Abstract
Significance: Mitochondria are structurally and biochemically diverse, even within a single type of cell. Protein complexes localized to the inner mitochondrial membrane synthesize ATP by coupling electron transport and oxidative phosphorylation. The organelles produce reactive oxygen species (ROS) from mitochondrial oxygen and ROS can, in turn, alter the function and expression of proteins used for aerobic respiration by post-translational and transcriptional regulation. Recent Advances: New interest is emerging not only into the roles of mitochondria in disease development and progression but also as a target for environmental toxicants. Critical Issues: Dysregulation of respiration has been linked to cell death and is a major contributor to acute neuronal trauma, peripheral diseases, as well as chronic neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease. Future Directions: Here, we discuss the mechanisms underlying the sensitivity of the mitochondrial respiratory complexes to redox modulation, as well as examine the effects of environmental contaminants that have well-characterized mitochondrial toxicity. The contaminants discussed in this review are some of the most prevalent and potent environmental contaminants that have been linked to neurological dysfunction, altered cellular respiration, and oxidation. Antioxid. Redox Signal. 23, 578–595.
Introduction
Mitochondria have increasingly emerged as a primary or secondary site of action for xenobiotics (defined as any foreign substance, see terminology Table 1). Mitochondria are responsible for a multitude of functions in the cell. In addition to producing ATP, these organelles are critical regulators of the synthesis and assembly of steroids, pyrimidines, heme, and iron-sulfur (Fe-S) clusters (102, 134). They also serve critical functions in homeostatic regulation of calcium, copper, manganese, and iron (6, 48, 133, 140). Finally, mitochondria are sources of reactive oxygen species (ROS) that can at one end of the spectrum be cytotoxic, and, at the other, serve as discreet signaling molecules (73). While this review focuses on effects of environmental agents on ATP production and ROS, xenobiotic exposure can also result in recruitment of pro-death molecules, release of proteins and chemicals that activate apoptosis or necrosis, as well as changes in the composition of the protein and lipid environment on the mitochondrial membrane that promote the engulfment of mitochondria by mitophagy. A review of the selective vulnerability of neuronal mitophagic signaling molecules to redox regulation was recently published (103).
Table 1.
Common Terminology of Environmental Exposures
| Term | Definition |
|---|---|
| Body burden | The amount of natural and man-made toxins that accumulate by contact, inhalation, and ingestion over the lifetime of a person |
| Carcinogen | Cancer-causing agent |
| Developmental/reproductive toxicant | Chemicals that damage developing fetus, child, or reproductive system |
| Endocrine disrupter | Chemicals that specially alter normal hormone function |
| Mutagen | Chemical that can cause mutations in DNA |
| Neurotoxin | Chemicals that specifically damage neurons within the central or peripheral nervous system |
| Teratogen | Chemicals that cause birth defects |
| Toxin | A naturally occurring poison |
| Toxicant | A man-made poison |
| Xenobiotic | Any foreign chemical or compound in the body |
Biomarkers of exposure, usually blood or urine levels of toxins or metabolites, reveal the extent to which geographical populations are exposed to a given toxicant. The US Center for Disease Control's Fourth National Report on Human Exposure to Environmental Chemicals examined more than 200 different environmental contaminants that were carcinogens, mutagens, teratogens, as well as reproductive toxins in a sampling of 2500 participants (137). Chemicals were selected based on prevalence of exposure to US populations, known or suspected health effects from exposure, and seriousness of the perceived threat based on scientific reports. Many of the compounds examined have historically been shown to be toxic to humans, such as cigarette smoke (CS) lead and methylmercury (MeHg). Table 2 displays the Agency for Toxic Substances and Disease's top 20 priority toxins that are determined to pose the most significant threat to human health. There remains, however, a dearth of information on the toxicity of many of the chemicals that US populations are exposed to daily.
Table 2.
The Agency for Toxic Substances and Disease Registry's 2013 Top 20 Priority Toxinsa
| Rank | Compound | CASRN |
|---|---|---|
| 1 | Arsenic | 007440-38-2 |
| 2 | Lead | 007439-92-1 |
| 3 | Mercury | 007439-97-6 |
| 4 | Vinyl chloride | 000075-01-4 |
| 5 | Polycholrinated biphenols | 001336-36-3 |
| 6 | Benzene | 000071-43-2 |
| 7 | Cadmium | 007440-43-9 |
| 8 | Benzo(a)pyrene | 000050-32-8 |
| 9 | Polycyclic aromatic hydrocarbons | 130498-29-2 |
| 10 | Benzo(b)fluoranthene | 000205-99-2 |
| 11 | Chloroform | 000067-66-3 |
| 12 | Aroclor 1260 | 011096-82-5 |
| 13 | DDT and P,P′-DDT | 000050-29-3 |
| 14 | Aroclor 1254 | 011097-69-1 |
| 15 | Dibenzo(a,h)anthracene | 000053-70-3 |
| 16 | Trichloroethylene | 000079-01-6 |
| 17 | Chromoium, hexavalent | 018540-29-9 |
| 18 | Dieldrin | 000060-57-1 |
| 19 | Phosphorus, white | 007723-14-0 |
| 20 | Hexachlorobutadiene | 000087-68-3 |
Adapted from www.atsdr.cdc.gov/spl/.
Given that environmental contaminants contribute to the development and progression of a wide range of diseases, from neurodegeneration, diabetes, obesity, cardiovascular disease, and lung diseases (28, 33, 93, 154, 156), it is essential that we identify xenobiotics and their public health risks in a more proactive manner. In this review, we examine the mitochondrial toxicity of three established classes of toxicants, CS, pesticides, and heavy metals, and identify the mechanisms that contribute to their toxicity. If environmental toxins alter mitochondrial respiration, oxidation, or function, identification of common themes or site of action of these diverse compounds could present new ways to rapidly assess risk potential.
Protein components of mitochondrial respiration
The mitochondria have a dual membrane system, where the mitochondrial matrix is surrounded by the inner membrane (IM) and the outer membrane (OM) is separated from the IM by the intermembrane space (IMS). The IM and OM differ in their membrane lipid and protein composition, and a complex of single and dual membrane-spanning proteins and transporters are critical regulators of mitochondrial function, dynamics, mitophagy, and apoptosis.
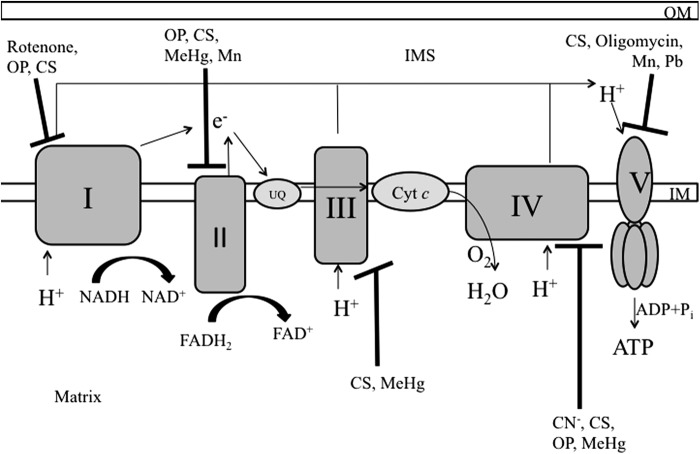
As a testament to the capacity of these lipids and proteins to form unique microenviroments within a single organelle, the mitochondrial matrix and the IMS are biochemically unique environments and the matrix is a far more reducing environment. The matrix contains the mitochondrial DNA, ribosomes, and enzymes involved in the Krebs cycle. In contrast, the IMS is more oxidizing and contains the Mia40-Erv1 pathway responsible for protein folding and complex assembly required for the respiratory complex function (181). The IM projections have finger-like structures, termed cristae, which contain the major respiratory complexes responsible for oxidative phosphorylation (OXPHOS) (Fig. 1).
FIG. 1.
Oxidative phosphorylation and xenobiotic sites of inhibition. Flow of electrons through the ETC and pumping out of protons leading to the formation of H2O and ATP are shown. Inhibition sites of the mitochondrial complexes by toxicants are represented by black blunted arrows. I, complex I; II, complex II; III, complex III; IV, complex IV; V, complex V; CN−, cyanide; Cyt c, cytochrome c; ETC, electron transport chain; UQ, ubiquinone.
OXPHOS and electron transport are coupled in a multistep process to create ATP. Complex I (NADH-coenzyme Q oxidoreductase) obtains electrons from NADH, while protons are expulsed from the matrix into the cristae lumen. Complex I is the largest of the respiratory complexes, and the presence of a flavin mononucleotide prosthetic group and eight Fe-S clusters are critical for complex I function. Electrons can enter the OXPHOS pathway via succinate, the substrate for complex II (succinate dehydrogenase). Both complex I and complex II pass their electrons to coenzyme Q (ubiquinone), a lipid-soluble carrier molecule that cycles between a fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and a fully reduced (ubiquinol) state. Complex III (coenzyme Q-cytochrome c oxidoreductase) accepts the electrons from ubiquinol, passing them to cytochrome c, a small lipid-soluble carrier hemeprotein. Complex III contains both heme and Fe-S clusters, which are used in removing the electrons from ubiquinol and transferring them to cytochrome c. From cytochrome c, electrons move down the redox gradient to complex IV (cytochrome c oxidase). Complex IV contains two hemes and two copper centers and transfers four electrons to one oxygen molecule to produce two molecules of water. At both the complex III and complex IV steps, additional protons are pumped from the matrix into the cristae lumen by the fall in electron potential energy as the electrons move down the electron transport chain (ETC). This creates a proton-motive force used to drive transport across the IM as well as power complex V (ATP synthase) to regenerate ATP from ADP+ and Pi.
While typically described as a step-by-step process, the organization and stochiometery of the respiratory components is not actually clear. Recently, data from Blue-Native gel electrophoresis have demonstrated interactions between the complexes to form respiratory supercomplexes or respirasomes (47, 158, 195). A variety of supercomplexes have been purified, consisting of complex I+complex III, complex III+complex IV, complex II+complex IV, or complex I+complex III+complex IV (55), giving rise to a “plastic” model for the organization. The presence of the freely diffusible ETC components should enable the production of ATP under conditions where supercomplexes are formed as well as when their formation is unfavorable. The conditions that promote supercomplex dissociation and the effects of having smaller versus larger complexes on net ATP synthesis and mitochondrial function and integrity remain to be determined but could account for the heterogenous response of mitochondria within a given cell exposed to a single exogenous stress.
Organizing the respiratory complexes into respirasomes confers several advantages, one of which is substrate channeling. Althoff et al. have reported a three-dimensional (3D) model of the I1III2IV1 respirasome, describing how one complex III monomer faces the lipid bilayer while the other is surrounded by complex I, creating a pathway for ubiquinone and cytochrome c to travel and shuttle electrons (2). Complex IV in the I1III2IV1 respirasome not only functions as the cytochrome c oxidase but also stabilizes and enhances the activity of both the complex I and complex III, as supercomplex I1III2 has less catalytic activity than I1III2IV1 (157). In addition, formation of supercomplexes has been shown to limit the production of ROS and to increase the stability of complex I (55).
Redox regulation of respiration
Oxygen is the final acceptor of electrons from the ETC to produce water; however, a small fraction of electrons combines with oxygen to form the superoxide anion, which can give rise to other ROS and react with nitric oxide (NO) to from reactive nitrogen species. Both superoxide anion and H2O2 can create hydroxyl radicals by the Haber–Weiss and Fenton reactions. Similarly, superoxide anion can combine with NO to form peroxynitrite (ONOO−). ROS production can account for between 0.25% and 11% of the oxygen consumed by mitochondria (4).
Superoxide and the lipid peroxidation byproducts of the radical are potent activators of proton conductance by mitochondrial uncoupling proteins, autophagic engulfment, and signaling molecules with essential roles in differentiation, adhesion, migration, and survival. The superoxide concentration in the mitochondrial matrix is believed to be 5- to 10-fold higher than in the cytoplasm (25). The superoxide anion is both relatively short-lived and membrane impermeable, posing the most acute danger to the lipids, proteins, and DNA contained within mitochondria itself. However, superoxide dismutates into the membrane-permeable H2O2 either spontaneously or through the action of superoxide dismutase (SOD) proteins. H2O2 can also act as a signaling molecule, activating redox-sensitive pathways involved in insulin release and signaling, hypoxic response, adipocyte differentiation, and the cell cycle (72, 73, 106, 182).
Mitochondria contain both enzymatic and nonenzymatic antioxidant systems. Glutathione (GSH) is the major intracellular thiol used for defense against and elimination of ROS, electrophiles, and xenobiotics, and mitochondrial GSH is believed to constitute 10%–15% of total cellular GSH (97). When GSH is oxidized by ROS or electrophiles, it forms a dimer oxidized glutathione (GSSG), which can be reduced by the action of glutathione reductase (GR) maintaining the redox status of the cell.
SOD enzymes dismutate superoxide into H2O2. H2O2 is converted into O2 and H2O by catalase, or it is acted on by glutathione peroxidase 1 (Gpx1), a seleno-enzyme that converts H2O2 into H2O through the oxidation of GSH to GSSG. Gpx4 is also present in the mitochondria; its preferred substrates are lipid hydroperoxides, which form as a result of oxidative damage to the membrane phospholipids (42). In addition, mitochondria contain the peroxiredoxins (Prx)3 and 5 along with their thioredoxin (Trx)2 enzyme partner. Prx3 is exclusive to mitochondria while Prx5 is also present in peroxisomes, the cytosol, and the nucleus (31, 185). Prxs oxidize H2O2 to cysteine−SOH, which reacts with another cysteine to form H2O and a disulfide bond. The disulfide bonds are reduced by Trx2, which cycles from a reduced to an oxidized state. Trx2 is then reduced by thioredoxin reductase (TrxR) by the oxidation of NADPH. These enzymes are important for maintaining the redox environment of the mitochondria and for responding to oxidative stress imposed by external environmental factors.
Mitochondrial Protein Modification by ROS
Proteins, nucleic acids, and lipids are vulnerable to oxidation. The oxidation of amino acids has been particularly well studied in neuronal respiration, where redox modification can alter protein binding, interaction, and translocation or confer previously unknown properties and binding partners. The amino acids cysteine and methionine contain reactive thiol groups that confer redox sensitivity to proteins. Similarly, the thiol containing amino acid histidine makes it a target for singlet O2 reactions. Other less reactive amino acids include tyrosine, phenylalanine, valine, proline, arginine, and tryptophan, which can be oxidized by oxygen radicals, forming peroxides and peroxyl radicals.
Mitochondrial proteins are rich in cysteine residues, with an estimated concentration of 60–90 mM exposed protein thiols in the organelle (147). Cysteines are nucleophilic and readily react with electrophilic ROS. The thiol group, under physiological pH, gives the amino acid a pKa of 8.5, resulting in a protonated, unreactive residue. Lower pKa are observed under specific microenvironments that favor a deprotonated state. This may occur when the cysteine is surrounded by alkaline amino acids within the 3D context of a protein (51).
The alkaline environment of the mitochondrial matrix suggests that the majority of thiols in the matrix are ionized. Unprotonated sulfur reacts with ROS to form sulfenic acids (−SOH), which are very short lived, either reacting with other cysteines to form disulfide bonds or further oxidized to the sulfinic (−SO2H) and sulfonic acids (−SO3H) by strong oxidants such as superoxide anion (85). Disulfide bonds may form within the context of the protein, between two separate proteins, or with GSH, a modification called S-glutathionylation. Both sulfinic and sulfonic acid formation are irreversible, the accumulation of which can lead to altered protein function, protein degradation, or cell death. Disulfide bridge formation and sulfenic acid modification are reversible, and they may be used as modulatory events by the mitochondria. Control of a protein's activity by redox modification is called a “redox switch.”
Redox modifications of proteins can serve as an essential protective mechanism in the mitochondrial respiratory complexes. There are numerous cysteine residues in complex I that are targets for oxidative modifications, particularly the Fe-S containing 51 kDa (NDUFV1) and 75 kDa (NDUSF1) subunits (35, 46). Both in vivo and in vitro studies suggest that oxidative thiol modifications to the ND3, NDUFV1, and NDUSF1 subunits result in a reduction of complex I catalytic activity (24, 35, 81, 139, 207). Excessive ROS exposure leads to additional cysteine modifications and further loss of Fe-S clusters. In this way, oxidative modification of these subunits acts as a brake, halting the further production of oxidants that could lead to irreversible modifications and damage. The exact mechanisms for this switch between active and inactive complex I are under investigation. Redox modulation of complex II and complex V has also been described. Data from ischemia/reperfusion mouse hearts and isolated bovine heart mitochondria have shown that there are cysteine residues in the 70 kDa SdhA subunit if complex II are targets for S-glutathionylation (36). S-glutathionylation increases electron transfer activity and decreases superoxide formation, while de-glutathionylation decreases catalytic activity (36). Under the conditions of dyssnchronous heart failure, cysteines in the F1 α-subunit of complex V can be either S-glutathionylated or form disulfide bridges, leading to decreased ATP production (192).
Mitophagy and ROS
Mitochondrial protein and lipid oxidation by ROS plays an essential role in controlling mitochondrial viability through both apoptotic and mitophagic signaling. Mitophagy, or mitochondrial specific autophagy, pathways cull individual mitochondria under normal conditions as well as when cells are stressed. Autophagy occurs in the autophagosome, a double-membrane organelle that forms around the damaged organelle. Autophagosomes are formed by a multistep process that involves the interplay of several autophagy-related proteins (Atg), including Atg4, Atg6/Beclin-1, Atg12, Atg5, Atg12, Atg16, Vps34, AMBRA1, Bif-1, Bcl-2, and LC3 system proteins [reviewed in Carlsson and Simonsen (30)]. In the PTEN-induced putative kinase 1 (PINK1)/parkin pathway of mitophagy, damaged mitochondria go through a dynamin-related protein 1-mediated fission event (199). The healthy mitochondrial fragment is fused with other healthy mitochondria, while the unhealthy fragment is targeted for the autophagasome.
Under basal conditions, mitochondria have IM-associated PINK1; however, reduction in the mitochondrial membrane potential leads to an accumulation of PINK1 on the OM (39). The relocalization of PINK1 recruits parkin, an E3 ubiquitin ligase, to the mitochondria. Parkin is then phosphorylated by PINK1, allowing for the ubiquitination of OM proteins, including Mfn1, Mfn2, and p62/SQSTM1 (53, 54). The presence of Parkin on the OM and the ubiquitination of OM proteins are believed to recruit the autophagy adaptor proteins LC3/GABARAP, leading to mitophagy (92). Alternatively, mitochondria contain autophagy receptors Bcl-2/adenovirus E1B 19-kDa protein-interacting protein 3 (Bnip3) and Nix, which can directly interact with LC3 system on the autophagosome to initiate mitophagy (44).
The redox modulation of mitophagy and the cross-talk with apoptosis is starting to be revealed. Antioxidant treatment, such as with N-acetylcysteine or catalase, has been shown to decrease the formation of autophagosomes (57, 159, 184). However, it is not clear whether the antioxidants quench the ROS and prevent damage from occurring or whether the treatments of antioxidants directly affect autophagy machinery. The Atg4 protein allows for the conversion of LC3-I to lipidated LC3-II and its insertion into the autophagosome. ATG4 is regulated by oxidation, as mutation of the critical Cys81 on Ath4 or treatment of cells with antioxidants prevented autophagosome formation (159). Increased oxidative stress has also been shown to inhibit mitophagy by modifying cysteine residues of Parkin, which are important for maintaining its solubility and function (196). The induction of apoptosis leads to the targeting of several autophagy proteins for cleavage, including Atg5, AMBRA1, and Beclin-1. Fragments of Atg5 have been observed to interact with Bcl-XL to trigger cytochrome c release and caspase activation (208).
Mitochondrial Toxicity of Specific Toxicants
Determining the use of environmentally relevant dosing relevant for human health is often difficult, as there may be no clear primary source of action of many known irritants, pathogens, and carcinogens. Xenobiotics toxicity is quite often associated with disruption of multiple signaling system disruption such as membrane permeabilization, DNA interchalation, cellular oxidation, or energetic dysfunction. The possibility that we can begin to dissect total body load of environmental stress has emerged as a field referred to as the “exposome,” which has only recently been made possible by next-generation mass spectrophotometric techniques and partnerships between the EPA, CDC, NIEHS, and scientists in academia and industry.
The susceptibility of the mitochondria to xenobiotics has been one of the leading areas of research based on data linking pesticide and antibiotic exposure to neuronal dysfunction. Rotenone is a naturally occurring compound that is produced by plants, but it has been in use as a potent insecticide since the 1800s. Rotenone, for example, is a potent complex I inhibitor that is toxic to both humans and other mammals as well as to fish and insects. At the other end of the ETC, cyanide exposure blocks Complex IV of respiration. Cyanide is a bioproduct of normal human and plant metabolism as well as is commonly used for industrial metal and chemical processing. Cyanide competes with oxygen for binding to the Fe-Cu center in complex IV, inhibiting its cytochrome c oxidase activity and making it one of the most toxic compounds to humans who are exposed through inhalation, ingestion, or absorption (183). Other xenobiotics can disrupt the mitochondrial proton gradient, rather than the ETC, by permeabilizing the IM, effectively uncoupling proton pumping from ATP synthesis. In this section, the toxicity to the mitochondrial respiratory complexes is examined for three groups of toxicants; heavy metals, pesticides, and CS. Inhibition sites for these toxicants are shown in Figure 1.
Cigarette Smoke
According to the Centers for Disease Control and Prevention, it is estimated that 18.1% of American adults (42.1 million) are current smokers and that CS accounts for one in every five deaths in the United States (138). While mainstream CS is one of the few environmental pollutants in which exposure is voluntary and preventable, second- and third-hand CS exposure often is not. CS not only is a risk factor for a wide variety of disease such as cancer, chronic obstructive pulmonary disease (COPD) but also has been linked to neurodevelopmental disability (16, 113, 119, 173, 188). CS is a complex mixture of around 4700 different chemical compounds and 1015–17 oxidants/free radicals per puff (Table 3), several of which are known to be toxic to mitochondria, such as Cd, acrolein, and 2-ethylpyridine (40, 168). CS generates 500 ppm NO, which can combine with the superoxide anion to form ONOO−. Acute exposure of CS in vivo in rats or in vitro to alveolar epithelial cells depletes intracellular GSH levels, mainly through formation of GSH conjugates without oxidation of GSH to GSSG (101, 142). However, in chronic smokers and patients with COPD, GSH levels in the lung are increased as compared with nonsmokers (118). This represents a protective response to the oxidant burden imposed by CS.
Table 3.
Common Constituents of Tobacco Smokea
| Chemical component | Commonly found in |
|---|---|
| 2-Ethylpyridine | Chemical syntheses |
| Acrolein | Product of burning organic matter |
| Acrylonitrile | Used in manufacture of plastics, resins, and nitriles |
| Arsenic | Common component of pesticides |
| Benzene | Used in the production of gasoline |
| Carbon monoxide | Product of vehicle exhaust, gas stoves, and coal heating processes |
| Cadmium | Major component of batteries |
| Chloroform | Found in pesticides, fumigants, and fire extinguisers |
| Chromium | Used in production of steel |
| Hexane | Used in glues |
| Hydrogen cyanide | Chemical weapons |
| Lead | Once used as a pigment in paint |
| Manganese | Used in production of steel and aluminum alloys |
| Mercury | Chemical manufacturing and fluorescent lamps |
| Naphtalene | Mothballs |
| Phenol | Used in production of plastics and detergents |
| Toluene | Used to produce paint thinners |
| Vinyl chloride | Production of PVC pipes, bottles, and some upholstery |
For a full list, refer Talhout et al. (177).
PVC, polyvinyl chloride.
Oxidants, such as CS, induce the antioxidant response through the nuclear factor erythroid 2-related factor-2 (Nrf2) pathway (94). When activated by oxidative stress, Nrf2 translocates to the nucleus, binds to the antioxidant response element, and transcribes mRNA for antioxidant enzymes, such as heme oxygenase, glutathione S-transferase, and Cu/Zn-SOD (120). Mice lacking Nrf2 are highly susceptible to CS-mediated inflammation, decreased antioxidant enzymes, and development of emphysema earlier than CS-exposed mice with functional Nrf2 (83, 144). Activation of Nrf2 has been observed in healthy smokers; Nrf2 levels and activity are decreased in COPD patients (61), creating a more oxidized environment.
Many of the components of CS are reactive aldehydes, such as acetaldehyde and acrolein, that directly modify proteins through Michael addition chemistry. Ongoing studies seek to determine whether CS directly modifies the critical cysteine switches that control complex I function or can disrupt supercomplexes.
Surprisingly, the role that mitochondria play in CS toxicity beyond a mediator of apoptosis has only recently received serious attention. In experiments comparing cigarette smoke extract (CSE), hexane-treated CSE (CSE void of lipophilic compounds), and water-filtered CSE (CSE lacking ROS), mitochondrial membrane potential and ATP levels were investigated in airway epithelial cells to determine whether there was mitochondrial dysfunction and which components of CS may be involved (186). Indeed, numerous reports of CS- or CS component-induced apoptosis describe activation of the internal apoptotic pathway, with mitochondrial membrane hyperpolarization, Bax accumulation, and cytochrome c release (5, 32, 143, 150). CSE decreased both membrane potential and ATP levels in a dose-dependent manner that was attenuated by hexane treatment. This suggests that the lipophilic compounds from CS can directly enter the cells and lead to mitochondrial dysfunction. Van der Toorn et al. demonstrated that lung cells depleted of functional mitochondria had less ROS generation after CSE treatment than controls (186), suggesting that mitochondria are significant sources of ROS generation during CS exposure.
Changes in the structure and OXPHOS function may contribute to CS-induced mitochondrial dysfunction. CSE increased fragmentation, branching, and density of the matrix and reduced the number of cristae in vitro in human bronchial cell epithelial cell line BEAS-2B and in primary bronchial epithelial cells derived from stage IV (most severe) COPD patients (80). Damage to the mitochondrial IM and vacuolization of the matrix was also observed in purified mouse brain mitochondria exposed to CSE (200). The effect of CS on OXPHOS components varies by cell type and exposure condition.
CSE inhibited both complexes I and II enzymatic activity, leading to decreased oxygen consumption in human primary bronchial epithelial cells (187). The CS component acrolein has been shown to inhibit enzymatic activity of complexes I, II, and III in primary and transformed human retinal cells (86). Treatment with the polyphenolic antioxidant resveratrol alleviated the acrolein-induced decline in oxygen consumption and increased the levels of Mn-SOD in human retinal ARPE-19 cell line (165). In vivo complex I enzymatic activity was decreased in lung and kidney extracts, unchanged in the heart, but increased in the liver of BALB/c mice exposed to CS for 4 consecutive days using a nose-only system (145). Complex IV was decreased in all four organs investigated (145). In a study where A/J mice were exposed to CS for 4 or 8 weeks, expression of complexes II, III, IV, and V was increased above control mice, while complex I was decreased for both exposures (1).
Expression of the respiratory complexes from lung tissue was also analyzed at 2 weeks after an 8 week exposure to determine whether changes in mitochondria would return to control levels after cessation of CS. Complexes II, III, IV, and V returned to control levels; however, complex I levels remained decreased (1). While these results agree in regards to complex I, the differences in complex IV may arise from examining enzymatic activity versus gene expression. In addition, mouse strain differences in inflammatory and oxidative stress responses to CS in the lung have been described (201), partially explaining the discrepancy. In agreement with Agarwal et al., Hoffmann et al. have found that chronic exposure to CSE increased the protein expression of complexes II, III, and IV in BEAS-2B cells (80). Future research may clarify the mechanisms involved in CS modulation of OXPHOS components across durations of exposure and under different disease states (COPD, pulmonary fibrosis, cancer, etc.) in human patients.
CS-induced reduction of mitochondrial membrane potential has been associated with the activation of autophagy (Fig. 2). Elevated levels of autophagy have been measured in rodents exposed to CS as well as in cell culture models (3, 82). Ito et al. have observed that knockdown of PINK1 or Parkin knockdown in vitro increased mitochondrial ROS production and cellular senescence (84). In both pulmonary epithelial cells and mice, CS stabilized the mitophagy regulator PINK1 (114). PINK1 deficiency both in vivo and in vitro protected against CS-induced necrosis (as measured by the phosphorylation of MLKL, a substrate for RIP3 in the necroptosis pathway) and mitochondrial dysfunction (114). In addition, PINK1 knockout mice showed decreased airspace enlargement, a marker for emphysema, after CS exposure (114).
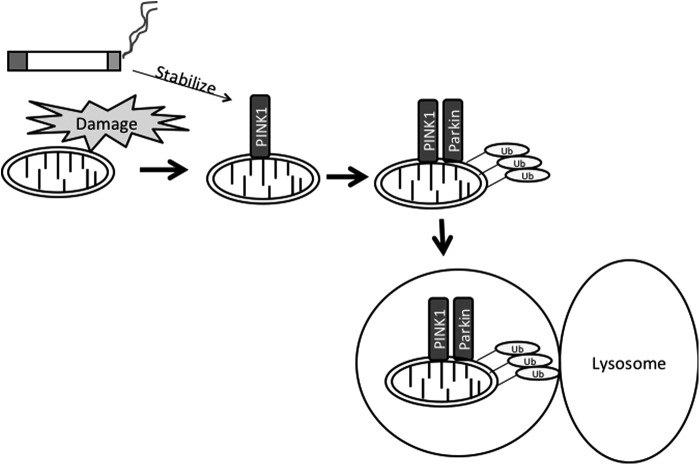
FIG. 2.
Proposed mechanism of CS-induced mitophagy. CS damages mitochondria leading to loss of membrane potential. In addition, CS stabilizes the accumulating PINK1 protein, which recruits the E3 ubiquitin ligase parkin. Mitochondrial proteins are ubiquitinated by parkin, allowing for mitochondria to be engulfed by isolation membranes that fuse with lysosomes. CS, cigarette smoke; PINK1, PTEN-induced putative kinase 1; Ub, ubiquitin.
The importance of mitochondrial health in developing treatments for CS exposure has recently been highlighted in a recent study involving transfer of induced pluripotent stem cells. Li et al. exposed rats to CS for 56 days and on days 29 and 43 intravenously administered human-induced pluripotent stem cell-derived mesenchymal stem cells (iPSC-MSCs) (100). Administration of iPSC-MSC decreased measures of alveolar damage and fibrosis after CS exposure (100). Interestingly, human mitochondria were observed in rat epithelial cells (100). In vitro mitochondrial transfer after iPSC-MSC administration occurred through a tunneling nanotube-dependent mechanism and prevented CS-induced alterations in ATP levels in BEAS-2B cells (100). While mitochondrial transfer may not account for all of the benefits of iPSC-MSC administration, it is clear that the transferred mitochondria were healthier than the control and maintained cellular energetics in response to CS (100).
Pesticides
Pesticides are a group of structurally unrelated chemicals that have the purpose of killing unwanted species, such as weeds (herbicides), rodents (rodenticides), fungi (fungicides), or insects (insecticides). While the chemicals are designed to target specific pathways in their target species, they often have unintended effects in nontarget species. The United States Environmental Protection Agency estimates that in 2007 there was 5211 million pounds of pesticides used globally (65). Clear links have been established between environmental exposure to pesticides and cancer (155, 163, 164), neurodegenerative diseases (Alzheimer's disease [AD] and Parkinson's disease [PD]) (9, 11, 153), asthma (76, 77, 161), diabetes (167, 176), sexual and reproductive dysfunction (64, 127, 146, 148, 194), and learning and developmental disorders (attention deficit hyperactivity disorder [ADHD]) (20, 135). The extent to which pesticide-induced mitochondrial dysfunction plays a role in many of these conditions is not clear.
Rotenone and complex I inhibiting pesticides
Rotenone is a naturally occurring toxin found in the Fabaceae plant family that is used as an insecticide and pesticide. It is highly lipophilic, crosses the blood–brain barrier easily, and induces pathological changes in the brain that resemble PD. While rotenone can cause these effects in rodents in experimental settings, it is not widely used in agriculture and has poor oral bioavailability toward limiting its likely contribution to sporadic PD. Rotenone is discussed here as an example of a complex I inhibitor. Other pesticides, including dihydrorotenone, fenazaquin, fenpyroximate, bullatacin, AE F119209, and tebufenpyrad (78, 125, 171, 178), share the same binding site on complex I as rotenone and are emerging as potentially interesting mediators of early onset neurodegeneration (125).
Rats exposed to rotenone cause uniform complex I inhibition in the brain (18, 29). Despite universal inhibition, only DAergic (dopaminergic) nigrostriatal neurons are selectively lost (29). This implies that DAergic neurons have an intrinsic sensitivity to mitochondrial dysfunction that is not present in other cell types. In addition, rat brains develop cytoplasmic inclusions that contain ubiquitin and α-synuclein, component of Lewy bodies in PD patients (18, 29). Rotenone also causes behavioral changes in rodents reminiscent of PD, such as bradykinesia, rigidity, and gait abnormalities (18). DAergic neurodegeneration in response to rotenone is accompanied by increased markers of oxidative stress, such as protein carbonylation, GSH depletion, and DNA damage (99, 193). Using the rotenone platform, or another pesticide with similar properties, may provide a critical set of tools to pair gene and environmental interactions in the study of mitochondrial failure in inherited and sporadic PD.
Rotenone exposures typically used in rodent studies to model PD produce brain concentrations of rotenone (∼30 nM) near the Ki for complex I inhibition (18). Epidemiological studies show that PD is more common in rural areas, where the increased prevalence is associated with the use of pesticides, herbicides, and heavy metals (58, 95). In addition to cell loss, postmortem PD brains have an accumulation of proteinaceous intracellular inclusions called Lewy bodies. PD is a heterogenous disease caused in 10%–20% of cases by an inherited defect in one in (currently) eight genes. These genes include parkin and PINK1, which are mitochondrial proteins. Mutation or deletion of these proteins has yet to reproduce the motor phenotype of PD, but animals exhibit some mild changes in mitochondrial morphology and function (79). In addition, PINK1 mutation in Drosophila have complex I impairment (190). Mutations in the antioxidant protein DJ-1 mutated in familial PD are impaired in scavenging mitochondrial-derived H2O2 (121). The short lifespan and limited cognitive and behavioral repertoire of mice limit their utility as models of PD, but they may provide a critical tool to pair environmental stressors, such as rotenone with a genetic form of PD to determine whether it results in early symptom onset.
Organophosphates
Organophosphates (OP) are a class of insecticides that target the enzyme acetylcholinesterase (AChE), which is an essential neurotransmitter and critical to function of the neuromuscular junction and cognitive function. OP have been implicated in AD, where acetylcholinergic-rich synapses die early in disease, and amyotrophic lateral sclerosis is a disease of the motor unit (28, 87). The most common environmental OP are malathion, parathion, methyl parathion, chlorpyrifos, diazinon, phosmet, dichlorvos, fenitrothion, azamethiphos, and azinphos methyl.
Release of acetylcholine into the synapse activates muscle cells and cholinergic neurons. AChE hydrolyzes acetylcholine into choline and acetic acid, allowing the cholinergic neuron to return to its resting state and terminates the muscle contraction. Inhibition of AChE by OP is irreversible, causing neurotoxicity. This mechanism is exploited to kill insects, but these compounds are also toxic to humans, as AChE is a conserved enzyme with the same function in both insects and mammals. Indeed, OP have been used as nerve agents for chemical warfare (e.g., sarin). Although OP degrade rapidly in the environment, traces of OP can be detected in food and drinking water (38, 180), making this class of xenobiotics particularly dangerous pre- and postnatally. Low levels of OP in food and drinking water have been linked to the ADHD and is being studied in other developmental disabilities (20, 109, 152).
Mitochondrial dysfunction induced by OP may contribute to physiological changes observed in neurodegenerative diseases. Concomitant with the mitochondrial complex impairment, Kaur et al. observed neuronal apoptosis and increased oxidative stress and inflammatory response after chronic exposure to a low dose of dichlorvos (91). OP are inhibitors of mitochondrial respiration in multiple tissues. Monocrotophos and dichorvos decreased complex I and complex II activities in cortex, cerebellum, and brain stem of rats (111). Complex I and complex IV were inhibited in hearts of rats exposed to mevinphos (202). Exposure to dichlorvos or parathion decreased the activities of complexes I, II, and IV in the brain and liver (19, 91, 115).
Heavy Metals
Metals constitute the bulk of the periodic table and are, therefore, abundant in our environment. Due to their unique chemistry and characteristics, such as reflectivity, malleability, ductility, and conductivity, metals are used for numerous purposes in nature and industry. While metals are beneficial in our day-to-day lives, the opposite side of the coin reveals the highly toxic nature of these elements. Toxicity to metals can result from malicious poisonings, environmental exposures, and occupational exposures. In addition, exposure to metals in the form of nanomaterials is gaining wider attention, as nanoparticles often behave differently depending on composition, size, and shape (151).
Lead
Lead (Pb) toxicity has been appreciated for centuries. It is ubiquitous in our environment, occurring naturally as ores with other metals; however, industrial sources account for the highest levels of Pb in our ecosystem. Pb contamination of polar regions of Greenland date back to 500 BC–300 AD, when it is estimated that nearly 400 tons of Pb were deposited in the environment by the ancient Greeks and Romans. Subsequently, Pb poisoning was first characterized and documented by early scientists and physicians (122). Current uses for Pb include electrodes for electrolysis and in automobile batteries, radiation shielding and reactor coolant, leaded glass, raw material for machinery, semiconductors, ammunition, polyvinyl chloride (PVC) plastics, sailing ballasts, solder, and devices to shield X-rays. Use of Pb in gasoline, paint, and ceramics has declined due to health concerns; however, surfaces covered in leaded paints are still present in older homes.
Exposure to Pb occurs primarily in the industrial setting or environmentally due to contamination. Children are of the utmost concern for environmental Pb exposure, as their developing nervous system is highly susceptible to the toxic effects of Pb. Pb affects several organ systems, most notably the skeletal system, hematopoietic system, kidneys, reproductive system, and both the peripheral and central nervous systems. Exposure to Pb has been associated with anemia, hypertension, sterility, osteoporosis, spontaneous abortions, and neurological problems, including hearing, learning, and cognitive impairments (56). Pb has also been implicated as a risk factor for the development of AD. High levels of bone and blood Pb are associated with decreased spatial copying skills, reduced pattern memory, and declines in cognitive functions associated with AD (129, 160). In addition, Pb exposure in rats increased amyloid precursor protein (APP) mRNA and aggregated Aβ, while Pb exposure in nonhuman primates increased amyloidogenesis, senile plaque deposition, and upregulated APP proteins (14, 15, 197).
Several molecular mechanisms are implicated in Pb toxicity, including generation of oxidative stress and disruption of Ca2+ homeostasis. On the molecular level, Pb2+ behaves similar to essential divalent metals, such as Ca2+, Zn2+, and Fe2+, and therefore gains access into the cell through molecular mimicry using Ca2+ transporters (108). Indeed, markers of Pb toxicity, such as lipid peroxidation and decreased mitochondrial antioxidant enzymes in the brain, were decreased in mice supplemented with either Ca2+ or Zn2+ (136).
While much of the literature has focused on Pb affecting Ca2+-dependent signaling pathways, the mitochondria has been implicated as a major site for Pb accumulation and induction of oxidative stress (172). Mitochondria play a major role in Ca2+ sequestration, while simultaneously Ca2+ is an important regulator of ATP production and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induction. The Ca2+ uniporter is utilized by Pb2+ to enter the mitochondria (198). Pb2+ substitution for Ca2+ causes Ca2+ dysregulation in the mitochondria, which can induce Ca2+ efflux and apoptosis (34, 75). Recently, generation of ROS and loss of mitochondrial membrane potential were shown to initiate apoptosis in not only Pb treated PC12 cells but also nonexposed bystander PC12 cells, which was mediated by gap junctions (71). This study suggests that Pb-induced mitochondrial dysfunction may elicit toxic effects across cells, allowing for damage to unexposed areas.
Pb has been shown to be a potent inducer of oxidative stress in multiple studies. Pb has been shown to cause oxidative damage to lipids and DNA (49, 128, 162, 206), resulting in leaky membranes and apoptosis. In a study performed in rats exposed in utero to a low dose of Pb, levels and activities of Cu/Zn-SOD, Mn-SOD, GPx1, and GPx4 were decreased in the hippocampus (13). Changes in mitochondrial antioxidant enzymes occur during in utero exposures to Pb and persist in rats past postnatal day 35 (59). Inhibition of these enzymes is due to the ability of Pb to substitute for the divalent metals necessary for enzymatic function. In addition, GSH levels were diminished by Pb exposure (13). While many antioxidant enzymes activities and levels are decreased by Pb, enzymes required for GSH synthesis and regulation (γ-GCS, GR, and GST) have been shown to be increased in the brain (175). This is believed to be a protective response to the toxic insult of Pb exposure. Increased oxidant burden imposed by Pb will have an inhibitory effect on mitochondrial function. Pb has been shown to decrease ATP levels and reduced Na+/K+ ATPase activity in rats (12). Mitochondrial oxygen consumption as well as mitochondrial structure, measured by number of cristae, was significantly altered in rods and cones of Pb-exposed mice (131).
Methylmercury
MeHg is a major environmental contaminant and potent neurotoxicant. Hg is present in the earth's crust and is deposited into our environment by volcanic activity and forest fires, while industrial processes (coal combustion, gold production, smelting, and cement production) and industrial waste are man-made sources. Release of Hg into the environment allows for the conversion of inorganic Hg into MeHg by bacteria. MeHg bioaccumulates and biomagnifies the food chain in aquatic environments, with the largest predatory fish (e.g., tuna, swordfish, and sharks) containing the highest levels of MeHg.
The neurotoxicity of MeHg has been recognized through large-scale poisonings. Effects of chronic MeHg exposure were made apparent after the Minamata incident in Japan, MeHg poisoning in Iraq from consumption of contaminated grain, as well as examination of island dwelling populations on the Seychelles and Faroes that have seafood-rich diets (45, 62). Prenatal exposure to MeHg is known to produce more severe effects than postnatal exposure. Neuropathological studies of victims of Minamata disease revealed that brain lesions were highly localized in individuals who had already reached adulthood at the time that exposure began. The focal lesions occur due to a loss of neurons in the granular layer of cerebellum as well as due to a loss of granular cells in the somatosensory, visual, and auditory cortical areas. This leads to a wide range of neurological problems, such as paresthesia, blurred vision, hearing impairment, olfactory and gustatory disturbances, slurred speech, ataxic gait, clumsiness, muscle weakness, dysarthria, irritability, memory loss, depression, and sleeping disturbance (45). Developmental exposure to MeHg leads to microcephaly and inhibition of neuronal migration, distortion of cortical layers, cerebellar abnormalities, alterations in neurotransmitter systems, and alterations in glial cells (41). In patients with congenital Minamata disease, however, the lesions were highly diffuse, occurring almost everywhere in the brain (37).
Oxidative stress is a major contributor of MeHg toxicity. MeHg is a soft electrophile and interacts with thiol (-SH) and selenol (-SeH) groups, forming stable complexes with defined stoichiometry. Combination of MeHg with the amino acid l-cysteine forms a conjugate that resembles methionine, and thus by molecular mimicry enters cells through the L-type large neutral amino-acid transporter 1 (LAT1) (7, 88, 166, 203). Transport of MeHg through LAT1 appears to be the main, and currently the only characterized, transporter for MeHg into the brain and studies have shown that methionine pretreatment is protective against MeHg toxic effects on cell viability and mitochondria in rat liver slices (149). Thiol groups are important functional moieties in antioxidant molecules and enzymes, such as GSH, thioredoxins, and glutaredoxins. MeHg interacts with GSH to form an excretable GS-HgCH3 complex (10), increasing the GSSG:GSH ratio and reducing the antioxidant capacity in both astrocytes and microglia (123, 191).
Alteration of the redox environment in the cell will have critical effects on the tightly regulated redox environments of mitochondrial compartments. Through direct binding to selenocysteine groups, MeHg inhibits the activity of GPx. GPx is responsible for detoxifying H2O2 through the oxidation of GSH to GSSG. Inhibition of GPx results in higher ROS levels and lipid peroxidation in vitro in SH-SY5Y cells and in mitochondria-enriched fractions from MeHg-exposed mice (50). Mitochondrial function as measured by the MTT assay was also decreased in these mice (50). Further studies revealed that MeHg also decreases GPx expression in a brain region-specific manner, with both GPx1 and GPx4 decreased in the cerebellum, while only GPx4 was decreased in the cerebrum (205). Supplementation with increased dietary selenium attenuated MeHg-induced decreases in GPx activity and expression in a zebrafish embryo model (130).
MeHg also decreases the protein expression of TrxR, greatly reducing the Trx activity in both the cytosol and mitochondria (21). TrxR transcription is controlled by Nrf2, which is induced by MeHg to promote the transcription of antioxidant genes (21, 123). It is unclear how MeHg decreases GPX or TrxR protein expression. Decreased antioxidant enzyme levels and activity by MeHg allow for an increase in ROS and damage to the mitochondria.
ROS production by MeHg has also been shown to occur through inhibition of the ETC of mitochondria. In rats exposed to a sub-acute dose of MeHg (10 mg/kg for 5 days), there is elevated mitochondrial oxygen consumption in the cerebellum and cerebrum, while complex II is inhibited in the cerebellum but not in the cerebrum (116, 117). This combination was associated with increased levels of superoxide radical (116, 117). Inhibition of complexes III and IV was observed in rat primary cerebellar granule neurons treated with MeHg (141). Concurrent with complex III and IV inhibition was an increase of mitochondrial-derived superoxide production, decreased ATP generation, disruption of mitochondrial membrane potential, and opening of MPTP (141). The discrepancy over which complexes are inhabited by MeHg in these studies may be due to comparisons between a whole brain region extract, containing multiple cell types, versus a pure cell culture system of one particular cell type. This suggests that there may be cell-specific responses to MeHg for respiratory complex function. Interestingly, in zebrafish, MeHg did not alter brain mitochondrial respiration and subunits of complex II showed a six-fold increase in expression after MeHg esposure (26).
After ROS generation and mitochondrial dysfunction, many labs have reported the induction of apoptosis after MeHg exposure, and it is currently proposed that induction of apoptosis is responsible for the neurodevelopmental changes that occur with MeHg toxicity. Numerous studies have examined whether the apoptotic pathway involved in MeHg toxicity is mitochondria dependent or independent. Studies in mouse hippocampus and cerebrum in vivo and neural progenitor cells and neuronal cell lines in vitro have shown cytochrome c release, caspase-dependent apoptosis (105, 169, 170, 193). Mitochondrial dysfunction and apoptosis from MeHg exposure has been directly related to MeHg-induced oxidative stress, as both N-acetylcysteine and lycopene have been shown to reverse mitochondrial damage and/or apoptotic events (105, 141).
Manganese
Unlike both Pb and MeHg, Mn is an essential metal. Mn is necessary for protein and energy metabolism, bone mineralization, metabolic regulation, and cellular protection from ROS, and it acts as a cofactor for many lectins, integrins, and enzymes, such as catalase and Mn-SOD. Exposure to Mn is primarily in the occupational setting, with industrial uses ranging from steel and stainless steel production, formation of aluminum alloys, as a cathode in alkaline batteries, and an antiknock agent in unleaded gasoline (methylcyclopentadienyl Mn tricarbonyl), and it is incorporated into fungicides, such as maneb and mancozeb.
Occupational exposure to Mn is the primary cause of human Mn intoxication, predominantly from welding, smelting, or the creation of fine dusts. Exposure to excessive levels of Mn results in a parkinsonian-like condition called manganism. Manganism presents with symptoms similar to PD, such as rigidity, dystonia, tremor, posture instability, bradykinesia, and dementia. However, the neuropathology between the two diseases is strikingly different. Manganism is characterized by a loss of neurons in the globus pallidus and substantia nigra pars reticulata. In addition, the putamen, caudate nucleus, and the subthalamic nucleus can also be damaged. In contrast, PD mainly affects the substantia nigra pars compacta and locus coeruleus; The DAergic neurons in these brain regions are typically spared in manganism (126). Lewy body protein aggregates present in PD are not present in manganism (132).
Similar to Pb, a great extent of the toxicity of Mn derives from the atomic similarity between Mn2+ and Ca2+ (69). Ca2+ is transported into mitochondria through the Ca2+ uniporter and RaM (rapid mode) and effluxed via Na+-independent, Na+-dependent mechanisms, and MPTP (70). The Ca2+ uniporter has been shown to transport Mn2+ into the mitochondria (69). Export of Mn2+ is by the Na+-independent efflux mechanism, which is less active than the Na+-dependent efflux mechanism in many tissues, such as the brain (52). This leads to an accumulation of Mn2+ in mitochondria and long half-lives of Mn in tissues. Ca2+ activates OXPHOS by driving the activity of three tricarboxcylic acid (TCA) cycle dehydrogenases (pyruvate, isocitrate, and α-ketoglutarate dehydrogenases) to produce increased amounts of NADH while simultaneously increasing the amount of ROS produced (112). Substitution of Mn2+ for Ca2+ has been observed in TCA cycle dehydrogenases, leading to decreased NADH levels in liver, brain, and heart mitochondria (68, 107). Mn2+ can also directly interact with complex II and complex V, leading to decreased ATP production (68, 209). Impaired energy metabolism has also been observed in vivo in rats injected with Mn into the striatum, with a 51% reduction in ATP occurring alongside excitotoxic lesions and decreases in the neurotransmitters dopamine (DA), GABA, and substance P (23).
In addition to its Ca2+ mimetic effects, Mn is known to cause oxidative stress. ROS levels are increased in brains of neonatal rats exposed to Mn (22), which can be blocked through the use of antioxidants. N-acetylcysteine co-treatment prevented pathological astrocytic changes in the brain of rats exposed to a sub-acute dose of Mn (74). Both N-acetylcysteine and GSH can prevent Mn-induced cytotoxicity in DAergic cells (174). It is important to note that Mn2+ cannot generate hydroxyl radicals from hydrogen peroxide and/or superoxide via Fenton-type or Haber–Weiss-type reactions.
A mechanism for Mn-induced production of ROS in the brain involves DA oxidation. DA is one of the most abundant catecholamines in the brain and is sensitive to oxidation to form leukoaminochrome-o-quinone (63). The extracellular reaction of Mn3+ with DA leads to the reduction to Mn2+ and the formation of aminochrome. Aminochrome reacts with NAD(P)H and O2 inside DAergic cells to produce leukoaminochrome-o-quinone and superoxide radical (104). The DA-o-quinone may also form a semiquinone radical and generate more ROS. Production of radicals by this mechanism can lead to increased ROS, depletion of NAD(P)H, lipid peroxidation, and inactivation of enzymes by oxidizing thiol groups or direct modification of amino acids on proteins. As previously discussed, mitochondrial respiratory complexes have numerous cysteine groups that are sensitive to redox state and may thus be altered by Mn-induced oxidative stress. Studies in cell lines have shown that oxidative stress generated by high Mn exposure induces the opening of the MPTP, resulting in apoptosis (98, 179). In vivo, loss of neurons in the cortex of Mn-exposed cynomolgus monkeys is due to apoptosis (67).
Concluding Remarks
The components of the ETC are highly organized and coordinated for the efficient production of ATP. This relies not only on the availability of substrates but also on the right redox conditions of the matrix and IMS. Deviations of these conditions can have serious consequences. The mitochondria as a target for xenobiotics are starting to be fully appreciated. Toxins perturb the delicate redox environment or directly interact with components of the ETC to alter OXPHOS and ATP production. The use of antioxidants can relieve some of the mitochondrial dysfunction associated with environmental exposures, which may be beneficial for treatment of toxicity or diseases associated with a particular toxin. Here, we have described links between mitochondrial function, environmental exposures, and disease states, highlighting the susceptibility of the nervous system (summarized in Table 4). In future studies, it will be important to determine whether toxicity of a specific xenobiotic is directly due to toxicity to the mitochondria or whether the mitochondria is damaged as a secondary event after exposure. This may give insight into the role of mitochondria and environmental exposures in disease.
Table 4.
Tissue Concentrations of Central Nervous Systems Select Toxicants in Central Nervous Systems Diseases
| Toxin | CNS effects | Species | Observed tissue concentrations | References |
|---|---|---|---|---|
| MeHg | Focal brain lesionsa | Human | 4.5–50 μg/g Hg in hair | (204) |
| Neurodevelopmental disordersb | Human | 10–20 ppm Hg in maternal hair | (43) | |
| DAergic neurodegeneration | Caenorhabdtis elegans | 1 ng Hg/g protein | (110) | |
| Mouse primary cultured mesencephalic cells | 1 μM | (60) | ||
| Mn | DAergic neurodegeneration | C. elegans | 5–10 nmol Mn/1000 worms | (8, 17) |
| Macaca fascicularis | 65–85 μg/L Mn in blood | (66, 189) | ||
| 0.220–3.25 μg/g Mn brain | ||||
| OP | ADHD | Human | 24.4–186.0 nM total DAP in urine | (20, 109) |
| 78.6–109.0 nM total DAP in blood | ||||
| Pb | AD | M. fascicularis | 19–26 μg/dl blood Pb | (197) |
| Rat | 46.43±1.95 μg/dl blood Pb | (15) | ||
| Cognitive decline | Human | <10 μg/dl blood Pb | (27, 96) | |
| ALS | Human | 14.9–20.5 μg/g bone Pb | (89, 90) | |
| 5.2 μg/dl blood Pb | ||||
| Rotenone | DAergic neurodegeneration | Rat | 20–30 nM rotenone in brain | (18) |
Resulting in the following neurological problems: paresthesia, blurred vision, hearing impairment, olfactory and gustatory disturbances, slurred speech, ataxic gait, clumsiness, muscle weakness, dysarthria, irritability, memory loss, depression, and sleeping disturbances.
Microcephaly and inhibition of neuronal migration, distortion of cortical layers, cerebellar abnormalities, alterations in neurotransmitter systems, and alterations in glial cells.
AD, Alzheimer's disease; ADHD, attention deficit hyperactivity disorder; ALS, amyotrophic lateral sclerosis; CNS, central nervous systems; DAergic, dopaminergic; DAP, dialkyl phosphate metabolite; MeHg, methylmercury; OP, organophosphates.
Abbreviations Used
- 3D
three-dimensional
- AChE
acetylcholinesterase
- AD
Alzheimer's disease
- ADHD
attention deficit hyperactivity disorder
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- CNS
central nervous systems
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CSE
cigarette smoke extract
- DA
dopamine
- DAergic
dopaminergic
- DAP
dialkyl phosphate metabolite
- ETC
electron transport chain
- γ-GCS
gamma glutamate-cysteine ligase
- Gpx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSSG
oxidized glutathione
- GST
glutathione s-transferase
- IM
inner membrane
- IMS
intermembrane space
- iPSC-MSCs
induced pluripotent stem cell-derived mesenchymal stem cells
- LAT1
L-type large neutral amino acid transporter 1
- MeHg
methylmercury
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor-2
- OM
outer membrane
- ONOO−
peroxynitrite
- OP
organophosphates
- OXPHOS
oxidative phosphorylation
- PD
Parkinson's disease
- PINK1
PTEN-induced putative kinase 1
- Prx
peroxiredoxin
- PVC
polyvinyl chloride
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TCA
tricarboxcylic acid
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- Ub
ubiquitin
- UQ
ubiquinone
Acknowledgments
Support was provided in part by grants NIH R01 ES07331 and NIH R01 ES10563.
References
- 1.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, and Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. American journal of physiology. Lung Cell Mol Physiol 303: L889–L898, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Althoff T, Mills DJ, Popot JL, and Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J 30: 4652–4664, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An CH, Wang XM, Lam HC, Ifedigbo E, Washko GR, Ryter SW, and Choi AM. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol 303: L748–L757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, and Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoshiba K, Tamaoki J, and Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 281: L1392–L1401, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Arciello M, Gori M, and Balsano C. Mitochondrial dysfunctions and altered metals homeostasis: new weapons to counteract HCV-related oxidative stress. Oxid Med Cell Longev 2013: 971024, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aschner M. and Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res 462: 31–39, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, and Aschner M. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PLoS One 4: e7792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, and Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol 157: 409–414, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Ballatori N. and Clarkson TW. Biliary secretion of glutathione and of glutathione-metal complexes. Fundam Appl Toxicol 5: 816–831, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, and Carvalho F. Pesticides exposure as etiological factors of Parkinson's disease and other neurodegenerative diseases-A mechanistic approach. Toxicol Lett 230: 85–103, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Baranowska-Bosiacka I, Gutowska I, Marchetti C, Rutkowska M, Marchlewicz M, Kolasa A, Prokopowicz A, Wiernicki I, Piotrowska K, Baskiewicz M, Safranow K, Wiszniewska B, and Chlubek D. Altered energy status of primary cerebellar granule neuronal cultures from rats exposed to lead in the pre- and neonatal period. Toxicology 280: 24–32, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Baranowska-Bosiacka I, Gutowska I, Marchlewicz M, Marchetti C, Kurzawski M, Dziedziejko V, Kolasa A, Olszewska M, Rybicka M, Safranow K, Nowacki P, Wiszniewska B, and Chlubek D. Disrupted pro- and antioxidative balance as a mechanism of neurotoxicity induced by perinatal exposure to lead. Brain Res 1435: 56–71, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Basha MR, Murali M, Siddiqi HK, Ghosal K, Siddiqi OK, Lashuel HA, Ge YW, Lahiri DK, and Zawia NH. Lead (Pb) exposure and its effect on APP proteolysis and Abeta aggregation. FASEB J 19: 2083–2084, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, and Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci 25: 823–829, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behl M, Rao D, Aagaard K, Davidson TL, Levin ED, Slotkin TA, Srinivasan S, Wallinga D, White MF, Walker VR, Thayer KA, and Holloway AC. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ Health Perspect 121: 170–180, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedetto A, Au C, and Aschner M. Manganese-induced dopaminergic neurodegeneration: insights into mechanisms and genetics shared with Parkinson's disease. Chem Rev 109: 4862–4884, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, and Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Binukumar BK, Bal A, Kandimalla R, Sunkaria A, and Gill KD. Mitochondrial energy metabolism impairment and liver dysfunction following chronic exposure to dichlorvos. Toxicology 270: 77–84, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Bouchard MF, Bellinger DC, Wright RO, and Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 125: e1270–e1277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branco V, Godinho-Santos A, Goncalves J, Lu J, Holmgren A, and Carvalho C. Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free Radic Biol Med 73: 95–105, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Brenneman KA, Cattley RC, Ali SF, and Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology 20: 477–487, 1999 [PubMed] [Google Scholar]
- 23.Brouillet EP, Shinobu L, McGarvey U, Hochberg F, and Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol 120: 89–94, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, and Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadenas E. and Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Cambier S, Gonzalez P, Mesmer-Dudons N, Brethes D, Fujimura M, and Bourdineaud JP. Effects of dietary methylmercury on the zebrafish brain: histological, mitochondrial, and gene transcription analyses. Biometals 25: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Canfield RL, Henderson CR, Jr., Cory-Slechta DA, Cox C, Jusko TA, and Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348: 1517–1526, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon JR. and Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124: 225–250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, and Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis 34: 279–290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson SR. and Simonsen A. Membrane dynamics in autophagosome biogenesis. J Cell Sci 128: 193–205, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Chae HZ, Kang SW, and Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol 300: 219–226, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Chang SS, Jiang WW, Smith I, Glazer C, Sun WY, Mithani S, and Califano JA. Chronic cigarette smoke extract treatment selects for apoptotic dysfunction and mitochondrial mutations in minimally transformed oral keratinocytes. Int J Cancer 126: 19–27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaput JP, Perusse L, Despres JP, Tremblay A, and Bouchard C. Findings from the Quebec Family Study on the Etiology of Obesity: Genetics and Environmental Highlights. Curr Obes Rep 3: 54–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavez E, Jay D, and Bravo C. The mechanism of lead-induced mitochondrial Ca2+ efflux. J Bioenerg Biomembr 19: 285–295, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Chen CL, Rawale S, Chen CA, Zweier JL, Kaumaya PT, and Chen YR. Peptide-based antibodies against glutathione-binding domains suppress superoxide production mediated by mitochondrial complex I. J Biol Chem 285: 3168–3180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YR, Chen CL, Pfeiffer DR, and Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem 282: 32640–32654, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Choi BH. The effects of methylmercury on the developing brain. Prog Neurobiol 32: 447–470, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury MA, Jahan I, Karim N, Alam MK, Abdur Rahman M, Moniruzzaman M, Gan SH, and Fakhruddin AN. Determination of carbamate and organophosphorus pesticides in vegetable samples and the efficiency of gamma-radiation in their removal. Biomed Res Int 2014: 145159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum Mol Genet 19: R28–R37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Church DF. and Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarkson TW. and Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36: 609–662, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Cole-Ezea P, Swan D, Shanley D, and Hesketh J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free Radic Biol Med 53: 488–497, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Cox C, Clarkson TW, Marsh DO, Amin-Zaki L, Tikriti S, and Myers GG. Dose-response analysis of infants prenatally exposed to methyl mercury: an application of a single compartment model to single-strand hair analysis. Environ Res 49: 318–332, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Ding WX. and Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393: 547–564, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekino S, Susa M, Ninomiya T, Imamura K, and Kitamura T. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 262: 131–144, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Engelhard J, Christian BE, Weingarten L, Kuntz G, Spremulli LL, and Dick TP. In situ kinetic trapping reveals a fingerprint of reversible protein thiol oxidation in the mitochondrial matrix. Free Radic Biol Med 50: 1234–1241, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Eubel H, Heinemeyer J, Sunderhaus S, and Braun HP. Respiratory chain supercomplexes in plant mitochondria. Plant Physiol Biochem 42: 937–942, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Farina M, Avila DS, da Rocha JB, and Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int 62: 575–594, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferlemi AV, Avgoustatos D, Kokkosis AG, Protonotarios V, Constantinou C, and Margarity M. Lead-induced effects on learning/memory and fear/anxiety are correlated with disturbances in specific cholinesterase isoform activity and redox imbalance in adult brain. Physiol Behav 131: 115–122, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, and Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med 47: 449–457, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Gallogly MM, Starke DW, and Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal 11: 1059–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavin CE, Gunter KK, and Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J 266: 329–334, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, and Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, and Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Genova ML. and Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta 1837: 427–443, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Gidlow DA. Lead toxicity. Occup Med 54: 76–81, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Giordano S, Darley-Usmar V, and Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol 2: 82–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman SM. Environmental toxins and Parkinson's disease. Annu Rev Pharmacol Toxicol 54: 141–164, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Gottipolu RR. and Davuljigari CB. Perinatal exposure to lead: reduction in alterations of brain mitochondrial antioxidant system with calcium supplement. Biol Trace Elem Res 162: 270–277, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Gotz ME, Koutsilieri E, Riederer P, Ceccatelli S, and Dare E. Methylmercury induces neurite degeneration in primary culture of mouse dopaminergic mesencephalic cells. J Neural Transm 109: 597–605, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Goven D, Boutten A, Lecon-Malas V, Marchal-Somme J, Amara N, Crestani B, Fournier M, Leseche G, Soler P, Boczkowski J, and Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 63: 916–924, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Grandjean P, Satoh H, Murata K, and Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect 118: 1137–1145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graumann R, Paris I, Martinez-Alvarado P, Rumanque P, Perez-Pastene C, Cardenas SP, Marin P, Diaz-Grez F, Caviedes R, Caviedes P, and Segura-Aguilar J. Oxidation of dopamine to aminochrome as a mechanism for neurodegeneration of dopaminergic systems in Parkinson's disease. Possible neuroprotective role of DT-diaphorase. Pol J Pharmacol 54: 573–579, 2002 [PubMed] [Google Scholar]
- 64.Greenlee AR, Arbuckle TE, and Chyou PH. Risk factors for female infertility in an agricultural region. Epidemiology 14: 429–436, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Grube A, Donaldson D, Kiely T, and Wu L. Pesticide Industry Sales and Usage 2006 and 2007 Market Estimates. Washington, DC: U.S. Environmental Protection Agency, 2011, pp. 1–33 [Google Scholar]
- 66.Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, and Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J Neurochem 107: 1236–1247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, and Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem 105: 1948–1959, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunter TE, Gerstner B, Lester T, Wojtovich AP, Malecki J, Swarts SG, Brookes PS, Gavin CE, and Gunter KK. An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicol Appl Pharmacol 249: 65–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunter TE. and Puskin JS. Manganous ion as a spin label in studies of mitochondrial uptake of manganese. Biophys J 12: 625–635, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunter TE. and Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta 1787: 1291–1308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo S, Zhou J, Chen X, Yu Y, Ren M, Hu G, Liu Y, and Zou F. Bystander effects of PC12 cells treated with Pb(2)(+) depend on ROS-mitochondria-dependent apoptotic signaling via gap-junctional intercellular communication. Toxicol Lett 229: 150–157, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, and Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Hamanaka RB. and Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35: 505–513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hazell AS, Normandin L, Norenberg MD, Kennedy G, and Yi JH. Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment. Neurosci Lett 396: 167–171, 2006 [DOI] [PubMed] [Google Scholar]
- 75.He L, Poblenz AT, Medrano CJ, and Fox DA. Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem 275: 12175–12184, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Henneberger PK, Liang X, London SJ, Umbach DM, Sandler DP, and Hoppin JA. Exacerbation of symptoms in agricultural pesticide applicators with asthma. Int Arch Occup Environ Health 87: 423–432, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hernandez AF, Parron T, and Alarcon R. Pesticides and asthma. Curr Opin Allergy Clin Immunol 11: 90–96, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Higgins DS, Jr., and Greenamyre JT. [3H]dihydrorotenone binding to NADH: ubiquinone reductase (complex I) of the electron transport chain: an autoradiographic study. J Neurosci 16: 3807–3816, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoepken HH, Gispert S, Morales B, Wingerter O, Del Turco D, Mulsch A, Nussbaum RL, Muller K, Drose S, Brandt U, Deller T, Wirth B, Kudin AP, Kunz WS, and Auburger G. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis 25: 401–411, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Hoffmann RF, Zarrintan S, Brandenburg SM, Kol A, de Bruin HG, Jafari S, Dijk F, Kalicharan D, Kelders M, Gosker HR, Ten Hacken NH, van der Want JJ, van Oosterhout AJ, and Heijink IH. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res 14: 97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, and Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J Biol Chem 283: 24801–24815, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, and Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys 500: 203–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, and Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 10: 1113–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Ito S, Araya J, Hara H, Kurita Y, Kobayashi K, Takasaka N, Wakui H, Yoshii Y, Minagawa S, Kojima J, Numata T, Shimizu K, Kawaishi M, Kaneko Y, Nakayama K, and Kuwano K. PINK1-Parkin pathway-mediated mitophagy is involved in cigarette smoke extract (CSE)-induced cellular senescence in human bronchial epithelial cells (HBEC). Eur Respir J 44: 3242, 2014 [Google Scholar]
- 85.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, and van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, and Liu J. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Invest Ophthalmol Vis Sci 48: 339–348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson FO. and Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 30: 761–765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kajiwara Y, Yasutake A, Adachi T, and Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol 70: 310–314, 1996 [DOI] [PubMed] [Google Scholar]
- 89.Kamel F, Umbach DM, Hu H, Munsat TL, Shefner JM, Taylor JA, and Sandler DP. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener Dis 2: 195–201, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Kamel F, Umbach DM, Munsat TL, Shefner JM, Hu H, and Sandler DP. Lead exposure and amyotrophic lateral sclerosis. Epidemiology 13: 311–319, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Kaur P, Radotra B, Minz RW, and Gill KD. Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicology 28: 1208–1219, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Kawajiri S, Saiki S, Sato S, Sato F, Hatano T, Eguchi H, and Hattori N. PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett 584: 1073–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Kelishadi R. and Poursafa P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care 44: 54–72, 2014 [DOI] [PubMed] [Google Scholar]
- 94.Knorr-Wittmann C, Hengstermann A, Gebel S, Alam J, and Muller T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic Biol Med 39: 1438–1448, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Langston JW. Epidemiology versus genetics in Parkinson's disease: progress in resolving an age-old debate. Ann Neurol 44: S45–S52, 1998 [DOI] [PubMed] [Google Scholar]
- 96.Lanphear BP, Dietrich K, Auinger P, and Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep 115: 521–529, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact 163: 54–67, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, and Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther 313: 46–55, 2005 [DOI] [PubMed] [Google Scholar]
- 99.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, and Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278: 8516–8525, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, and Lian Q. Mitochondrial transfer of induced pluripotent stem cells-derived mscs to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol 51: 455–465, 2014 [DOI] [PubMed] [Google Scholar]
- 101.Li XY, Donaldson K, Rahman I, and MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med 149: 1518–1525, 1994 [DOI] [PubMed] [Google Scholar]
- 102.Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, and Muhlenhoff U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823: 1491–1508, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Lizama-Manibusan B. and McLaughlin B. Redox modification of proteins as essential mediators of CNS autophagy and mitophagy. FEBS Lett 587: 2291–2298, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lloyd RV. Mechanism of the manganese-catalyzed autoxidation of dopamine. Chem Res Toxicol 8: 111–116, 1995 [DOI] [PubMed] [Google Scholar]
- 105.Lu TH, Hsieh SY, Yen CC, Wu HC, Chen KL, Hung DZ, Chen CH, Wu CC, Su YC, Chen YW, Liu SH, and Huang CF. Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicol Lett 204: 71–80, 2011 [DOI] [PubMed] [Google Scholar]
- 106.Mailloux RJ, Fu A, Robson-Doucette C, Allister EM, Wheeler MB, Screaton R, and Harper ME. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J Biol Chem 287: 39673–39685, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malthankar GV, White BK, Bhushan A, Daniels CK, Rodnick KJ, and Lai JC. Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem Res 29: 709–717, 2004 [DOI] [PubMed] [Google Scholar]
- 108.Marchetti C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol 2013: 184360, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, and Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect 118: 1768–1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Finley EJ, Caito S, Slaughter JC, and Aschner M. The Role of skn-1 in methylmercury-induced latent dopaminergic neurodegeneration. Neurochem Res 38: 2650–2660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masoud A, Kiran R, and Sandhir R. Impaired mitochondrial functions in organophosphate induced delayed neuropathy in rats. Cell Mol Neurobiol 29: 1245–1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCormack JG, Halestrap AP, and Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 113.Messner B. and Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 34: 509–515, 2014 [DOI] [PubMed] [Google Scholar]
- 114.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, Hashimoto S, Ryter SW, and Choi AM. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest 124: 3987–4003, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moreno AJ. and Madeira VM. Interference of parathion with mitochondrial bioenergetics. Biochim Biophys Acta 1015: 361–367, 1990 [DOI] [PubMed] [Google Scholar]
- 116.Mori N, Yasutake A, and Hirayama K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol 81: 769–776, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Mori N, Yasutake A, Marumoto M, and Hirayama K. Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria. J Toxicol Sci 36: 253–259, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Morrison D, Rahman I, Lannan S, and MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 159: 473–479, 1999 [DOI] [PubMed] [Google Scholar]
- 119.Moylan S, Jacka FN, Pasco JA, and Berk M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav 3: 302–326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muller T. and Hengstermann A. Nrf2: friend and foe in preventing cigarette smoking-dependent lung disease. Chem Res Toxicol 25: 1805–1824, 2012 [DOI] [PubMed] [Google Scholar]
- 121.Mullett SJ. and Hinkle DA. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J Neurochem 117: 375–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Needleman H. Lead poisoning. Annu Rev Med 55: 209–222, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, Cai J, and Aschner M. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci 116: 590–603, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.This reference has been deleted.
- 125.Okun JG, Lummen P, and Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase). J Biol Chem 274: 2625–2630, 1999 [DOI] [PubMed] [Google Scholar]
- 126.Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann N Y Acad Sci 1012: 209–223, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Oliva A, Spira A, and Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod 16: 1768–1776, 2001 [DOI] [PubMed] [Google Scholar]
- 128.Pal PB, Sinha K, and Sil PC. Mangiferin, a natural xanthone, protects murine liver in Pb(II) induced hepatic damage and cell death via MAP kinase, NF-kappaB and mitochondria dependent pathways. PLoS One 8: e56894, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Payton M, Riggs KM, Spiro A, 3rd, Weiss ST, and Hu H. Relations of bone and blood lead to cognitive function: the VA Normative Aging Study. Neurotoxicol Teratol 20: 19–27, 1998 [DOI] [PubMed] [Google Scholar]
- 130.Penglase S, Hamre K, and Ellingsen S. Selenium prevents downregulation of antioxidant selenoprotein genes by methylmercury. Free Radic Biol Med 75C: 95–104, 2014 [DOI] [PubMed] [Google Scholar]
- 131.Perkins GA, Scott R, Perez A, Ellisman MH, Johnson JE, and Fox DA. Bcl-xL-mediated remodeling of rod and cone synaptic mitochondria after postnatal lead exposure: electron microscopy, tomography and oxygen consumption. Mol Vis 18: 3029–3048, 2012 [PMC free article] [PubMed] [Google Scholar]
- 132.Perl DP. and Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol 66: 675–682, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Pizzo P, Drago I, Filadi R, and Pozzan T. Mitochondrial Ca(2)(+) homeostasis: mechanism, role, and tissue specificities. Pflugers Arch 464: 3–17, 2012 [DOI] [PubMed] [Google Scholar]
- 134.Poderoso C, Duarte A, Cooke M, Orlando U, Gottifredi V, Solano AR, Lemos JR, and Podesta EJ. The spatial and temporal regulation of the hormonal signal. Role of mitochondria in the formation of a protein complex required for the activation of cholesterol transport and steroids synthesis. Mol Cell Endocrinol 371: 26–33, 2013 [DOI] [PubMed] [Google Scholar]
- 135.Polanska K, Jurewicz J, and Hanke W. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit/hyperactivity disorder in children. Int J Occup Med Environ Health 26: 16–38, 2013 [DOI] [PubMed] [Google Scholar]
- 136.Prasanthi RP, Devi CB, Basha DC, Reddy NS, and Reddy GR. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci 28: 161–167, 2010 [DOI] [PubMed] [Google Scholar]
- 137.Prevention CfDCa. Fourth Report on Human Exposure to Environmental Chemicals. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2009 [Google Scholar]
- 138.Prevention CfDCa. Current Cigarette Smoking Among Adults-United States, 2005–2012. MMWR Morb Mortal Wkly Rep 63: 29–34, 2014 [PMC free article] [PubMed] [Google Scholar]
- 139.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, and Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A 106: 10764–10769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prohaska JR. and Gybina AA. Intracellular copper transport in mammals. J Nutr 134: 1003–1006, 2004 [DOI] [PubMed] [Google Scholar]
- 141.Qu M, Nan X, Gao Z, Guo B, Liu B, and Chen Z. Protective effects of lycopene against methylmercury-induced neurotoxicity in cultured rat cerebellar granule neurons. Brain Res 1540: 92–102, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Rahman I, Li XY, Donaldson K, Harrison DJ, and MacNee W. Glutathione homeostasis in alveolar epithelial cells in vitro and lung in vivo under oxidative stress. Am J Physiol 269: L285–L292, 1995 [DOI] [PubMed] [Google Scholar]
- 143.Ramage L, Jones AC, and Whelan CJ. Induction of apoptosis with tobacco smoke and related products in A549 lung epithelial cells in vitro. J Inflamm 3: 3, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, and Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raza H, John A, and Nemmar A. Short-term effects of nose-only cigarette smoke exposure on glutathione redox homeostasis, cytochrome P450 1A1/2 and respiratory enzyme activities in mice tissues. Cell Physiol Biochem 31: 683–692, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L, Zhu H, Finnell RH, and Zhu T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci U S A 108: 12770–12775, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Requejo R, Hurd TR, Costa NJ, and Murphy MP. Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J 277: 1465–1480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rocheleau CM, Romitti PA, and Dennis LK. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol 5: 17–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roos DH, Puntel RL, Farina M, Aschner M, Bohrer D, Rocha JB, de Vargas and Barbosa NB. Modulation of methylmercury uptake by methionine: prevention of mitochondrial dysfunction in rat liver slices by a mimicry mechanism. Toxicol Appl Pharmacol 252: 28–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roy J, Pallepati P, Bettaieb A, Tanel A, and Averill-Bates DA. Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chem Biol Interact 181: 154–167, 2009 [DOI] [PubMed] [Google Scholar]
- 151.Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, and Oberdorster G. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J Toxicol Environ Health A 73: 445–461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, and Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 171: 593–601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salazar JG, Ribes D, Cabre M, Domingo JL, Sanchez-Santed F, and Colomina MT. Amyloid beta peptide levels increase in brain of AbetaPP Swedish mice after exposure to chlorpyrifos. Curr Alzheimer Res 8: 732–740, 2011 [DOI] [PubMed] [Google Scholar]
- 154.Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin Chest Med 35: 17–27, 2014 [DOI] [PubMed] [Google Scholar]
- 155.Samanic CM, De Roos AJ, Stewart PA, Rajaraman P, Waters MA, and Inskip PD. Occupational exposure to pesticides and risk of adult brain tumors. Am J Epidemiol 167: 976–985, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sargis RM. The hijacking of cellular signaling and the diabetes epidemic: mechanisms of environmental disruption of insulin action and glucose homeostasis. Diabetes Metab J 38: 13–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schafer E, Dencher NA, Vonck J, and Parcej DN. Three-dimensional structure of the respiratory chain supercomplex I1III2IV1 from bovine heart mitochondria. Biochemistry 46: 12579–12585, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Schagger H. and Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19: 1777–1783, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, and Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26: 1749–1760, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, Gordon PB, Links JM, and Todd AC. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology 55: 1144–1150, 2000 [DOI] [PubMed] [Google Scholar]
- 161.Schwartz DA. Gene-environment interactions and airway disease in children. Pediatrics 123 Suppl 3: S151–S159, 2009 [DOI] [PubMed] [Google Scholar]
- 162.Shafiq-ur R. Effect of lead on lipid peroxidation, phospholipids composition, and methylation in erythrocyte of human. Biol Trace Elem Res 154: 433–439, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Shakeel MK, George PS, Jose J, and Mathew A. Pesticides and breast cancer risk: a comparison between developed and developing countries. Asian Pac J Cancer Prev 11: 173–180, 2010 [PubMed] [Google Scholar]
- 164.Sharma T, Jain S, Verma A, Sharma N, Gupta S, Arora VK, and Dev Banerjee B. Gene environment interaction in urinary bladder cancer with special reference to organochlorine pesticide: a case control study. Cancer Biomark 13: 243–251, 2013 [DOI] [PubMed] [Google Scholar]
- 165.Sheu SJ, Liu NC, Ou CC, Bee YS, Chen SC, Lin HC, and Chan JY. Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci 54: 6426–6438, 2013 [DOI] [PubMed] [Google Scholar]
- 166.Simmons-Willis TA, Koh AS, Clarkson TW, and Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J 367: 239–246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod Toxicol 31: 297–301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smith CJ. and Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol 38: 637–646, 2000 [DOI] [PubMed] [Google Scholar]
- 169.Sokolowski K, Falluel-Morel A, Zhou X, and DiCicco-Bloom E. Methylmercury (MeHg) elicits mitochondrial-dependent apoptosis in developing hippocampus and acts at low exposures. Neurotoxicology 32: 535–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sokolowski K, Obiorah M, Robinson K, McCandlish E, Buckley B, and DiCicco-Bloom E. Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits. Dev Neurobiol 73: 936–949, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Song H, Liu Y, Xiong L, Li Y, Yang N, and Wang Q. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, oxime ester, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J Agric Food Chem 61: 8730–8736, 2013 [DOI] [PubMed] [Google Scholar]
- 172.Sousa CA. and Soares EV. Mitochondria are the main source and one of the targets of Pb (lead)-induced oxidative stress in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 98: 5153–5160, 2014 [DOI] [PubMed] [Google Scholar]
- 173.Stathopoulou A, Beratis IN, and Beratis S. Prenatal tobacco smoke exposure, risk of schizophrenia, and severity of positive/negative symptoms. Schizophr Res 148: 105–110, 2013 [DOI] [PubMed] [Google Scholar]
- 174.Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash LH, Aschner M, and Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology 25: 543–553, 2004 [DOI] [PubMed] [Google Scholar]
- 175.Struzynska L, Sulkowski G, Lenkiewicz A, and Rafalowska U. Lead stimulates the glutathione system in selective regions of rat brain. Folia Neuropathol 40: 203–209, 2002 [PubMed] [Google Scholar]
- 176.Swaminathan K. Pesticides and human diabetes: a link worth exploring? Diabet Med 30: 1268–1271, 2013 [DOI] [PubMed] [Google Scholar]
- 177.Talhout R, Schulz T, Florek E, van Benthem J, Wester P, and Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 8: 613–628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Talpade DJ, Greene JG, Higgins DS, Jr., and Greenamyre JT. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J Neurochem 75: 2611–2621, 2000 [DOI] [PubMed] [Google Scholar]
- 179.Tamm C, Sabri F, and Ceccatelli S. Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicol Sci 101: 310–320, 2008 [DOI] [PubMed] [Google Scholar]
- 180.Tang Q, Wang X, Yu F, Qiao X, and Xu Z. Simultaneous determination of ten organophosphate pesticide residues in fruits by gas chromatography coupled with magnetic separation. J Sep Sci 37: 820–827, 2014 [DOI] [PubMed] [Google Scholar]
- 181.Tienson HL, Dabir DV, Neal SE, Loo R, Hasson SA, Boontheung P, Kim SK, Loo JA, and Koehler CM. Reconstitution of the mia40-erv1 oxidative folding pathway for the small tim proteins. Mol Biol Cell 20: 3481–3490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, and Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14: 537–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tsubaki M. Fourier-transform infrared study of cyanide binding to the Fea3-CuB binuclear site of bovine heart cytochrome c oxidase: implication of the redox-linked conformational change at the binuclear site. Biochemistry 32: 164–173, 1993 [DOI] [PubMed] [Google Scholar]
- 184.Underwood BR, Imarisio S, Fleming A, Rose C, Krishna G, Heard P, Quick M, Korolchuk VI, Renna M, Sarkar S, Garcia-Arencibia M, O'Kane CJ, Murphy MP, and Rubinsztein DC. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum Mol Genet 19: 3413–3429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Van der Eecken V, Clippe A, Van Veldhoven PP, and Knoops B. Mitochondrial targeting of peroxiredoxin 5 is preserved from annelids to mammals but is absent in pig Sus scrofa domesticus. Mitochondrion 11: 973–981, 2011 [DOI] [PubMed] [Google Scholar]
- 186.van der Toorn M, Rezayat D, Kauffman HF, Bakker SJ, Gans RO, Koeter GH, Choi AM, van Oosterhout AJ, and Slebos DJ. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. American journal of physiology. Lung Cell Mol Physiol 297: L109–L114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, Koeter GH, van Oosterhout AJ, and Kauffman HF. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol 292: L1211–L1218, 2007 [DOI] [PubMed] [Google Scholar]
- 188.Vassallo R. Diffuse lung diseases in cigarette smokers. Semin Respir Crit Care Med 33: 533–542, 2012 [DOI] [PubMed] [Google Scholar]
- 189.Verina T, Schneider JS, and Guilarte TR. Manganese exposure induces alpha-synuclein aggregation in the frontal cortex of non-human primates. Toxicol Lett 217: 177–183, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vilain S, Esposito G, Haddad D, Schaap O, Dobreva MP, Vos M, Van Meensel S, Morais VA, De Strooper B, and Verstreken P. The yeast complex I equivalent NADH dehydrogenase rescues pink1 mutants. PLoS Genet 8: e1002456, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang L, Jiang H, Yin Z, Aschner M, and Cai J. Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol Sci 107: 135–143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang SB, Murray CI, Chung HS, and Van Eyk JE. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc Med 23: 14–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Watabe M. and Nakaki T. Mitochondrial complex I inhibitor rotenone-elicited dopamine redistribution from vesicles to cytosol in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther 323: 499–507, 2007 [DOI] [PubMed] [Google Scholar]
- 194.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, and Perera FP. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect 112: 1125–1132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wittig I, Carrozzo R, Santorelli FM, and Schagger H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1757: 1066–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 196.Wong ES, Tan JM, Wang C, Zhang Z, Tay SP, Zaiden N, Ko HS, Dawson VL, Dawson TM, and Lim KL. Relative sensitivity of parkin and other cysteine-containing enzymes to stress-induced solubility alterations. J Biol Chem 282: 12310–12318, 2007 [DOI] [PubMed] [Google Scholar]
- 197.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, and Zawia NH. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28: 3–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang X, Wang B, Zeng H, Cai C, Hu Q, Cai S, Xu L, Meng X, and Zou F. Role of the mitochondrial Ca(2)(+) uniporter in Pb(2)(+)-induced oxidative stress in human neuroblastoma cells. Brain Res 1575: 12–21, 2014 [DOI] [PubMed] [Google Scholar]
- 199.Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, and Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A 105: 7070–7075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang YM. and Liu GT. Injury of mouse brain mitochondria induced by cigarette smoke extract and effect of vitamin C on it in vitro. Biomed Environ Sci 16: 256–266, 2003 [PubMed] [Google Scholar]
- 201.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, and Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 202.Yen DH, Chan JY, Tseng HP, Huang CI, Lee CH, Chan SH, and Chang AY. Depression of mitochondrial respiratory enzyme activity in rostral ventrolateral medulla during acute mevinphos intoxication in the rat. Shock 21: 358–363, 2004 [DOI] [PubMed] [Google Scholar]
- 203.Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, and Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem 107: 1083–1090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yorifuji T, Tsuda T, Takao S, Suzuki E, and Harada M. Total mercury content in hair and neurologic signs: historic data from Minamata. Epidemiology 20: 188–193, 2009 [DOI] [PubMed] [Google Scholar]
- 205.Zemolin AP, Meinerz DF, de Paula MT, Mariano DO, Rocha JB, Pereira AB, Posser T, and Franco JL. Evidences for a role of glutathione peroxidase 4 (GPx4) in methylmercury induced neurotoxicity in vivo. Toxicology 302: 60–67, 2012 [DOI] [PubMed] [Google Scholar]
- 206.Zhang H, Wei K, Zhang M, Liu R, and Chen Y. Assessing the mechanism of DNA damage induced by lead through direct and indirect interactions. J Photochem Photobiol B 136: 46–53, 2014 [DOI] [PubMed] [Google Scholar]
- 207.Zhang L, Xu H, Chen CL, Green-Church KB, Freitas MA, and Chen YR. Mass spectrometry profiles superoxide-induced intramolecular disulfide in the FMN-binding subunit of mitochondrial Complex I. J Am Soc Mass Spectrom 19: 1875–1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhou F, Yang Y, and Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J 278: 403–413, 2011 [DOI] [PubMed] [Google Scholar]
- 209.Zwingmann C, Leibfritz D, and Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab 23: 756–771, 2003 [DOI] [PubMed] [Google Scholar]