Abstract
The aim of the present cross-sectional study was to investigate whether activation of the renin-angiotensin system in renovascular disease affects the cytochrome P450 ω/ω-1 hydroxylase (20-hydroxyeicosatetraenoic acid [20-HETE]) and epoxygenase (epoxyeicosatrienoic acids [EETs]) pathways of arachidonic acid metabolism in vivo, each of which interacts with angiotensin II. Plasma concentration and urinary excretion of 20-HETE and EETs and their metabolites, dihydroxyeicosatrienoic acids, were measured in urine and plasma by mass spectrometry in 10 subjects with renovascular disease, 10 with essential hypertension, and 10 healthy normotensive subjects (control subjects), pair-matched for gender and age. Vascular and renal function were evaluated in all of the subjects. Plasma 20-HETE was highest in subjects with renovascular disease (median: 1.20 ng/mL; range: 0.42 to 1.92 ng/mL) compared with subjects with essential hypertension (median: 0.90 ng/mL; range: 0.40 to 2.17 ng/mL) and control subjects (median: 0.45 ng/mL; range: 0.14 to 1.70 ng/mL; P<0.05). Plasma 20-HETE significantly correlated with plasma renin activity in renovascular disease (rs=0.67; n=10; P<0.05). The urinary excretion of 20-HETE was significantly lower in subjects with renovascular disease (median: 12.9 μg/g of creatinine; range: 4.4 to 24.9 μg/g of creatinine) than in control subjects (median: 31.0 μg/g of creatinine; range: 11.9 to 102.8 μg/g of creatinine; P<0.01) and essential hypertensive subjects (median: 35.9 μg/g of creatinine; range: 14.0 to 72.5 μg/g of creatinine; P<0.05). Total plasma EETs were lowest, as was the ratio of plasma EETs to plasma dihydroxyeicosatrienoic acids, an index of epoxide hydrolase activity, in renovascular disease (ratio: 2.4; range: 1.2 to 6.1) compared with essential hypertension (ratio: 3.4; range: 1.5 to 5.6) and control subjects (ratio: 6.8; range: 1.4 to 18.8; P<0.01). In conclusion, circulating levels of 20-HETE are increased and those of EETs are decreased in renovascular disease, whereas the urinary excretion of 20-HETE is reduced. Altered cytochrome P450 arachidonic acid metabolism may contribute to the vascular and tubular abnormalities of renovascular disease.
Keywords: eicosanoids, 20-HETE, EETs, DHETs, renal artery stenosis, hypertension, angiotensin II
In the past 2 decades, the view that the cytochrome P450 (CYP) monooxygenase pathway of arachidonic acid (AA) metabolism affects blood pressure and contributes to the development of hypertension is supported by studies in several experimental models.1,2 However, little information is available on possible contributions of CYP products to essential hypertension (EH) and human renal vascular hypertension.3 The role of angiotensin II (Ang II) in the development of hypertension and ischemic nephropathy in the experimental model of the 2-kidney 1-clip hypertension is evident, because a fall in renal perfusion pressure triggers renin release. Vasoconstriction, vascular remodeling, glomerulosclerosis, and interstitial matrix deposition implicate an array of mediators that operate downstream from Ang II.4
CYP monooxygenases are expressed in the renal vasculature and nephron,5 generating primarily 20-hydroxyeicosatetraenoic acid (20-HETE) by ω- and ω-1 hydroxylases and 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs) by epoxygenases. The several individual isozymes that produce 20-HETE and EETs are independently regulated. For example, the rat 2C23 epoxygenase isozyme responds to a salt load by increasing expression and activity to augment synthesis of antipressor EETs.6 20-HETE exhibits both pressor and antipressor activities.7,8 20-HETE mediates renal circulatory autoregulation9 and contributes to pressure natriuresis.10 One or more EETs may serve as endothelial-derived hyperpolarizing factors.11,12 EETs are metabolized rapidly by epoxide hydrolases to dihydroxyeicosatrienoic acids (DHETs), which are generally viewed as biologically inactive with a few exceptions.13 Inhibition of soluble epoxide hydrolase (sEH) produces increased EETs with decreased blood pressure in experimental and genetic hypertension in rats 14–16
Ang II stimulates 20-HETE synthesis in renal microvessels7,17 and decreases EET levels by downregulating epoxygenases and increasing their degradation by increasing expression and activity of sEH.18,19 These studies provide the rationale to the hypothesis that occasioned the present study; namely, metabolism of AA via CYP monooxygenases is altered in renovascular disease (RVD). To assess whether altered generation of eicosanoids derived from CYP AA metabolism occurs with activation of the renin-angiotensin system, we set up a cross-sectional study to measure 20-HETE, EET, and DHET levels in plasma and urine by paired analyses in hypertensive subjects with RVD that were compared with these levels in normotensive control subjects and those with EH.
Materials and Methods
Subjects
Three groups of subjects, pair-matched for gender and age, were studied: patients with RVD (n=10), patients with EH (n=10), and normotensive subjects (n=10). Hypertensive patients were recruited among those admitted to the hypertension unit of the Department of Internal Medicine, University Hospital of Verona. Diagnosis of RVD was based on angiographic evidence of severe stenosis (exceeding 70% of the lumen diameter) of a renal artery in hypertensive patients.20
All of the subjects with RVD and EH were on antihypertensive treatment to normalize blood pressure. Those drugs, having a direct effect on the activity of the renin-angiotensin system, angiotensin-converting enzyme inhibitors, and Ang II type 1 receptor antagonists, were withdrawn ≥2 months before the study.
For a detailed description of selection and exclusion criteria, as well as clinical characteristics of patients and control subjects, please see the data supplement available at http://hyper.ahajournals.org.
Study Protocol
The protocol consisted of clinical evaluation, blood pressure measurement, analysis of endothelial function and vascular profile, and blood and urine sampling. Both patients with RVD and EH were hospitalized during the study and received a diet containing ≈150 mmol of sodium per day.
The urinary excretion of eicosanoids was evaluated from overnight urine collections (from 8 pmPM to 8 am). The timing and total volume were recorded, and the samples (2 mL) of urine were stored at −80°C for the assay of derivates of AA by CYP metabolism after the addition of deuterated 20-HETE (Cayman Chemical Co), DHET, 8,9-EET, 11,12-EET, and 14,15-EET (Biomol) as internal standards.
Blood samples were collected from a peripheral vein for the measurement of plasma renin activity (PRA) in patients with RVD, EH subjects, and control subjects. Before blood sampling, patients were kept in the supine position for ≥1 hour. Blood samples were also taken to determine plasma glucose, total lipids and high-density lipoprotein cholesterol, homocysteine, and creatinine. Blood pressure was recorded (the mean of 3 readings) between 8 am and 10 pm using an oscillometric device (TM-2551, A&D Instruments) with the subjects in the supine position for 30 minutes. Body mass index was calculated (kilograms per meter squared). Intima-media thickness of the common carotid artery was measured. Endothelial function was investigated according to the model of flow-mediated vasodilatation.21 Applanation tonometry was performed at carotid level using PulsePen (Dia-Tecne).22 For a detailed description of the methods used in the analysis of endothelial function and carotid arteries, please see the data supplement.
The study protocol had been approved previously by the ethics committee of the University Hospital in Verona. Informed written consent was obtained from all of the subjects.
Measurements of Eicosanoids
Free eicosanoids were directly extracted with 2 mL of ethyl acetate twice from plasma samples (0.4 mL) after adding internal standards (DHETs-d8 4.8 ng, 20-HETE-d6 4 ng, and EET-d8 12 ng) and acidified to pH 4 with acetic acid. For analysis of total eicosanoids, plasma was extracted with 2 mL of CHCl3/CH3OH (2:1) containing 0.1 mmol/L of triphenylphosphine as an antioxidant according to the method of Karara et al.23 After frequent vortexing for 30 minutes, the mixtures were centrifuged at 2000g for 10 minutes, and the chloroform layer was transferred to another tube, dried under gentle nitrogen blow, and subjected to hydrolysis using 1 mL of 1 mol/L sodium hydroxide at room temperature for 90 minutes. The reaction mixtures were adjusted to pH 4 with 1 mol/L of HCl and extracted with 2 mL of ethyl acetate twice.
The urine samples (2 mL each) with internal standards added were first incubated with 0.2 mg of β-glucuronidase from Escherichia coli (in 0.075 mol/L of potassium phosphate buffer [pH 6.8] containing 1 g/L of BSA) for 2 hours at 37°C, and the samples were then acidified to pH 6 with 10% acetic acid.24 The treated urine samples were applied to preconditioned Bond Elut-Certify II columns (Varian), washed with 2 mL of methanol/water (1:1), and then collected with 2 mL of hexane/ethyl acetate/acetic acid (75:25:1) for eicosanoid analysis.
The combined ethyl acetate extracts for each sample were dried and dissolved in methanol for high-performance liquid chromatography separation and gas chromatography/mass spectrometry analyses as described.25 The ions m/z of 319 and 327 were monitored for endogenous and deuterium (d8)-labeled EETs, the ions m/z of 391 and 397 were monitored for 20-HETE, and the ions m/z of 481 and 489 were monitored for DHETs. The amounts of eicosanoids were calculated from their peak area ratios according to standard curves, respectively. Three plasma samples of each group were analyzed by liquid chromatography/mass spectrometry to estimate the EET composition as described.25
Additional Laboratory Tests
PRA was measured using a radioimmunoassay to quantify the amount of angiotensin I generated during 1 to 3 hours of incubation of plasma at 37°C and pH 5.7. The sensitivity of the assay was 0.25 ng/mL per hour, and its interassay variability was <11%.26 Plasma homocysteine was measured by high-performance liquid chromatography. Tumor necrosis factor-α was measured by enzyme immunoassay (R&D Systems). The biochemical profile of the studied subjects was determined using a Technicon DAX 96 automated analyzer (Technicon Instruments).
Statistical Analysis
The Kruskall-Wallis test was used to compare variables in the 3 groups of subjects. The Dunn test was applied to posthoc pairwise comparisons. The Fisher's exact test was also applied when appropriate. Spearman coefficient (rs) was calculated to quantify correlation between variables. P<0.05 was considered statistically significant. Data are expressed as the median and range in the text and as individual values in the figure, unless differently indicated.
Results
The baseline characteristics of the 3 groups of subjects are detailed in Table S1. No statistically significant differences were observed in lipid profile, plasma glucose, and inflammatory markers displayed in Table S1. Systolic and diastolic blood pressures were not different in the 3 groups of subjects; neither were the hemodynamic and vascular profiles shown in Table S2, except for plaques of the internal carotid arteries, more frequently observed in RVD.
Hypertensive subjects with RVD differed significantly from EH and control subjects in having a higher plasma creatinine (RVD: 109.17 μmol/L; range: 71.6 to 163.5 μmol/L; EH: 80.8 μmol/L; range: 52.1 to 95.4 μmol/L; control subjects: 67.6 μmol/L; range: 60.0 to 102.5 μmol/L; P<0.05, RVD versus control subjects) and a lower creatinine clearance (RVD: 50.1 mL/min; range: 29.3 to 168.7 mL/min; EH: 79.9 mL/min; range: 51.5 to 134.5 mL/min; control subjects: 84.8 mL/min; range: 42.0 to 170.3 mL/min; P<0.05, RVD versus control subjects; Table S1). PRA was not significantly different in the 3 groups and was not significantly lower in RVD and EH patients receiving β-blockers than in patients receiving other antihypertensive drugs. However, PRA exceeded 0.5 ng/mL per hour in 6 subjects with RVD but only in 2 with EH and 1 normotensive subject.
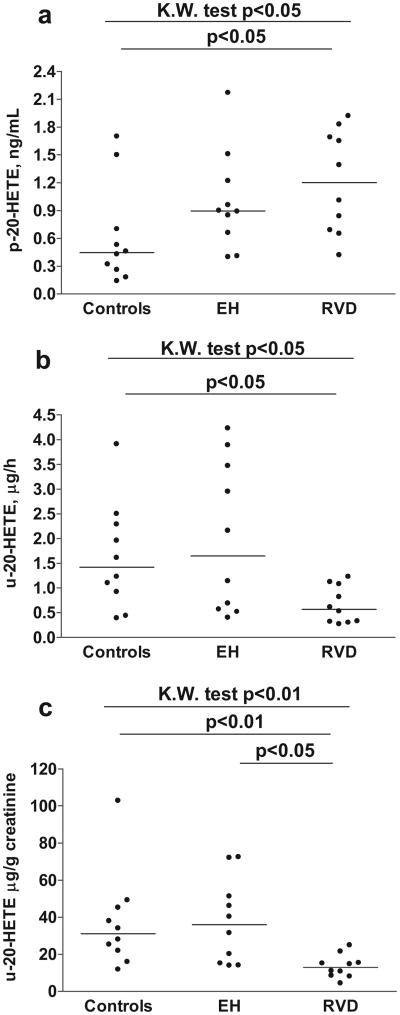
Plasma and urinary levels of 20-HETE were significantly different among the 3 groups; subjects with RVD demonstrated the highest plasma and the lowest urinary values of 20-HETE (Figure 1A and 1B). Plasma 20-HETE in control subjects was one third that of RVD, whereas urinary excretion of 20-HETE in control subjects was 2- to 3-fold higher. The urinary excretion of 20-HETE was reduced in patients with RVD whether expressed as micrograms per hour (Figure 1B) or when factored for urinary excretion of creatinine (Figure 1C).
Figure 1.
Statistically significant differences were observed in plasma concentration (A) and urinary excretion (B and C) of 20-HETE between patients with RVD and healthy control subjects. n.s. indicates not significant.
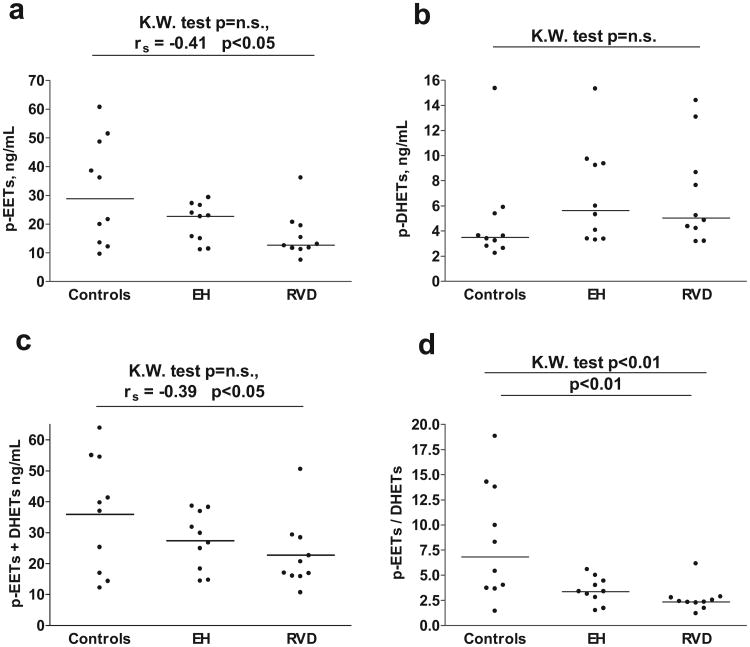
Plasma EET concentrations were significantly correlated with hypertensive status (rs=0.41; P=0.026; Figure 2A): the lowest value occurred in subjects with RVD (7.39 ng/mL) and the highest in control subjects (60.60 ng/mL). An estimation of the relative total epoxygenase activity of the 3 groups tested is available by obtaining the sum of plasma EETs+DHETs for each group, which was also significantly correlated with hypertensive status; viz, control 32.5; EH 30.4; and RVD 18 ng/mL plasma (rs=0.39; P=0.035; Figure 2C). The measurements of plasma EETs in control and RVD subjects reflect total EETs, ie, free and esterified. Plasma-free EETs were low, accounting for ≤3% of the total according to Karara et al,23 which is in agreement with our findings, because these low levels of EETs were usually below the level of detection (0.5 ng/mL plasma) by gas chromatography/mass spectrometry in control and RVD subjects. The exception was the EH group, which exhibited plasma levels of free EETs between 0.9 and 10 ng/mL of plasma (4.7± 1.1 ng/mL of plasma, mean±SD), perhaps reflecting the reported increase in activity of plasma phospholipase A2 in hypertensive rats.27
Figure 2.
Plasma concentration of EETs (A) and the sum of plasma EETs and plasma DHETs (C), as well as EETs:DHETs ratio (D) were lowest in patients with RVD. Plasma concentrations of DHET (B) were not statistically different in patients with RVD, EH, and healthy controls. n.s. indicates not significant.
Three plasma samples from each group were analyzed by liquid chromatography/mass spectrometry to detect regioisomeric composition of EETs. This analysis failed to discern differences among the 3 groups in their regioisomeric composition; the 14,15-EET predominated and the 11,12-EET was least. The average levels of 14,15-, 11,12-, and 8,9-EETs in the control subjects were 3.9±0.9, 2.0±0.9, and 4.7±1.3 ng/mL, respectively. In EH subjects, the average values were 3.7±0.6, 1.6±0.4, and4.1±0.2 ng/mL, and in RVD subjects,3.3±0.4, 0.6±0.1, and 2.4±0.7 ng/mL. The levels of 5,6-EET cannot be accurately estimated because of its lability.
Plasma concentrations of DHET, a major metabolite of EETs, were not significantly different in the 3 groups of subjects (Figure 2B). The ratio of plasma EETs/plasma DHETs provides an index of the activity of sEH. When the ratio is decreased (Figure 2D), metabolism of EETs is increased, a factor contributing to elevation of blood pressure.15,28 The lowest ratio occurred in subjects with RVD, whereas that of control subjects was 3-fold greater. EETs were undetectable in the urine of almost all of the samples (only 3 samples contained 0.05 to 0.25 ng/mL of EETs), whereas excretion of DHETs was similar in the 3 groups (RVD: 0.07 μg/h; range: 0.02 to 0.22; EH: 0.07 μg/h; range: 0.02 to 0.15 μg/h; control: 0.06 μg/h; range: 0.03 to 0.16 μg/h; please see Figure S1E).
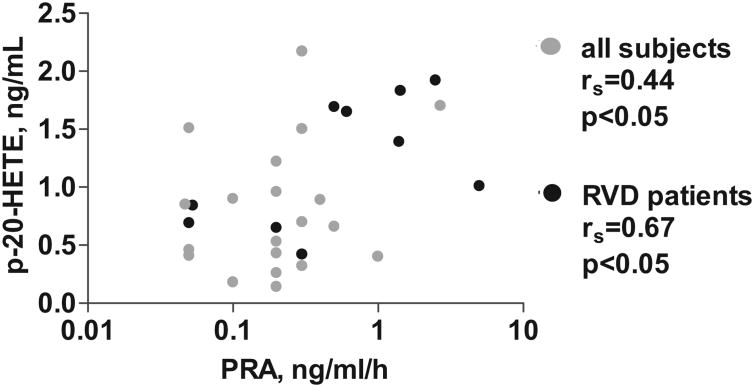
A statistically significant positive correlation between plasma 20-HETE and PRA was observed within the group of patients with RVD, as well as the whole study population (Figure 3). Plasma 20-HETE and plasma DHETs were positively correlated only when all of the subjects were included (rs=0.538; P=0.002; please see Figure S2). Similarly, a positive correlation was observed between plasma 20-HETE and plasma concentration of homocysteine (rs=0.503; P=0.005) and creatinine (rs=0.460; P<0.02).
Figure 3.
Statistically significant correlation was observed between plasma 20-HETE and PRA either when all of the studied subjects were considered (n=30) or when only data from patients with RVD were analyzed. Black dots indicate patients with RVD (n=10).
Discussion
The present study identified differences between subjects with RVD and those with either EH or normal blood pressure (control subjects) in terms of disparities between the 2 groups in their plasma levels and urinary excretion of EETs and 20-HETE and metabolism of EETs to DHETs. Subjects with RVD exhibited increased plasma 20-HETEs, reduced excretion of 20-HETE, decreased plasma EETs, and increased plasma DHETs. The present study does not offer proof of a causal role of these CYP-derived eicosanoid abnormalities in the development of RVD and hypertension. However, because EETs and 20-HETE are essential components of renal vascular and transport mechanisms that regulate blood pressure,29 their alterations in RVD can be conditionally linked to elevation of blood pressure, as the following analysis will attempt to disclose.
Although PRA decreases soon after the development of renal artery stenosis,4 Ang II still represents a primary stimulus via activation of Ang II type 1 receptor for the release of a number of bioactive compounds, including aldosterone, endothelin, and eicosanoids,30 that independently contribute to vasoconstriction, salt and water retention, vascular remodeling, renal fibrosis, and the decline in glomular filtration rate that ultimately determines the progression of the disease.4 Furthermore, studies on 2-kidney, 1-clip renovascular hypertension in rats have shown that blocking the renin-angiotensin system prevented hypertension indefinitely.31 In the present study, PRA correlated positively with plasma levels of 20-HETE in subjects with RVD (Figure 3). Ang II can activate the release of renal 20-HETE by stimulating the synthesis of 20-HETE at critical sites in the kidney: the preglomerular microvessels that determine renovascular resistance7,8,17,32 and a nephron segment, the medullary thick ascending limb, that contributes decisively to salt and water balance.29 The pressor response to increases in circulating Ang II can be attenuated by inhibition of 20-HETE synthesis.7 Furthermore, overexpression of an ω-hydroxylase isozyme, which increased the production of 20-HETE in blood vessels, caused endothelial dysfunction.33
Either deficient or excessive renal production of 20-HETE was reported to elevate blood pressure. In the spontaneously hypertensive rat, increased renal 20-HETE contributes to elevation of blood pressure,34 whereas in the salt-sensitive Dahl rat, reduced 20-HETE synthesis also elevates blood pressure.35 The prohypertensive effects of either increased or decreased renal 20-HETE production depends on the principal site, either a nephron segment such as the TAL (decreased 20-HETE) or renal microvessels (increased 20-HETE) as the afferent arteriole,29 that exhibits altered synthesis of 20-HETE. Increased synthesis of 20-HETE by preglomerular microvessels elevates renovascular resistance, reducing intravascular pressures in the medullary circulation, promoting movement of fluid into the vasa rectae with expansion of extracellular fluid volume and elevation of blood pressure.36 By way of contrast, 20-HETE, which inhibits Na+-K+-2Cl− cotransporter activity in the TAL,37 will promote Na+ reabsorption and elevate blood pressure when deficient, as in the Dahl salt-sensitive rat.38 The 4 isoforms/isozymes of ω-hydroxylase that synthesize 20-HETE29 are differentially localized in the kidney and individually regulated, eg, the 4A8 isoform is regulated by testosterone.39 In addition, catalytic activities of the individual ω- and ω-1-hydroxylase isozymes vary by as much as 40-fold.29
Decreased urinary concentration of 20-HETE (Figure 1B), associated with RVD, may reflect diminished 20-HETE production by nephron segments, the TAL and proximal tubules. As 20-HETE functions in these segments to inhibit Na+ reabsorption,29,37,40 a deficiency will promote Na+ reabsorption and elevate blood pressure as occurs in the Dahl salt-sensitive rat.38 The elevated plasma concentrations of 20-HETE in RVD occurred with decreased urinary excretion of 20-HETE (Figure 1A through 1C) and probably reflects the multiple pathways for disposition of 20-HETE, namely, incorporation of 20-HETE into phospholipids, ω- and β-oxidation, and transformation of 20-HETE via cyclooxygenase into prostaglandin analogs.41,42 In addition, separate sites of origin, vasculature and renal tubules, can account for differences in plasma and urinary levels of 20-HETE. In humans, 20-HETE is excreted primarily as a glucuronide conjugate,43 as we have confirmed in the present study. This pathway of 20-HETE excretion was not found in the rat but is greatly increased in human hepatic cirrhosis.44
EETs in blood are primarily acylated at the sn2 position of phospholipids and capable of being released by phospholipase A2.23 EETs are avidly incorporated into cellular phospholipids and “released at rates exceeding those for arachidonic acid.”45 Endothelial cells are a major site of EET incorporation, a process greatly enhanced by preventing hydrolysis of EETs by sEH,13 because DHETs show negligible incorporation into phospholipids.45 Thus, high levels of unbound EETs in the endothelium13 presumably will increase circulating EETs that are mainly esterified in the phospholipids of circulating lipoproteins23 (Figure 2A).
Plasma concentrations of DHETs are lowest in control subjects (Figure 2B), presumably reflecting decreased activity of sEH contributing to the elevated plasma concentrations of EETs in control subjects (Figure 2A). Vascular-free EETs can be increased by release from endothelial phospholipids; eg, bradykinin stimulates phospholipase activity to increase unbound EETs that exert antipressor effects by relaxing vascular smooth muscle.46 On the other hand, the low levels of tissue/plasma EET concentrations in RVD may result from Ang II upregulating vascular endothelial sEH activity.19 Diminished EET levels will augment 20-HETE vasoconstrictor activity, because 11,12-EET functions as a physiological antagonist of 20-HETE.47 Deficiency of 11,12-EET has also been linked to salt-sensitive hypertension, because 11,12-EET inhibits the epithelial sodium channel in the rat collecting duct.48 Moreover, impairment of 11,12-EET synthesis in mice produced by disruption of the gene-controlling EET synthase caused salt-sensitive hypertension.49
Perspectives
This study supports the hypothesis that CYP-dependent AA metabolism is altered by RVD. Decline in the urinary excretion of 20-HETE in RVD (Figure 2C) was suggested to reflect decreased 20-HETE production by the TAL and proximal tubules that may contribute to increased Na+ reabsorption in these tubular segments.37 Circulating 20-HETE was positively correlated with PRA in RVD (Figure 3) and may contribute to elevation of blood pressure by increasing vasomotion and enhancing vascular reactivity.29 Circulating EETs incorporated into phospholipids achieved plasma concentrations 2- to-3-fold higher in control subjects than in those with RVD (Figure 2A) and serve as sources of unbound EETs mobilized by vasoactive hormones to dilate blood vessels.13 Finally, the reduced ratio of plasma EETs: DHETs (Figure 2C) in RVD and EH subjects; vis a vis control subjects, suggests increased sEH activity that will reduce EET levels and, thereby, decrease antipressor activity in RVD and EH (Figure 2A). In conclusion, the present study sets the stage for further studies to address the involvement of the CYP pathway in primary and secondary forms of hypertension.
Supplementary Material
Acknowledgments
We thank Angela G. Zhu, Magdalena Mamczur, and Maurizio Degan for their technical support in eicosanoid analyses and Gail Anderson for preparation of the article.
Sources of Funding: This study was supported by grants from the Italian Ministry of the University and Scientific Research (cofinanziamento 60%; to P.M. and A.L.), Philip Morris USA Inc and Philip Morris International (to H.J.), National Institutes of Health program project grant 34300 (to J.C.M.) and HL-25394 (to J.C.M.), and European Community's Sixth Framework Program (Eicosanox, LSMH-CT-2004-005033; to P.P.).
Footnotes
Disclosures: None.
References
- 1.McGiff JC, Quilley J. 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr Opin Nephrol Hypertens. 2001;10:231–237. doi: 10.1097/00041552-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Sarkis A, Lopez B, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids in hypertension. Curr Opin Nephrol Hypertens. 2004;13:205–214. doi: 10.1097/00041552-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Elbekai RH, El-Kadi AOS. Cytochrome P450 enzymes: central players in cardiovascular health and disease. Pharmacol Ther. 2006;112:564–587. doi: 10.1016/j.pharmthera.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112:1362–1374. doi: 10.1161/CIRCULATIONAHA.104.492348. [DOI] [PubMed] [Google Scholar]
- 5.Quilley J, McGiff JC. Multiple roles of eicosanoids in blood pressure regulation. In: Lipp GYH, Hall J, editors. Comprehensive Hypertension. Philadelphia, PA: Mosby Elsevier; 2007. pp. 377–395. [Google Scholar]
- 6.Capdevila JH, Wei S, Yan J, Karara A, Jacobson HR, Falck JR, Guengerich FP, DuBois RN. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem. 1992;267:21720–21726. [PubMed] [Google Scholar]
- 7.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 8.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 9.Zou AP, Imig JD, Kaldunski M, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol. 1994;266:F275–F282. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Galicia M, Frohlich B, Roman RJ. Induction of P4504A activity improves pressure-natriuresis in Dahl S rats. Hypertension. 1998;31:232–236. doi: 10.1161/01.hyp.31.1.232. [DOI] [PubMed] [Google Scholar]
- 11.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 12.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci. 2007;28:32–38. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am J Physiol. 1999;277:H2098–H2108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol. 2007;293:F342–F349. doi: 10.1152/ajprenal.00004.2007. [DOI] [PubMed] [Google Scholar]
- 15.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 16.Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys. 2007;47:87–98. doi: 10.1385/cbb:47:1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension. 2003;41:709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 19.Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JYJ, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morganti A, Marana I, Airoldi F, Alberti C, Nador B, Palatresi S. Renovascular hypertension. Clinical and diagnostic clues. Ann Urol (Paris) 1999;33:137–143. [PubMed] [Google Scholar]
- 21.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 22.Salvi P, Lio G, Labat C, Ricci E, Pannier B, Benetos A. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens. 2004;22:2285–2293. doi: 10.1097/00004872-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Karara A, Wei S, Spady D, Swift L, Capdevila JH, Falck JR. Arachidonic acid epoxygenase: structural characterization and quantification of epoxyeicosatrienoates in plasma. Biochem Biophys Res Commun. 1992;182:1320–1325. doi: 10.1016/0006-291x(92)91877-s. [DOI] [PubMed] [Google Scholar]
- 24.Rivera J, Ward N, Hodgson J, Puddey IB, Falck JR, Croft KD. Measurement of 20-hydroxyeicosatetraenoic acid in human urine by gas chromatography-mass spectrometry. Clin Chem. 2004;50:224–226. doi: 10.1373/clinchem.2003.025775. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, McGiff JC, Quilley J, Sacerdoti D, Reddy LM, Falck JR, Zhang F, Lerea KM, Wong PY. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J Biol Chem. 2004;279:36412–36418. doi: 10.1074/jbc.M403962200. [DOI] [PubMed] [Google Scholar]
- 26.Morganti A, Grassi G, Giannattasio C, Bolla G, Turolo L, Saino A, Sala C, Mancia G, Zanchetti A. Effect of angiotensin converting enzyme inhibition on cardiovascular regulation during reflex sympathetic activation in sodium-replete patients with essential hypertension. J Hypertens. 1989;7:825–835. doi: 10.1097/00004872-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendis MR, Dhalla NS. Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol. 2003;19:1525–1536. [PubMed] [Google Scholar]
- 28.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 29.McGiff JC, Quilley J. 20-HETE and the kidney: resolution of old problems and new beginnings. Am J Physiol. 1999;277:R607–R623. doi: 10.1152/ajpregu.1999.277.3.R607. [DOI] [PubMed] [Google Scholar]
- 30.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 31.DeForrest JM, Knappenberger RC, Antonaccio MJ, Ferrone RA, Creekmore JS. Angiotensin II is a necessary component for the development of hypertension in the two kidney, one clip rat. Am J Cardiol. 1982;49:1515–1517. doi: 10.1016/0002-9149(82)90373-3. [DOI] [PubMed] [Google Scholar]
- 32.Chu ZM, Croft KD, Kingsbury DA, Falck JR, Reddy KM, Beilin LJ. Cytochrome P450 metabolites of arachidonic acid may be important mediators in angiotensin II-induced vasoconstriction in the rat mesentery in vivo. Clin Sci (Lond) 2000;98:277–282. [PubMed] [Google Scholar]
- 33.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 34.Xu F, Straub WO, Pak W, Su P, Maier KG, Yu M, Roman RJ, Ortiz De Montellano PR, Kroetz DL. Antihypertensive effect of mechanism-based inhibition of renal arachidonic acid omega-hydroxylase activity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R710–R720. doi: 10.1152/ajpregu.00522.2001. [DOI] [PubMed] [Google Scholar]
- 35.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol. 1994;267:R579–R589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 36.Imig JD, Zou AP, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 37.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 38.Hoagland KM, Maier KG, Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension. 2003;41:697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- 39.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 40.Escalante BA, McGiff JC, Oyekan AO. Role of cytochrome P-450 arachidonate metabolites in endothelin signaling in rat proximal tubule. Am J Physiol Renal Physiol. 2002;282:F144–F150. doi: 10.1152/ajprenal.0064.2001. [DOI] [PubMed] [Google Scholar]
- 41.Cheng MK, McGiff JC, Carroll MA. Renal arterial 20-hydroxyeicosatetraenoic acid levels: regulation by cyclooxygenase. Am J Physiol Renal Physiol. 2003;284:F474–F479. doi: 10.1152/ajprenal.00239.2002. [DOI] [PubMed] [Google Scholar]
- 42.Kaduce TL, Fang X, Harmon SD, Oltman CL, Dellsperger KC, Teesch LM, Gopal VR, Falck JR, Campbell WB, Weintraub NL, Spector AA. 20-hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem. 2004;279:2648–2656. doi: 10.1074/jbc.M306849200. [DOI] [PubMed] [Google Scholar]
- 43.Prakash C, Zhang JY, Falck JR, Chauhan K, Blair IA. 20-Hydroxyeicosatetraenoic acid is excreted as a glucuronide conjugate in human urine. Biochem Biophys Res Commun. 1992;185:728–733. doi: 10.1016/0006-291x(92)91686-k. [DOI] [PubMed] [Google Scholar]
- 44.Sacerdoti D, Balazy M, Angeli P, Gatta A, McGiff JC. Eicosanoid excretion in hepatic cirrhosis. Predominance of 20-HETE. J Clin Invest. 1997;100:1264–1270. doi: 10.1172/JCI119640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem. 1992;267:3686–3690. [PubMed] [Google Scholar]
- 46.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ Res. 1997;81:258–267. doi: 10.1161/01.res.81.2.258. [DOI] [PubMed] [Google Scholar]
- 47.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price EJ, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.