 | 246.9 |
| 648.1 | |||
| 92 | Urethral stricture | 598 | 598.9 |
| 753.6 | |||
| 93 | Urinary tract infection | 590 | 590.9 |
| 595 | 595.9 | ||
| 599.0 | |||
| 646.5 | 646.6 | ||
| 771.82 | |||
| V13.02 | |||
| 94 | Urticaria | 708 | 708.9 |
| 995.1 | |||
| 95 | Uterine prolapse | 618.1 | 618.4 |
| 96 | Vaginitis | 112.1 | |
| 131.00 | 131.01 | ||
| 616.1 | |||
| 623.5 | |||
| 627.3 | |||
| 97 | Valvular heart disease | 391.1 | |
| 391.9 | |||
| 394 | 397.9 | ||
| 424 | 424.9 | ||
| 98 | Varicose veins | 454 | 454.9 |
| 99 | Vertiginous syndromes | 386 | 386.9 |
| 780.4 | |||
| 100 | Viral exanthem | 051 | 059.x |
| 74.3 | |||
| 101 | Warts | 78.1 | |
| 102 | Chronic kidney disease | 403 | 404.x |
| 581 | 582.x | ||
| 585 | 586 | ||
| V42.0 | |||
| V45.11 | V45.12 | ||
| V56.xx | |||
| 103 | Abdominal aortic aneurysm | 441.xx | |
All data were imported nightly from the practice EHR to a relational clinical data repository (CDR) located behind the firewall of each organization using proprietary software mapping tools developed by QED Clinical, Inc. d/b/a CINA. The CINA software used for ETL were tools that were already in place and being used by each organization to produce point of care clinical decision support reports and population management reports. The CDR, updated nightly, provided a near real-time source of standardized and codified data used in point-of-care clinical decision and the audit and feedback reports, as well as the periodic data extractions used for this report. CINA, having a Business Associate Agreement (BAA) already in place with each organization, served as our data transfer agent, providing us with the limited data sets required for the analyses reported here.
Data validation by CINA
Since this research was conducted on data extracted and translated from the EHR to a secondary CDR by our data transfer agent, CINA, it was imperative to understand and validate the data received through a multi-step process. Data validation was largely the responsibility of CINA as the ETL vendor in place at each organization prior to the initiation of this project. Because CINA provides software tools that utilize data from the CDR in the course of clinical care and decision-making, CINA has several processes in place to ensure the reliability and validity of the data that is contained within the CDR. Data reliability testing by CINA includes the following: 1) Patient-level sampling comparing the data imported into the CDR with the source data as it is represented in the EHR; 2) Daily use in clinical practice of the data in the CDR through the point-of-care clinical decision support tool and population management tools provided by CINA; and 3) Data reliability testing with each data extraction for research analysis.
Data validation exercises by the investigators
These data validation studies included: 1) An assessment data distribution for continuous variables to identify implausible or non-physiologic values, 2) Comparisons of distributions of continuous variables by organization to look for problems with units (i.e., English/metric), differing analytic methods, and mapping anomalies and 3) An examination of the distribution of categorical responses to look for clinically conflicting findings. These data validation studies were done independently of CINA, but the results were shared with CINA for wider data quality improvement.
We constructed tables of the distributions of each continuous variable that included the value, number and percent of each observation, and cumulative percentage; additionally we found that viewing graphs of these distributions as a group very useful. Using our clinical judgment and the proportions of values in the tails of the distributions, we excluded the following values from further analysis: systolic BP >260 mm Hg or <50, diastolic BP >200 or<0, height >90″ or <45, weight >500 lbs. or <50, creatinine >20 mg/dl or <0.2, total cholesterol >450 mg/dl or <50, LDL >300 or <10, and HDL-cholesterol >150 or <5. The proportion of values deleted varied from 0.005% for systolic BP to 0.5% for creatinine.
On comparing distributions of data by clinic, we discovered a few anomalies, one of which was due to one organization using a different cholesterol fractionation technique, and others probably due to mapping variances. These anomalies were corrected in most cases by examining the organization-specific field names. There is no way to check the accuracy of ICD-9 coding of comorbidities in the EHR, which enter into the calculation of guideline concordance and its risk-adjustment, short of manual chart review – a nearly impossible task for 232,172 patients; therefore we accepted the ICD9 coding without editing or verification.
A value for BP in the preceding two years was missing in only 2.2% (5,049/232,272) of patients; a value for LDL in the preceding five years was missing for in 41.1% (95,401/232,172); height and weight were missing in 9.0% and 2.7%, respectively. These missing values were not imputed, meaning that the sample sizes in the multivariable models were reduced (Appendix Tables 4 and 5).
Appendix Table 4.
Forward Logistic Regression Model of Patient-Level Factors with Blood Pressure Non-Control per JNC7 Guideline.
| Covariate | Parameter Estimate | p-value | Odds Ratio (95% CI) | Cumulative C-index |
|---|---|---|---|---|
| Intercept | −3.1521 | <.0001 | ||
| Hypertension | 1.7286 | <.0001 | 5.63 (5.46 – 5.81) | 0.735 |
| Diabetes mellitus | 1.5772 | <.0001 | 4.84 (4.64 – 5.06) | 0.764 |
| Body mass index (kg/m2) | ||||
| <18.5 (underweight) | −0.0544 | .45 | 0.95 (0.82 – 1.09) | 0.802 |
| 18.5 – 24.9 (normal) | Reference | |||
| 25.0 – 29.9 (overweight) | 0.4171 | <.0001 | 1.52 (1.46 – 1.58) | 0.802 |
| 30.0 – 34.9 (obesity, class I) | 0.7208 | <.0001 | 2.06 (1.97 – 2.14) | 0.802 |
| 35.0 – 39.9 (obesity, class II) | 0.9440 | <.0001 | 2.57 (2.44 – 2.71) | 0.802 |
| >40.0 (obesity, class III) | 1.1896 | <.0001 | 3.29 (3.09 – 3.50) | 0.802 |
| Number of Visits | ||||
| 1 – 2 | Reference | |||
| 3 – 4 | −0.2921 | <.0001 | 0.75 (0.72 – 0.77) | 0.806 |
| 5 – 6 | −0.3149 | <.0001 | 0.73 (0.69 – 0.77) | 0.806 |
| >6 | −0.3348 | <.0001 | 0.72 90.68 – 0.75) | 0.806 |
| Age (years) | ||||
| 18 – 40 | Reference | |||
| 41 – 60 | −0.3831 | <.0001 | 1.47 (1.42–1.52) | 0.812 |
| 61 – 80 | 0.6374 | <.0001 | 1.89 (1.81 – 1.98) | 0.812 |
| >80 | 0.9475 | <.0001 | 2.58 (2.38 – 2.79) | 0.812 |
| Male | 0.3835 | <.0001 | 1.47 (1.43 – 1.51) | 0.817 |
| Hyperlipidemia | −0.2812 | <.0001 | 0.75 (0.73 – 0.78) | 0.818 |
| Kidney disease, chronic | 0.8324 | <.0001 | 2.30 (2.09 – 2.53) | 0.819 |
| Ischemic heart disease | −0.4755 | <.0001 | 0.62 (0.58 – 0.67) | 0.820 |
| Prostatitis and BPH | −0.3367 | <.0001 | 0.71 (0.67 – 0.76) | 0.821 |
Number of observations read: 227,123
Number of observations used: 209,582
Appendix Table 5.
Forward Logistic Regression Model of Patient-Level Factors Associated with LDL-Cholesterol Non-Control per the NCEP Guideline.
| Covariate | Parameter Estimate | p-value | Odds Ratio (95% CI) | Cumulative C- Index |
|---|---|---|---|---|
| Intercept | −3.3493 | <.0001 | ||
| Hyperlipidemia | 0.9236 | <.0001 | 2.52 (2.43 – 2.61) | 0.654 |
| Diabetes mellitus | 0.8920 | <.0001 | 2.44 (2.33 – 2.56) | 0.682 |
| Body mass index (BMI) | ||||
| <18.5 (underweight) | 0.0269 | 0.78 | 1.03 (0.85 – 1.25) | 0.710 |
| 18.5 – 24.9 (normal) | Reference | |||
| 25.0 – 29.9 (overweight) | 0.4519 | <.0001 | 1.57 (1.50 – 1.65) | 0.710 |
| 30.0 – 34.9 (obesity, class I) | 0.6028 | <.0001 | 1.83 (1.73 – 1.92) | 0.710 |
| 35.0 – 39.9 (obesity, class II) | 0.6231 | <.0001 | 1.86 (1.75 – 1.99) | 0.710 |
| ≥40.0 (obesity, class III) | 0.6632 | <.0001 | 1.94 (1.79 – 2.10) | 0.710 |
| Age | ||||
| 18 – 40 | Reference | |||
| 41 – 60 | 0.5888 | <.0001 | 1.80 (1.72 – 1.89) | 0.719 |
| 61 – 80 | 0.4737 | <.0001 | 1.61 (1.52 – 1.70) | 0.719 |
| >80 | 0.5006 | <.0001 | 1.65 (1.48 – 1.83) | 0.719 |
| Cerebral vascular disease/CVA | 0.9993 | <.0001 | 2.72 (2.49 – 2.96) | 0.726 |
| Male | 0.2206 | <.0001 | 1.25 (1.20 – 1.29) | 0.731 |
| Number of visits/year | ||||
| 1 – 2 | Reference | |||
| 3 – 4 | −0.1678 | <.0001 | 0.85 (0.81 – 0.88) | 0.731 |
| 5 – 6 | −0.1625 | <.0001 | 0.85 (0.80 – 0.90) | 0.731 |
| >6 | −0.2212 | <.0001 | 0.80 (0.76 – 0.84) | 0.731 |
| Alcohol & drug abuse | 0.3537 | <.0001 | 1.42 (1.34 – 1.52) | 0.733 |
| Anemia | −0.3073 | <.0001 | 0.74 (0.68 – 0.79) | 0.733 |
| Hypertension | 0.1848 | <.0001 | 1.20 (1.16 – 1.25) | 0.734 |
Number of observations read: 136,771
Number of observations used: 131,589
Data security and privacy protection
Each of the ten participating organizations signed a data use agreement allowing the use of their data; this agreement specifies the data elements used and that HIPAA identifiers, with the exception of dates of service, were deleted prior to transfer to a secure server within the Department of Family Medicine, University of Colorado School of Medicine.
IRB
The protocol for the CRRLC, a waiver of informed consent, and a waiver of HIPAA authorization have been approved by the Colorado Multiple Institutional Review Board (COMIRB) and an IRB sponsored by the American Academy of Family Physicians representing all participating clinics.
Statistical analyses
We used previous research and our clinical judgment to select the 31 listed variables listed in Table 1 to describe the cohort and to develop risk adjustment models using stepwise logistic regression with BP or LDL non-control as the dependent variables. The cumulative c-index was computed after each step.
Table 1.
Population Characteristics for All 232,172 Patients.
| Selected Patient Characteristics and Risk-Adjustment Variables | PREVALENCE | |
|---|---|---|
| Number | Percent | |
| Adverse drug effect | 1,134 | 0.49 |
| Age (years) | ||
| 18 – 40 | 95,639 | 41.2 |
| 41 – 60 | 94,978 | 40.9 |
| 61 – 80 | 35,971 | 15.5 |
| >80 | 5,584 | 2.4 |
| Alcohol or drug abuse | 11,326 | 4.9 |
| Anemia | 10,442 | 4.5 |
| Body mass index (kg/m2) | ||
| <18.5 (underweight) | 3,557 | 1.5 |
| 18.5 – 24.9 (normal) | 67,834 | 29.2 |
| 25.0 – 29.9 (overweight) | 73,852 | 31.8 |
| 30.0 – 34.9 (Obesity, Class I) | 39,744 | 17.1 |
| 35.0 – 39.9 (Obesity, Class II) | 16,041 | 6.9 |
| ≥40.0 (Obesity, Class III) | 9,967 | 4.3 |
| Missing | 21,177 | 9.1 |
| Cataract/aphakia | 4,081 | 1.8 |
| Cerebral vascular disease or CVA | 3,612 | 1.6 |
| Congestive heart failure | 1,518 | 0.7 |
| Depression or anxiety | 50,822 | 21.9 |
| Diabetes mellitus | 14,804 | 6.4 |
| Ischemic heart disease | 6,007 | 2.6 |
| Hepatitis or mononucleosis | 2,356 | 1.0 |
| Hyperlipidemia | 65,343 | 28.1 |
| Hypertension | 58,849 | 25.3 |
| Kidney disease, chronic | 2,667 | 1.1 |
| Male gender | 101,184 | 43.6 |
| Medical or surgical aftercare | 12,871 | 5.5 |
| Neoplasm, benign | 26,926 | 11.6 |
| Neoplasm, malignant | 3,308 | 1.4 |
| Obesity | 16,089 | 6.9 |
| Peripheral vascular disease | 1,835 | 0.8 |
| Personality disorders | 192 | 0.1 |
| Pulmonary disease, chronic obstructive | 3,709 | 1.6 |
| Prostatitis/BPH | 7,821 | 3.4 |
| Psychosocial problem | 1,463 | 0.6 |
| Respiratory tract infection, acute lower | 22,820 | 9.8 |
| Respiratory tract infection, acute upper | 47,984 | 20.7 |
| Rhinitis, chronic | 49,977 | 21.5 |
| Routine health maintenance | 126,419 | 54.5 |
| Schizophrenia or affective psychosis | 5,660 | 2.4 |
| Visits (number per year) | ||
| 1 – 2 | 141,393 | 60.9 |
| 3 – 4 | 47,948 | 20.7 |
| 5 – 6 | 17,764 | 7.7 |
| >6 | 25,067 | 10.8 |
We calculated the risk of each patient for BP or LDL non-control using the parameter estimates from each model and summed this expected risk by clinic (E), which was compared with the observed number non-control patients (O) in each clinic as the O/E ratio. For ease of clinical interpretation we converted the O/E ratio to a risk-adjusted percent non-control by multiplying each clinic’s O/E ratio by the observed mean rate of non-control for all patients across all clinics. We calculated an achievable benchmark of care patterned after the work of Kiefe and colleagues 18–21, except clinics were rank-ordered by their risk-adjusted non-control rates. Our achievable benchmark is the weighted average non-control rate for the top-ranked clinics providing care for approximately 10% of all patients.
RESULTS
Participants
Table 1 shows the population characteristics. The mean (S.D.) age was 45.6 (15.7) years; body mass index (BMI) 27.7 (6.2) kg/M2, and number of visits within two years 3.4 (5.0).
Unadjusted BP non-control (Figure 1)
There was no BP measurement within the preceding two years in 2.2% (5,049/232,172) of patients. Overall, 16.0% (36,418/227,123) of patients with measured BPs had uncontrolled BP. For patients without diabetes or CKD, 12.8% (26,948/210,783) had uncontrolled BP (≥140/90). For patients with diabetes or CKD, 58.0% (9,470/16,340) had uncontrolled BP (≥130/80).
Risk-adjustment of BP non-control
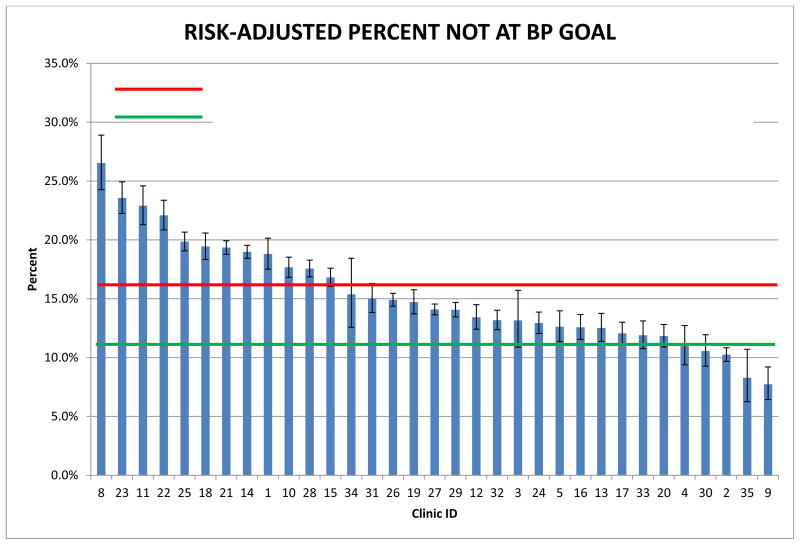
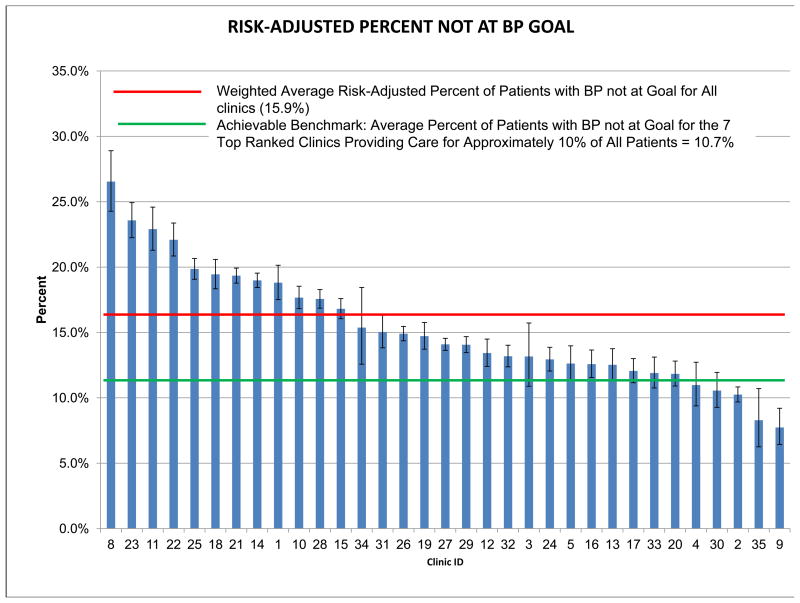
The multivariable model of patient-level variables associated with BP non-control is shown in Appendix Table 4. The c-index for the full model was 0.822, while the c-index for the first ten variables entering the model, which were used in the risk adjustment of BP non-control, was 0.821. Appendix Figure 1 shows the risk-adjusted percent non-control by clinic, which varied from a high of 26.5% to a low of 7.7% with a weighted average across all clinics of 15.9%. The achievable benchmark was 10.7% non-control. Twenty-eight (85%) of the 33 clinics had non-control rates with 95% confidence intervals higher than this benchmark.
Appendix Figure 1.
Risk-Adjusted Percent of Patients with Blood Pressure Not Controlled by Clinic.
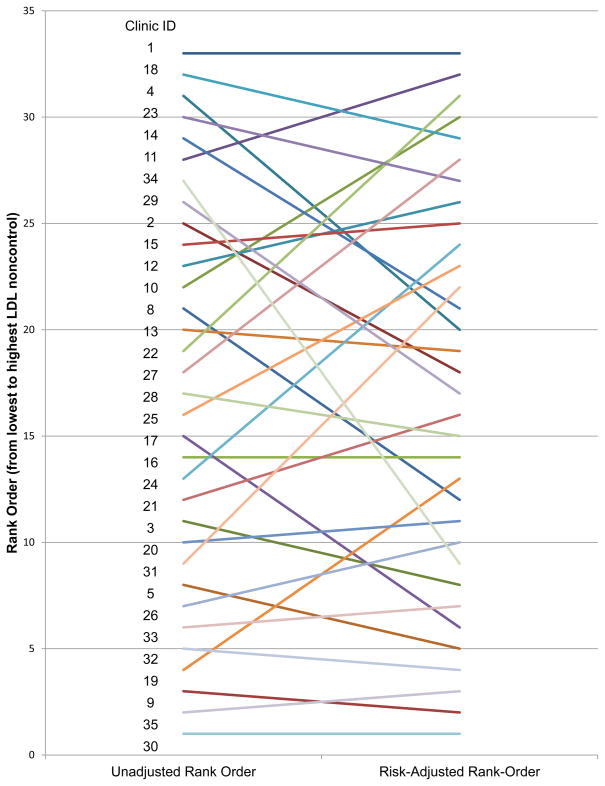
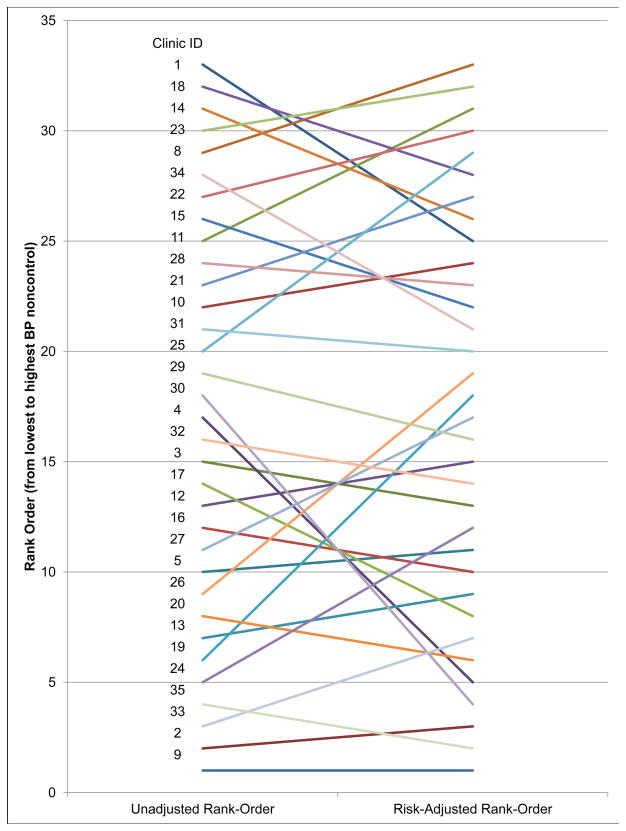
Figure 4 shows the considerable differences the rank-order of clinics by the unadjusted percent BP non-control rate versus the rank-order of the risk-adjusted non-control rate, with four clinics changing rank-order by ≥10 places, 8 clinics changing rank-order between 5 and 9 places, and 21 clinics changing rank-order ≤4 places.
Figure 4.
Comparison of Rank-Order of Clinics by Unadjusted and Risk-Adjusted Blood Pressure Control.
Unadjusted LDL non-control (Figure 4)
LDL measurements within the preceding 5 years, the maximum interval between measurements recommended by the NCEP14, were not retrievable electronically from the EHR for 41.1% of patients. Overall, 14.9% (20,391/136,771) of patients with measurements had uncontrolled LDL. The degree of LDL non-control varied markedly with patient risk from 36.1% for highest risk patients with CHD or CHD equivalent, to 28.3% for intermediate risk patients with no CHD or CHD equivalent but two or more risk factors, to 6.8% for low risk patients.
Risk-adjustment of LDL non-control
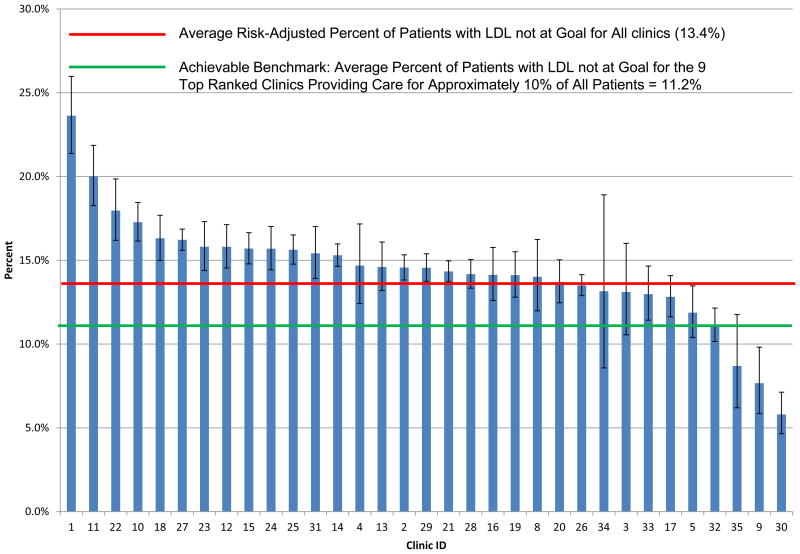
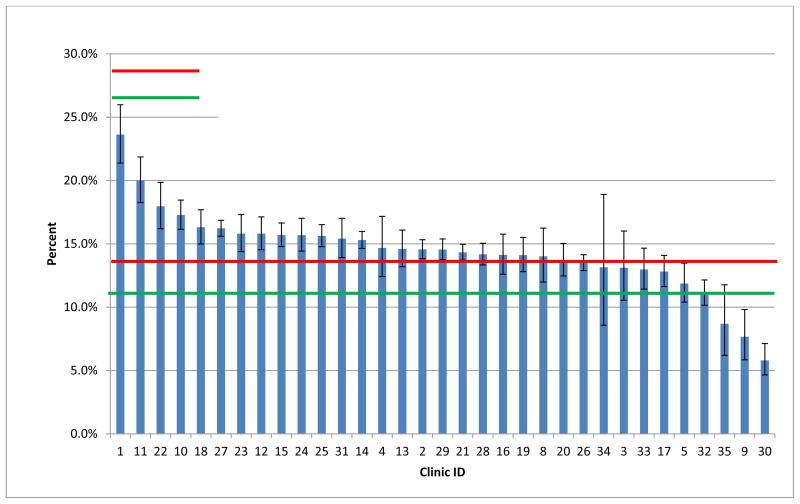
Variables predictive of LDL non-control from a logistic regression model are shown in Appendix Table 5. The c-index for the full model was 0.737; the cumulative c-index for the first ten variables used for risk-adjustment is 0.734. Figure 5 shows the risk-adjusted percent LDL non-control by clinic, which varied from 5.8% to 23.6%. The mean non-control for all clinics was 13.4%, while the three best performing clinics set the benchmark at 11.2%; 26 of the 33 (79%) clinics had non-control rates with 95% confidence intervals higher than this benchmark.
Figure 5.
Risk-Adjusted Percent of Patients with LDL-Cholesterol Not Controlled by Clinic.
Again, there were marked differences in clinic rank-order based on the non-adjusted non-control rate from that based on the risk-adjusted non-control rate (Figure 4). Six clinics experienced a change in rank order of more than ≥10 places, while 8 clinics changed rank-order between 5 and 10 places, and 19 changed rank order ≤4 places. This is due to differences in the distribution of variables predictive of BP and LDL control by clinic (Appendix Tables 4 and 5). Failure to risk-adjust could lead clinics to attribute high non-control rates to the often non-mutable characteristics of their patients (e.g., age, gender, a diagnosis of diabetes, etc.) and preclude making changes in processes or structures of care.
DISCUSSION
Key Results
While the EHRs of only 2.2% of patients were missing all BP values within the two previous years, 41.1% were missing LDL values within the previous five years. Of patients with known values, 16.0% and 14.9% failed to meet guideline recommendations for BP control and LDL control, respectively; however, a large majority of clinics had non-control rates in excess of that achieved by the best performing clinics, indicating substantial room for improvement. Ranking of clinics by risk-adjusted non-control rates was markedly different from that by unadjusted non-control rates, indicating the importance of risk adjustment.
Strengths and Limitations
The strengths of this study include the large primary care patient population, the inclusion of all patients within a clinic ≥18 years of age and with ≥1 clinic visit within preceding two years, inexpensive electronic data collection, risk-adjustment of the clinic-level outcomes of BP and LDL non-control, and the electronic assessment of patient-level non-control per JNC7 and NCEP.
Limitations include 1) Limited clinic-level data precludes comparison of characteristics of high and low outlier clinics; 2) The very incomplete data on race/ethnic status; 3) A large proportion of patients (41.1%) had no LDL measurement within the preceding five years retrievable as a discrete data field from the EHR; 4) The participating clinics are not representative of the full range of U.S. ambulatory care; 5) Some providers question the validity of EHR data; and 6) The absence of data on MACE.
Missing LDL data
The reliability of our assessment of LDL control must be interpreted in the light that 41.1% of patients had no LDL value available in a discrete EHR field in the preceding five years. We recognize that an LDL value measured at another health care organization may have been recorded as a text note, but we made no attempt to retrieve data from text notes. More importantly, numeric data buried in a past text note is also difficult for the care provider or organization to retrieve. A companion paper currently under review will report on timeliness of measurement of BP and LDL
Representativeness of patient population
The health care organizations included in this study are not a representative sample of U.S. ambulatory care in the sense that there is no representation of other major models of ambulatory care delivery, such as private integrated systems like Kaiser, government integrated systems like the VA, and community health centers providing care for the large underserved segment of our population. Although, we do not have the data, we believe that DARTNet clinics are at least somewhat representative of private, non-integrated, fee-for services clinics. The 33 clinics in the present study include urban, academic-affiliated clinics; suburban and rural clinics; and vary in size from a single physician supported by one or two paraprofessionals to group practices of 30 or more primary care physicians in multiple suburban locations.
Doubts about validity of EHR data
It is our view that the EHR will play an increasingly critical role in both the delivery of health care and the assessment of that delivery. The EHR has an enormous advantage over the paper record in cost-effectively aggregating data on large groups of patients. In a recent supplement to Medical Care on Electronic Data Methods, Randhawa from the Center for Outcomes and Evidence, Agency for Healthcare Research and Quality, expressed this view more cogently: “The challenge of addressing complex questions, such as what affects patient outcomes in a real-world clinical setting, demands a scalable electronic infrastructure that can provide high-quality, clinically rich, prospective, multi-site data for generating internally valid and generalizable conclusions in a timely and efficient manner.”22 The present paper comes from the Distributed Ambulatory Care Research in Therapeutics Network (DARTNet) initially funded by AHRQ to respond to the challenge posed by Randhawa.
We delivered electronically to the point of care patient-specific clinical decision support, which consisted of graphical displays of BP, LDL, and all antihypertensive, and antihyperlipidemic prescriptions over time. Additionally, audit and feedback of aggregate clinical data similar to that shown in Figures 1, 2, 3, and 5 were provided to all care providers on two occasions. While our care provider surveys showed only a minority to have regularly used these reports, we received virtually no expressions of concern regarding the validity of these data. Before instituting the clinical decision support, these reports were reviewed and approved by the Steering Committee consisting of four care providers from participating clinics and two academically based physicians. Unfortunately, our time series assessment of guideline concordance showed little change, which we now attribute to our failure to adequately engage the care providers. We are planning to report those data in a separate manuscript.
Figure 3.
Risk-Adjusted Percent of Patients with Blood Pressure Not Controlled by Clinic.
Data quality
It is common practice to perform extensive validation of data manually abstracted from the paper medical record for clinical research. Validation methods include: 1) Cross checking important concepts against several sources of data, 2) Checking for illogical data combinations (e.g., pregnancy in a male), 3) Assessing the accuracy of diagnostic coding by comparing the narrative record against standardized definitions, 4) Conducting inter- and intra-observer variability assessments, and 5) Excluding unreasonable values in distributions of continuous data. We did only the last, because 1) through 4) are not routinely performed when working with EHR data, since the data as it exists in the EHR is the same data that is being used for clinical decision making; therefore, the practice and provider have a medical / legal obligation for accuracy. Also, laborious and expensive data validation negates an important advantage of EHR data, the ability to collect and analyze data on large numbers of patients inexpensively and quickly. In the context of the present study, the ultimate of data validation should come in the form of credibility of the results to care providers and improvement of patient outcomes. Finally, in a literature search we were unable to find publications of validation of ambulatory care EHR data against source data.
The use of electronic data collection to assess guideline concordance
We have demonstrated the ability to assess guideline concordance using electronic data collection in 232,172 patients in 33 clinics comprising 10 private, fee-for-services, health care organizations with disparate EHRs. Despite daily feed-back of patient-specific clinical decision support and two cycles of audit and feedback, no credibility issues have been raised by participants in this study.
The costs of data collection and management per patient over two years of $2.98 and $4.31 based on the grant’s direct and combined direct and indirect costs, respectively, are not intended as a formal cost analysis, but as an estimate only. The ultimate value of electronically supported interventions to reduce MACE must compare the costs of delivery of the intervention to the cost savings from reduced MACE.
BP and LDL non-control rates
The rates of BP and LDL non-control in this study are better than those previously reported. Gillespie, reporting on NHANES data from 2005 – 2008 on 10,037 adults aged ≥18 years, found that 20.3% (2,108/10,037) had uncontrolled hypertension defined as BP ≥140/906. We found 16.0% to have uncontrolled BP using the JNC7 definition (<130/80 for patients with diabetes or chronic kidney disease, <140/90 otherwise); if we applied the NHANES definition, the non-control rate was 13.5%. There are several possible explanations for the lower non-control rates in our study: 1) CRRLC patients are being seen in fee-for-service clinics, meaning that they have a primary care provider and are likely of higher socio-economic status in contrast to the NHANES sample specifically designed to be representative of the U.S population; 2) Similarly, the racial-ethnic distribution in our population may also be different in a direction favoring better BP control than that of NHANES; and 3) BP control may have improved from the time of NHANES data collection (2005 – 2008) to that of this report (2009 – 2010).
Also reporting on NHANES data from 2005 – 2008 and using the same NCEP criteria as we used, Kuklina, et al., found that 21.2% had uncontrolled LDL23, compared to the 14.9% we found. In addition to the caveats listed for hypertension above, 41.1% of our overall population did not have a LDL measurement within five years as recommended by NCEP and were excluded; this could lead to a large bias in our results.
Risk-adjustment of adverse postoperative outcomes as a quality measure in surgery has become common since its introduction more than two decades ago24–29. Risk-adjusted outcomes as a measure of quality in surgery have been validated against data from site visits29;30, and are now widely accepted in surgical care. Processes of care (e.g., prescribing a statin for patients with CHD), surrogate outcomes (e.g., BP and LDL measures), and true outcomes (e.g., mortality) are being used increasingly to assess the quality of non-surgical care. While mortality is often adjusted for patient risk, we have been unable to find published reports in which comparisons of guideline concordance between providers have been adjusted for patient factors associated with concordance. Our multivariable models show that comorbidity has important effects on both BP and LDL control. Clinics with a disproportionate number of these patients may be unfairly ranked higher by unadjusted non-control rates, as these risk factors are relatively immutable.
Generalizability
This study should be generalizable to fee-for-service primary care clinics using EHRs. Care should be taken in applying these results to primary care in other settings, such as integrated healthcare systems or federally qualified health clinics providing care to the underserved.
Clinical and research implications
Although the BP and LDL non-control rates in this study appear to be better than those in reports based on the most recent NHANES data6;23, this is not a reason for complacency. The 16.0% of primary care patients with uncontrolled BP and 14.9% with uncontrolled LDL represent substantial opportunities to reduce the morbidity, mortality, and the costs of care due to MACE. This reduction in mortality, morbidity and costs of care needs to be demonstrated in a large scale randomized trial; achieving the large sample size needed (~600,000 patients) will require electronically facilitated data collection and interventions, as we have demonstrated here.
Figure 6.
Comparison of Rank-Order of Clinics by Unadjusted and Risk-Adjusted LDL-Cholesterol Control.
Appendix Figure 2.
Risk-Adjusted Percent of Patients with LDL-Cholesterol Not Controlled by Clinic.
Acknowledgments
This work was supported by The National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) Grant 1RC1HL101071-01.
CRRLC Steering Committee: Edward Bujold, MD, Family Medical Center, Granite Falls NC; Cynthia Croy, MD, Family Health Center of Joplin, Joplin, MO; Michael Ho, MD, PhD, Denver VA Medical Center and University of Colorado School of Medicine, Denver, CO; Winston Liaw, MD, Fairfax Family Practice, Fairfax, VA; Jamie Reedy, MD, Westfield Family Practice at Summit Medical Group, Westfield, NJ; Stephen Ross, MD, University of Colorado School of Medicine, Aurora, CO.
Footnotes
Conflict of interest: Dr. Pace has a COI statement on file; other authors report no COI.
Reference List
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement From the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 4.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie C, Kuklina EV, Briss PA, Blair NA, Hong Y. Vital Signs: Prevalence, Treatment, and Control of Hypertension -- United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1–60. [PubMed] [Google Scholar]
- 7.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 8.Fryar CD, Hirsch R, Eberhardt MS, Yoon SS, Wright JD. Hypertension, high serum total cholesterol, and diabetes: racial and ethnic prevalence differences in U.S. adults, 1999–2006. NCHS Data Brief. 2010:1–8. [PubMed] [Google Scholar]
- 9.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–372. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby AM, Pace W, Bryan C, et al. Comparative effectiveness research in DARTNet primary care practices: point of care data collection on hypoglycemia and over-the-counter and herbal use among patients diagnosed with diabetes. Med Care. 2010;48:S39–S44. doi: 10.1097/MLR.0b013e3181ddc7b0. [DOI] [PubMed] [Google Scholar]
- 11.Pace WD, Cifuentes M, Valuck RJ, Staton EW, Brandt EC, West DR. An electronic practice-based network for observational comparative effectiveness research. Ann Intern Med. 2009;151:338–340. doi: 10.7326/0003-4819-151-5-200909010-00140. [DOI] [PubMed] [Google Scholar]
- 12.Pace WD, West DR, Valuck RJ, Cifuentes M, Staton EW. Effective Health Care Research Reports. Rockville, MD: Agency for Healthcare Research and Quality; Jul 28, 2009. Distributed Ambulatory Research in Therapeutics Network (DARTNet): Summary Report (Prepared by University of Colorado DEcIDE Center under Contract No. HHSA29020050037I TO2.) p. 14. [Google Scholar]
- 13.Chobanian AV. NIH Publication No. 04-5230. National Heart, Lung, and Blood Institute; 2004. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: Complete Report. Ref Type: Report. [DOI] [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Tierney WM, Overhage JM, Takesue BY, et al. Computerizing guidelines to improve care and patient outcomes: the example of heart failure. J Am Med Inform Assoc. 1995;2:316–322. doi: 10.1136/jamia.1995.96073834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneeweiss R, Rosenblatt RA, Cherkin DC, Kirkwood CR, Hart G. Diagnosis clusters: a new tool for analyzing the content of ambulatory medical care. Med Care. 1983;21:105–122. doi: 10.1097/00005650-198301000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Pace WD, Dickinson LM, Staton EW. Seasonal variation in diagnoses and visits to family physicians. Ann Fam Med. 2004;2:411–417. doi: 10.1370/afm.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiefe C, Woolley TW, Allison JJ, Box JB, Craig AS. Determining Benchmarks: A Data-Driven Search for the Best Achievable Performance. Clin Perfor Qual Health Care. 1994;2:190–194. [Google Scholar]
- 19.Kiefe CI, Weissman NW, Allison JJ, Farmer R, Weaver M, Williams OD. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care. 1998;10:443–447. doi: 10.1093/intqhc/10.5.443. [DOI] [PubMed] [Google Scholar]
- 20.Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. J Am Med Asso. 2001;285:2871–2879. doi: 10.1001/jama.285.22.2871. [DOI] [PubMed] [Google Scholar]
- 21.Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract. 1999;5:269–281. doi: 10.1046/j.1365-2753.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 22.Randhawa GS, Slutsky JR. Building sustainable multi-functional prospective electronic clinical data systems. Med Care. 2012;50 (Suppl):S3–S6. doi: 10.1097/MLR.0b013e3182588ed1. [DOI] [PubMed] [Google Scholar]
- 23.Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol--United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:109–114. [PubMed] [Google Scholar]
- 24.Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50:12–26. doi: 10.1016/0003-4975(90)90073-f. [DOI] [PubMed] [Google Scholar]
- 25.Marshall G, Grover FL, Henderson WG, Hammermeister KE. Assessment of predictive models for binary outcomes: an empirical approach using operative death from cardiac surgery. Stat Med. 1994;13:1501–1511. doi: 10.1002/sim.4780131502. [DOI] [PubMed] [Google Scholar]
- 26.Grover FL, Johnson RR, Shroyer AL, Marshall G, Hammermeister KE. The Veterans Affairs Continuous Improvement in Cardiac Surgery Study. Ann Thorac Surg. 1994;58:1845–1851. doi: 10.1016/0003-4975(94)91725-6. [DOI] [PubMed] [Google Scholar]
- 27.Hammermeister KE, Johnson R, Marshall G, Grover FL. Continuous assessment and improvement in quality of care. A model from the Department of Veterans Affairs Cardiac Surgery. Ann Surg. 1994;219:281–290. doi: 10.1097/00000658-199403000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:328–340. [PubMed] [Google Scholar]
- 29.Khuri SF, Daley J, Henderson W, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- 30.Daley J, Forbes MG, Young GJ, et al. Validating risk-adjusted surgical outcomes: site visit assessment of process and structure. National VA Surgical Risk Study. J Am Coll Surg. 1997;185:341–351. [PubMed] [Google Scholar]