Abstract
The biochemical events surrounding ischemia reperfusion injury in the acute setting are of great importance to furthering novel treatment options for myocardial infarction and cardiac complications of thoracic surgery. The ability of certain drugs to precondition the myocardium against ischemia reperfusion injury has led to multiple clinical trials, with little success. The isolated heart model allows acute observation of the functional effects of ischemia reperfusion injury in real time, including the effects of various pharmacological interventions administered at any time-point before or within the ischemia-reperfusion injury window. Since brief periods of ischemia can precondition the heart against ischemic injury, in situ aortic cannulation is performed to allow for functional assessment of non-preconditioned myocardium. A saline filled balloon is placed into the left ventricle to allow for real-time measurement of pressure generation. Ischemic injury is simulated by the cessation of perfusion buffer flow, followed by reperfusion. The duration of both ischemia and reperfusion can be modulated to examine biochemical events at any given time-point. Although the Langendorff isolated heart model does not allow for the consideration of systemic events affecting ischemia and reperfusion, it is an excellent model for the examination of acute functional and biochemical events within the window of ischemia reperfusion injury as well as the effect of pharmacological intervention on cardiac pre- and postconditioning. The goal of this protocol is to demonstrate how to perform in situ aortic cannulation and heart excision followed by ischemia/reperfusion injury in the Langendorff model.
Keywords: Medicine, Issue 101, Ischemia reperfusion, isolated heart, cardiac preconditioning, infarct staining, histone deacetylase, cardiac function
Introduction
Elucidation of the events underlying the cardiac response to both ischemia and reperfusion are essential in improving the treatment of myocardial infarction1 and cardiac surgical procedures that require aortic cross-clamping2. While in vivo models of ischemia reperfusion injury allow very useful endpoint analysis, they are not as effective for studying the functional effects of ischemia reperfusion injury acutely in real time. Additionally, in vivo ischemia reperfusion models generally produce significant variability in infarct size, and direct delivery of drug to the heart at the time of reperfusion is challenging. The utilization of a Langendorff isolated heart system for studying ischemia reperfusion injury allows for real-time functional assessment of pharmacological treatments, uniform area of infarcted tissue, and instantaneous delivery of drug directly to the myocardium.
First described by Oscar Langendorff in 18953, the Langendorff isolated heart is a robust model for studying ischemia reperfusion injury, having been used in ischemia reperfusion research for the last 40 years4,5. Here, some modifications are made to optimize the isolated heart for functional analysis. In situ cannulation of the aorta while the heart is beating ensures that the heart does not experience ischemic preconditioning, which would alter the results of ischemia reperfusion trials6. To facilitate this, a tracheotomy is performed, allowing ventilation and ensuring physiological stability of the rat during surgery. The heart is then attached to a glass water-jacketed spiral column through which Krebs Henseleit buffer is delivered via retrograde perfusion directly into the aorta. A saline-filled balloon is inserted into the left ventricle and attached to a pressure transducer, which allows for real time measurement of pressures from within the ventricle and calculation of multiple functional parameters. At the conclusion of the experiment, the heart is flushed with cold saline to arrest contraction and flash frozen in liquid nitrogen to enable downstream analysis of DNA, RNA and protein levels. Thus modified, the Langendorff perfused heart serves as an effective system for direct monitoring of the physiological effect of pharmacological interventions at any time acutely during the ischemia reperfusion injury.
Protocol
All procedures listed here have been approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. The experiments described here are acute, non-survival experiments. As such, there is no use of eye ointment and a sterile operating suite is not required. Euthanasia is achieved by exsanguination during harvesting of the heart.
1. Experimental Preparation
Set up either a constant pressure or constant flow Langendorff Perfusion Apparatus4.
- Prepare 4 L of modified Krebs Henseleit Buffer (in mM: 112 NaCl, 5 KCl, 1.2 MgSO4, 1 K2HPO4, 1.25 CaCl2, 25 NaHCO3, 11 D-glucose, 0.2 octanoic acid, pH = 7.4).
- Make 10x stock buffers.
- Dissolve NaCl, KCl, MgSO4, and K2HPO4 in 3 L ultrapure water.
- Dissolve CaCl2 in 500 ml ultrapure water with 40 ml of 1 N HCl.
- Slowly add CaCl2 solution to the buffer made in 1.2.1.1. Add enough ultrapure water to total 4 L. Allow to stir for 1 hr before filtering through a 0.8 µm filter. Store at 4 °C.
- Dissolve NaHCO3 in 4 L ultrapure water. Allow to stir for 1 hr before filtering through at 0.8 µm filter. Store at 4 °C
- Combine 400 ml 10x Krebs Henseleit buffer with 400 ml 10x NaHCO3 buffer. Add enough ultrapure water to total 4 L. Dissolve glucose and octanoic acid while stirring. Filter through 0.8 µm filter.
- Prepare system for heart.
- Add 2 L of filtered buffer to a 4 L bottle placed 38 in above the stopcock attached to the aortic cannula. This will deliver a perfusion pressure of 70-80 mmHg to the heart, which is the recommended perfusion pressure4.
- Insert gas dispersion bubbler into the buffer in the bottle.
- Run the buffer through the system, being sure to eliminate any bubbles from inside the tubing.
- Turn on gas (95% O2/5% CO2) at least 30 min before heart is to be placed on the system. Use a gas flow probe and blood gas analyzer to verify oxygen saturation.
- Prepare surgical supplies and balloon.
- For harvesting of heart, prepare one pair of 6.75” surgical scissors, one pair of 4.5” scissors, two pairs of hemostats, two sets of caramalt forceps, two syringes with 27 G needles, 2 pieces of 0-guage silk suture (about 15 cm in length), one 1/16 inch barbed cannula, and one tracheal cannula.
- To assemble tracheal cannula, glue a short piece of 1/16 inch diameter plastic tubing to a Y-connector.
- To assemble balloon apparatus: attach a gavage needle to a piece of 1/16 inch tubing, then attach tubing to pressure transducer housing. A small syringe attached to the other side of the pressure transducer housing will allow inflation and deflation of the balloon.
- Cut a square of plastic wrap and place tip of a gavage needle in the center. Wrap the plastic wrap around the gavage needle.
- Fill balloon with saline and tie off with two sutures. Inflate balloon to ensure there are no leaks. The volume of saline within the inflated balloon should be roughly 200 µl, but can vary depending on the size of the rat.
- Alternatively, use a latex finger cot in place of the plastic wrap. Place gavage needle within finger cot, fill with saline and tie off.
2. Harvest the heart
Restrain a Sprague Dawley rat using a decapicone or other manual restraint device. Weigh rat.
Administer ketamine/xylazine mixture (0.85 mg/kg ketamine and 0.15 mg/kg xylazine) via intraperitoneal injection to anesthetize rat. This dose of anesthetic will provide sufficient anesthesia for 20-40 min, but the procedure should be completed as quickly as possible after anesthesia is confirmed.
Confirm proper degree of anesthesia by testing toe pinch reflex.
- Tracheotomy
- Remove pelt from mid-forepaw to base of jaw, using hemostats to elevate pelt and scissors to make incision. Keep the pelt elevated to separate it from the underlying muscle, allowing one to cut along the pelt and remove it.
- Using hemostats, lift glandular tissue and make a horizontal incision in fascia inferior to the glandular tissue. Use hemostats to spread incision open, taking care not to nick the jugular veins.
- Using hemostats, pinch muscle overlying trachea, and using scissors make a transverse incision in the muscle just below the tip of the hemostats. Using hemostats, spread tissue apart to reveal trachea.
- Carefully insert hemostats under trachea, clearing fascia while progressing by gently advancing and retracting hemostats.
- Once hemostats are successfully placed behind the trachea, pinch 0-0 silk suture with hemostats and hemisect the trachea with a small, sharp pair of scissors.
- Insert tracheal cannula into the hemisected trachea, pull suture behind the trachea, and tie the suture around the cannulated trachea, anchoring the suture to the Y-groin of the cannula with a surgeons knot.
- Using a 27 G needle, inject 1,000 U/kg heparin into the jugular vein and allow to circulate for 30 sec.
- Thoracotomy
- Remove pelt from mid-abdomen to mid-forepaw region by pinching with hemostats and cutting with scissors.
- Make a ¾ inch transverse incision into the abdominal muscle wall, just below the diaphragm.
- Make a vertical incision up the abdominal wall and chest wall, cutting along the sternum.
- Cut the diaphragm back bilaterally, then spread ribcage to reveal heart, clamp ribcage with hemostats to ensure it remains spread.
- Using hemostats, gently remove thymus, exposing the ascending aorta.
- Tease hemostats through aortic loop, advancing and retracting hemostats and gently spreading fascia to allow tip of hemostats through.
- Use hemostats to pull 0-0 silk suture behind ascending aorta, tie suture into a loose half-square knot.
- Turn stopcock to start flow of perfusion buffer, hemisect aorta and immediately insert cannula into aortic lumen. Rapidly cinch a half-square knot and complete the knot, tightening thoroughly.
- Use scissors to dissect heart away from the great vessels, remove heart from chest and attach to perfusion column.
- Trim away excess lung tissue and allow heart to equilibrate on column for about 15 min before insertion of balloon. Use a normal flow rate of buffer through the heart of 10-20 ml/min.
3. Langendorff Perfusion and Ischemia Reperfusion Injury
- Placement of LV pressure balloon
- Deflate balloon while rolling into a cone shape and turn stopcock to ensure balloon remains deflated.
- Excise left atrium with a small pair of scissors to expose access to mitral valve.
- Insert balloon through left atrium and mitral valve into left ventricle.
- Start pressure monitoring software (LabChart Pro).
- Inflate balloon by opening stopcock and gently increasing amount of saline in balloon until diastolic pressure reading from micromanometer exceeds zero.
- To check length-tension response, inflate balloon slowly to a diastolic pressure of 75 mmHg, deflate slowly, repeat once.
- Set diastolic pressure to 10 mmHg by modulating the amount of saline in the balloon. Turn stopcock to seal balloon at this level of inflation, and maintain closed pressure system between LV balloon and pressure transducer.
- Lower heart into heated chamber and cover chamber with plastic.
- Administration of Ischemia Reperfusion Injury
- Plug the bottom of the heated chamber and allow chamber to fill with effused buffer.
- When buffer has submerged heart, turn stopcock to stop the flow of buffer into the heart, inducing global ischemia. Ischemia time can vary depending on the goals of the experiment.
- After 30 min of ischemia, turn stopcock to restore flow of buffer into heart, initiating reperfusion.
- Allow reperfusion of heart for 1 hr before terminating the experiment. Use variable reperfusion times, depending on the goals of the experiment.
- Termination of Experiment and Harvesting of Tissue
- Using side port of stopcock, administer 10 ml of phosphate buffered saline at 4 °C into the heart at a rate of 10 ml/min to arrest the heart.
- Remove heart from perfusion column, trim away remaining atrial tissue.
- Place heart into a stainless steel tissue slicing matrix, cover with Parafilm, and place at -80 °C for 8 min, or until the heart has a marshmallow-like consistency.
- Remove block from freezer and insert razorblades into slicing matrix to slice heart into 2 mm slices. Tissue blocks can also be purchased with slice sizes other than 2 mm.
- Remove slices one at a time and drop into liquid nitrogen to flash freeze for biochemical studies. Retain the third ventricular slice from the apex for infarct staining.
- Remove tissue from liquid nitrogen and store at -80 °C until use in biochemical experiments.
- Infarct Staining
- Incubate the third ventricular slice in 10 ml of 1% w/v triphenyltetrazolium chloride (TTC) at 37 °C for 15 min. Note: Incubation in TTC can be performed for 15 or 30 min with no change in effectiveness15.
- Transfer tissue slice from TTC to 10% buffered formalin and allow to incubate at room temperature overnight. For optimal results, hearts should be taken out of formalin and imaged within 24 hr4.
- Photograph stained slice and use software such as ImageJ to estimate infarct size according to manufacturer’s protocol. Take photographs as soon as possible after fixation, as the dye will fade over time.
Representative Results
The left ventricular balloon apparatus allows for real-time monitoring of the pressure developed by the contracting left ventricle (Figure 1). As described previously7, this pressure trace can be used to calculate many of the parameters of ventricular function. These calculations can be made in the baseline phase as well as the reperfusion phase, averaged over multiple traces within each group, and compared in order to determine whether the pharmacological intervention resulted in cardiac preconditioning, as we have done previously9. One such parameter is the developed pressure, calculated as the difference between the systolic pressure and the end diastolic pressure. The developed pressure in normal perfused rat hearts can range from 70 to 130 mmHg (Figure 1A). After an ischemic insult, the developed pressure is reduced and the end diastolic pressure elevates (Figure 1B). When the rats are administered a known preconditioning agent such as the class I and IIb HDAC inhibitor SAHA (vorinostat)18 prior to excising the heart, the reduction in developed pressure and the elevation of end diastolic pressure associated with ischemia reperfusion injury are attenuated (Figure 1C). Other measures of left ventricular function, such as the rate of pressure generation (dP/dtmax), the rate of pressure relaxation (-dP/dtmax), and the rate pressure product (RPP) can be directly obtained or calculated from the software output (Figure 2). The ischemic phase can also be monitored in real-time, with the apparent cessation of pressure generation within minutes of the onset of ischemia. Ischemic contraction can also be monitored by measuring the time to onset of contraction and the time to peak contraction.
Triphenyltetrazolium chloride (TTC) is commonly used to differentiate between metabolically active and inactive tissue, and is used here for infarct staining. Once absorbed into the tissue TTC is reduced by metabolic enzymes, turning the active tissue red. Inactive tissue does not reduce TTC, and as such will stain white8. Langendorff perfused rat hearts that have not been subjected to ischemic injury do not show any white areas (not shown), while the hearts subjected to ischemia reperfusion injury show substantial areas stained white, indicating infarcted tissue (Figure 3).
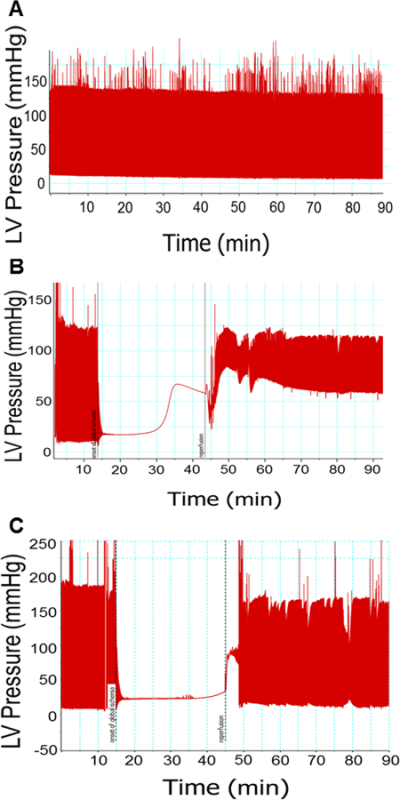 Figure 1: Representative pressure traces from left ventricular balloon. Pressure recorded by the LV balloon upon ventricular contraction. Hearts not subjected to ischemia (A) experience a minor loss of contractile ability over time. Hearts subjected to ischemia (B) show immediate loss of pressure generation, followed by tonic contraction. Upon reperfusion, these hearts experience elevated EDP and decreased developed pressure. Hearts preconditioned with SAHA and subjected to ischemia (C) show attenuation of ischemia reperfusion injury. N = 1 per group.
Figure 1: Representative pressure traces from left ventricular balloon. Pressure recorded by the LV balloon upon ventricular contraction. Hearts not subjected to ischemia (A) experience a minor loss of contractile ability over time. Hearts subjected to ischemia (B) show immediate loss of pressure generation, followed by tonic contraction. Upon reperfusion, these hearts experience elevated EDP and decreased developed pressure. Hearts preconditioned with SAHA and subjected to ischemia (C) show attenuation of ischemia reperfusion injury. N = 1 per group.
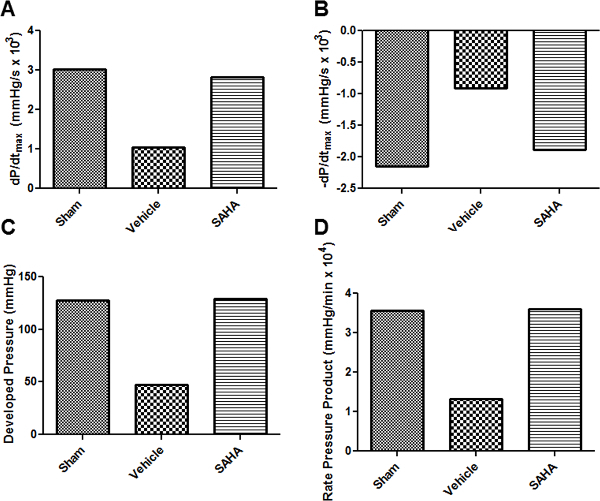 Figure 2: Calculated parameters of ventricular function. Rate of pressure generation (A), rate of pressure relaxation (B), developed pressure (C), and rate pressure product (D) are some parameters of ventricular function that can be calculated from the pressure monitoring software.
Figure 2: Calculated parameters of ventricular function. Rate of pressure generation (A), rate of pressure relaxation (B), developed pressure (C), and rate pressure product (D) are some parameters of ventricular function that can be calculated from the pressure monitoring software.
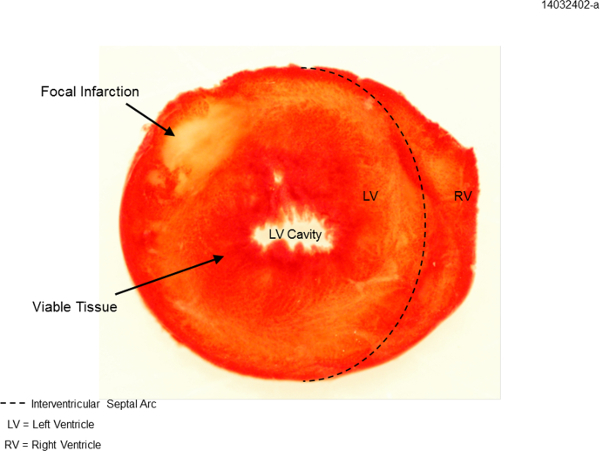 Figure 3: TTC staining for infarct area. Example ventricular cross-section after ischemia reperfusion injury. Dotted line delineates left ventricle (LV) from right ventricle (RV). Focal infarction is indicated by white area, whereas viable tissue is indicated by red-staining.
Figure 3: TTC staining for infarct area. Example ventricular cross-section after ischemia reperfusion injury. Dotted line delineates left ventricle (LV) from right ventricle (RV). Focal infarction is indicated by white area, whereas viable tissue is indicated by red-staining.
Discussion
The isolated perfused rat heart can be successfully used to study the effect of pharmacological intervention on cardiac preconditioning in ischemia reperfusion injury9. However, there are some essential steps to the procedure that must be standardized in order to ensure reproducible results. Maintaining a temperature of 37.4 °C within the system is critical, as even mild hypothermia and hyperthermia can cause cardiac preconditioning10,11. The overall time that elapses from injection of anesthetic to the excision of the heart must be kept to a minimum, as prolonged exposure to ketamine may interfere with cardiac preconditioning12. Timely cannulation of the aorta in situ is essential to preventing the development of hypoxia within the heart or the exsanguination of the animal prior to heart excision. Overall, the time from first incision to removal of the heart should be no longer than 6-8 min. The left ventricular balloon must be checked for leaks before each experiment, and replaced if necessary. The system must be meticulously maintained, including flushing the entire apparatus with distilled water after each run and replacing worn-out tubing as necessary to prevent leakage or contamination of the interior of the tubing.
The isolated heart may be modified for any number of novel uses, including fluorescence imaging13, NMR Spectroscopy7, and optical mapping14 among many others. The system can also be modified to perfuse hearts from different animals, including mice. This modification is especially useful as it allows for experiments using transgenic mice. To modify the system for mouse hearts, smaller cannulae and a smaller perfusion column must be used, in addition to other modifications. Detailed descriptions of how to utilize the Langendorff method for mouse hearts have been published elsewhere16,17. Other modifications of this protocol include the administration of various drugs at different time points. When investigating the ability of a drug to cause pharmacological preconditioning or post-conditioning, it is essential to administer the drug at different time points in relation to the ischemia reperfusion injury. The drug may be administered to the animal before the heart is excised, or mixed into the buffer either before the heart is placed on the column or during the ischemic phase so that the drug is present upon reperfusion. Alternatively, a side-port can be utilized to administer a bolus of drug at any time point during the protocol. Additionally, the protocol may be modified to change the duration of ischemia, reperfusion, or both. This allows the analysis of functional and biochemical data at multiple time points and can be used to track the time course of the acute effect of a drug. If the reperfusion period is altered, it is important to note that at least 60 minutes of reperfusion are necessary for TTC staining to effectively delineate infarct area15.
The main limitation of the isolated heart model in terms of ischemia reperfusion injury is that it does not account for many of the systemic factors that are present in the setting of ischemia reperfusion in vivo. This elimination of systemic influence must be accounted for in the analysis of data generated using the Langendorff model, but does not preclude the ability of the model to answer novel questions about the response of myocytes, fibroblasts, and endothelial cells to ischemia and subsequent reperfusion. The isolated heart model allows for complete manipulation of many of the variables affecting ischemia reperfusion injury in addition to real time analysis of the functional effects on the heart over short time intervals. The data generated by using the isolated heart system is invaluable in understanding the pharmacological preconditioning or post-conditioning of the heart in response to various pharmaceutical interventions.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This publication was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant Number UL1 TR000062. Further support was provided by VA merit award BX002327-01 to DRM. DJH was supported by NIH/NCATS Grant Number TL1 TR000061 and by NIH Grant Number T32 GM008716. SEA was supported by NIH Grant Number T32 HL07260.
References
- Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction. American Heart Journal. 2014;167(5):637–645. doi: 10.1016/j.ahj.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Beyersdorf F. The use of controlled reperfusion strategies in cardiac surgery to minimize ischaemia/reperfusion damage. Cardiovascular Research. 2009;83(2):262–268. doi: 10.1093/cvr/cvp110. [DOI] [PubMed] [Google Scholar]
- Langendorff O. Untersuchugen am überlebenden Säugethierherzen. Pflügers Archives. 1895;61:291–307. [Google Scholar]
- Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: The langendorff technique of isolated heart perfusion. Journal of Molecular and Cellular Cardiology. 2011;50(6):940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Tyers GF, Todd GJ, Neely JR, Waldhausen JA. The mechanism of myocardial protection from ischemic arrest by intracoronary tetrodotoxin administration. The Journal of Thoracic and Cardiovascular Surgery. 1975;69(2):190–195. [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Kolwicz SC, Tian R. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. The Journal of Visualized Experiments. 2010. [DOI] [PMC free article] [PubMed]
- Fishbein MC, et al. Early phase acute myocardial infarct size quantification: Validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. American Heart Journal. 1981;101(5):593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- Aune SE, Herr DJ, Mani SK, Menick DR. Selective inhibition of class I but not class IIb histone deacetylases exerts cardiac protection from ischemia reperfusion. Journal of Molecular and Cellular Cardiology. 2014;72:138–145. doi: 10.1016/j.yjmcc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon DM, et al. The protective role of heat stress in the ischaemic and reperfused rabbit myocardium. Journal of Molecular and Cellular Cardiology. 1992;24(8):895–907. doi: 10.1016/0022-2828(92)91102-b. [DOI] [PubMed] [Google Scholar]
- Khaliulin I, et al. Temperature preconditioning of isolated rat hearts--a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. The Journal of Physiology. 2007;581(Pt 3):1147–1161. doi: 10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molojavyi A, et al. Effects of ketamine and its isomers on ischemic preconditioning in the isolated rat heart) Anesthesiology. 2001;94(4):623–629. doi: 10.1097/00000542-200104000-00016. [DOI] [PubMed] [Google Scholar]
- Asfour H, et al. NADH fluorescence imaging of isolated biventricular working rabbit hearts. The Journal of Visualized Experiments. 2012. [DOI] [PMC free article] [PubMed]
- Sill B, Hammer PE, Cowan DB. Optical mapping of langendorff-perfused rat hearts. The Journal of Visualized Experiments. 2009. [DOI] [PMC free article] [PubMed]
- Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in langendorff rat model. Cardiovascular Drugs and Therapy. 2009;23(4):327–331. doi: 10.1007/s10557-009-6176-5. [DOI] [PubMed] [Google Scholar]
- Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: Characteristics and cautions. Clinical and Experimental Pharmacology and Physiology. 2003;30(11):867–878. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- Reichelt ME, Willems L, Hack BA , Peart JN, Headrick JP. Cardiac and coronary function in the langendorff-perfused mouse heart model. Experimental Physiology. 2009;94(1):54–70. doi: 10.1113/expphysiol.2008.043554. [DOI] [PubMed] [Google Scholar]
- Xie M, et al. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129(10):1139–1151. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]


