Abstract
Vascular endothelial growth factor receptor-1 (VEGFR-1)/Flt-1 is a transmembrane tyrosine kinase receptor for VEGF-A, VEGF-B, and placental growth factor (PlGF). VEGFR-1 is an enigmatic molecule whose precise role in postnatal angiogenesis remains controversial. Although many postnatal and adult studies have been performed by manipulating VEGFR-1 ligands, including competitive binding by truncated VEGFR-1 protein, neutralization by antibodies, or specific ligand overexpression or knockout, much less is known at the level of the receptor per se, especially in vivo. Perplexingly, while VEGFR-1 negatively regulates endothelial cell differentiation during development, it has been implied in promoting angiogenesis under certain conditions in adult tissues, especially in tumors and ischemic tissues. Additionally, it is unclear how VEGFR-1 is involved in vascular maturation and maintenance of vascular quiescence in adult tissues. To facilitate further investigation, we generated a conditional knockout mouse line for VEGFR-1 and characterized angiogenesis in postnatal and adult mice, including angiogenesis in ischemic myocardium. We discuss these findings in the context of the interplay between VEGF family members and their receptors, and summarize various mouse models in the VEGF pathway.
Keywords: VEGF, Flt-1, VEGFR-1, Flk-1, VEGFR-2, angiogenesis, vasculogenesis
1. Introduction
VEGFR-1 was originally identified by Shibuya and coworkers as fms-like tyrosine kinase, or Flt-1 (1). Subsequent studies demonstrated that Flt-1 was a receptor for vascular endothelial growth factor (VEGF-A) (2). Flt-1, now also known as VEGFR-1, was found to be most strongly expressed in vascular endothelial cells (3). The Vegfr-1 gene contains two alternative polyadenylation sites: one within intron 13 and another after exon 30, the last exon of the gene. The alternative transcripts encode two isoforms: soluble VEGFR-1 (sVEGFR-1) which lacks the transmembrane (TM) and cytoplasmic kinase domains, and a full length transmembrane VEGFR-1 which displays weak kinase activity upon VEGF-A binding (4). A related receptor, fetal liver kinase (Flk-1) was also identified as a VEGF-A receptor and is now commonly referred to as VEGFR-2 (5). Intriguingly, although VEGF-A binds to VEGFR-1 with an approximately ten-fold higher affinity than it does to VEGFR-2, the former interaction only weakly activates VEGFR-1 kinase activity, whereas VEGFR-2 exhibits robust tyrosine kinase activity upon VEGF-A binding (6). In vitro studies indicated that VEGFR-2 but not VEGFR-1 is required for endothelial cell proliferation, migration and survival (7-9).
To assess its biological function, Vegfr-1 null (-) allele was created by replacing the signal peptide coding sequence in exon 1 with E. coli lacZ gene (10). Vegfr-1-/- (flt-1-/-) embryos displayed uncontrolled endothelial cell differentiation, with an overcrowded population of endothelial cells forming severely disorganized vascular patterns, and died in utero at E8.5 (10, 11). In contrast, Vegfa+/- as well as Vegfr-2-/- (flk-1-/-) mutations severely limited endothelial cell differentiation and prevented the formation of vascular structures, resulting in embryonic lethality at E8.5-9.5 (12-15). Consistent with a requirement of VEGFR-2 signaling in vascular development, double knockout of neuropilin-1 and -2, which are VEGF-A co-receptors with roles in facilitating VEGF-A/VEGFR-2 interaction, led to Vegfr-2 null-like vascular defects (16). However, knockout of VEGFR-1 specific ligands including VEGF-B and placental growth factor (PlGF) did not have apparent impacts on embryonic vascular development (17-19).
In contrast to the embryonic lethality of Vegfr-1-/- knockout, targeted deletion of kinase domain alone did not affect vasculogenesis or angiogenesis in either embryos or adult tissues, suggesting that increased endothelial differentiation in Vegfr-1-/- embryos was unlikely due to lack of VEGFR-1 kinase signaling. Instead, a more likely function of VEGFR-1 may be to prevent VEGF-A/VEGFR-2 interaction through a sink-like function mediated by its high affinity binding to VEGF-A (20). Deletion of both VEGFR-1 TM and kinase domains led to in utero death at E8.5-9.0, with few blood vessels present in embryonic and yolk sac tissues (21). This phenotype suggests that the secretion of an additional amount of truncated VEGFR-1 may have further reduced VEGF-A/VEGFR-2 interaction to a level insufficient for normal development. Consistent with this interpretation, a previous study indicated that secreted VEGFR-1 may compete for VEGF-A more effectively than membrane-anchored VEGFR-1 (22). Alternatively, it is also possible that the TM domain in VEGFR-1 may be required for facilitating VEGFR-2 signaling by yet unknown mechanisms (21). Positive regulatory roles for VEGFR-1 signaling have been suggested in other studies as well. For example, loss of PlGF expression was associated with compromised angiogenesis in ischemic myocardium, implying that PlGF-induced VEGFR-1 signaling or heterodimerization with VEGFR-2 may be important for angiogenesis (23).
In addition to the Vegfr-1 knockout studies, many other related studies have been carried out, targeting different VEGF family members, isoforms, or receptors. In Table 1, we present a list of knockout mice in the VEGF pathway, which summarizes main phenotypes associated with different alleles.
Table 1.
Summary of mouse models in the VEGF pathway
| Gene | Type | Genotype | Phenotype | Ref |
|---|---|---|---|---|
| Vegfa | germline heterozygous knockout | Vegfa+/− | Vascular deficiency, embryonic lethality at E9.5 | (12, 13) |
| Vegfa | global conditional | VegfaloxP/loxP/MX-1-Cre | Impaired postnatal growth; some lethality | (31) |
| Vegfa | germline hypomorphic allele | Vegflo/lo | Impaired haematopoietic and endothelial differentiation | (32) |
| Vegfa | Vegfa 164/188 deletion | Vegfa120/120 | Reduced vascular growth, death before post-natal day 14 | (33) |
| Vegfa | Vegfa 120/188 deletion | Vegfa164/164 | Essentially normal | (33) |
| Vegfa | Vegfa 120/164 deletion | Vegfa188/188 | Impaired arterial development, ~half of embryos die in utero | (33) |
| Vegfa | endothelial knockout | Vegfalox/lox/VE-CAD-Cre | Endothelial cell apoptosis, premature postnatal death | (34) |
| Vegfr-1/Flt-1 | germline knockout with lacZ reporter | Flt-1−/− (Flt-1lcz/lcz) | Excessive endothelial cell production, lethal at E8.5 | (10) |
| Vegfr-1/Flt-1 | kinase domain knockout | Flt-1(TK−/−) | No apparent vascular defects | (20) |
| Vegfr-1/Flt-1 | global conditional | Vegf-1flox/flox/Rosa26CreERT2/+ | Increase in postnatal angiogenesis | (24) |
| Vegfr-1/Flt-1 | Knockout in podocytes | Nphs1-Cre+Flt1flox/flox | Reorganization of podocyte cytoskeleton, Kidney failure | (35) |
| Vegfr-1/Flt-1 | germline TM domain knockout | Flt-1TM-TK/TM-TK | Reduced vascular development, death at E8.5 in some embryos | (21) |
| Vegfr-2/Flk-1 | germline knockout with lacZ reporter | Flk-1−/− (Flk-1lcz/lcz) | Lack of vascular development, lethality at E8.5-9.5 | (14) |
| Vegfr-2/Flk-1 | germline Y1173F mutation | Flk-11173F/1173F | Lack of vascular development, death at E8.5-E9.5 | (36) |
| Vegfr-2/Flk-1 | endothelial knockout | Vegfr-2floxed/floxed/Cdh5(PAC)CreERT2T/+ | Reduced sprouting | (37) |
| Vegfr-3/Flt-4 | germline knockout | Vegfr3−/− | Vascular remodeling defects, death at E9.5-10.5 | (38) |
| Vegfr-3/Flt-4 | endothelial knockout | Vegfr-3floxed/floxed/PdgfbiCreT2 | Reduced tip to stalk cell conversion, hyper-sprouting | (39) |
| Plgf | germline knockout | Plgf−/− | Normal development, defective pathological angiogenesis | (19) |
| Vegfb | germline knockout | Vegfb−/− | Modest heart defects; reduced fat uptake | (17, 18, 40) |
| Nrp-1 | germline knockout | Nrp1−/− | Vascular malformation, nerve defects, death at E12.5 -13.5 | (41-43) |
| Nrp-2 | germline knockout | Nrp2−/− | Nerve guidance and innervation defects | (44, 45) |
| Nrp-1/Nrp-2 | germline double knockout | Nrp1−/−/Nrp2−/− | Lack of vascular development, embryonic lethality at E8.5 | (16) |
| Vegfc | germline knockout | Vegfc−/− (lcz) | Lack of lymphatic vessels, death at E15.5-17.5 | (46) |
| Vegfd | germline knockout | Vegfd−/− (lcz) | Essentially normal | (47) |
Recently, we reported that Cre-loxP mediated Vegfr-1 knockout in neonatal and adult mouse tissues led to increased angiogenesis of structurally and functionally normal blood vessels (24). Consistent with elevated angiogenesis, both tip cell formation and endothelial cell proliferation were increased. These changes were at least partially dependent on increased VEGFR-2 abundance and signaling, which may in turn result from increased VEGF-A accessibility in VEGFR-1 deficient tissues. Our findings indicate that a VEGF-A sink function appears to be the predominant role of VEGFR-1 during postnatal angiogenesis.
2. Materials
2.1. Recombinant DNA
VEGFR-1/Flt-1 BAC clone (RPCI-23-412O20, abbreviated as FltBac20)(ResGen, Life Technology, Grand Island, NY, USA) contained a 211,423 base pair (bp) fragment of chromosome 5 in the EcoRI site of the pBACe3.6 vector (25). The FltBac20 fragment starts from 2,755 bp downstream of exon 1, spans the rest of the Vegfr-1 gene (including most of the 25,253 bp long intron 1), and continues into the pomp gene.
E. coli strains (EL250 and EL350) (gifts from N. Copeland, NIH, Bethesda, MD, USA (26))
pL253 (gifts from N. Copeland (26)).
Molecular biology reagents, including competent cells, restriction enzymes, dNTPs, Taq polymerase, T4 DNA ligase and Klenow fragment, Pfu Turbo.
Gel extraction kits (Qiagen, Germantown, MD, USA).
Oligonucleotide primers.
2.2. ES cell culture
R1 embryonic stem (ES) cells from 129/S6 strains (gift from A. Nagy, Samuel Lunenfeld Research Institute, Toronto, Canada).
G4 embryonic stem (ES) cells, a hybrid cell line of C57BL/6 and 129/S6 strains (gift from A. Nagy, Samuel Lunenfeld Research Institute, Toronto, Canada).
ES cell media contained DMEM (Life Technologies, Grand Island, NY, USA) supplemented with 15% FBS (STEMCELL Technologies, Vancouver, Canada), streptomycin/penicillin, β-mercaptoethanol and leukemia inhibitory factor.
Mouse embryonic fibroblasts (MEFs).
Geneticin (G418/neomycin).
Ganciclovir.
FLPe expression vector (pCAGGSFLPe, gift from S. Dymecki, Harvard Medical School, Boston, MA, USA).
2.3. Tissue preparation and histology reagents
Embryos and yolk sac membranes.
Glutaraldehyde fixation solution (0.2% glutaraldehyde, 5 mM EGTA (pH 7.3), 2 mM MgCl2, and 0.1 M sodium phosphate (pH 7.3)).
Embryos/yolk sac washing solution: 100 mM phosphate buffer (pH7.3) supplemented with 2 mM MgCl2, 0.01% deoxycholate, and 0.02% Nonidet-P40.
X-Gal staining solution, prepared by 1:25 dilution of 5-bromo-4-chloro-3-indolyl-β-D-galactoside (Biosynth AG, Switzerland) from a 25 mg/ml stock in formamide into 100 mM phosphate buffer (pH7.3) containing 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide.
Ethanol gradient, consisting of a series of ethanol solutions at 70%, 85%, 85%, and 100% of ethanol.
Blocking solution: 1% BSA, 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA) in PBST (phosphate buffered saline (PBS) supplemented with 0.3% Triton X-100).
4% paraformaldehyde.
Zinc fixation solution (0.05% Calcium Acetate, 0.5% Zinc Acetate, 0.5% Zinc Chloride, 100 mM Tris-Cl, pH 7.4).
Antibodies: Anti-α-SMA conjugated to FITC (1:250, Sigma-Aldrich), anti-NG2 (1:300; Millipore), goat anti-rabbit conjugated to Alexa Fluor®-488 (1:300; Molecular Probes, Life Technologies, Grand Island, NY, USA), rat anti-mouse platelet/endothelial cell adhesion molecule (PECAM)-1 (Mec13.3; BD Biosciences, San Jose, CA, USA), biotinylated goat anti-rat IgG-HRP (Jackson ImmunoResearch, West Grove, USA), goat anti-PECAM-1 (M-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), biotinylated donkey anti-goat IgG-HRP (Jackson ImmunoResearch, West Grove, USA).
Isolectin B4 (IB4)-Alexa-594 or IB4-Alexa Fluor®-488 (1:200, Molecular Probes, Life Technologies, Grand Island, NY, USA).
ABC and DAB kits (Vector Labs, Burlingame, CA, USA).
Retinal mounting media (50% glycerol in PBS).
Fisher finest Premium Cover Glasses.
Nail polish (Revlon).
2.4. Mice
All procedures used in handling mice were approved by the Animal Care Committee at the University of Connecticut Health Center in compliance with Animal Welfare Assurance.
CD1 mice (Charles River, Wilmington, MA, USA).
Vegfr-2+/- (Flk-1+/-) mice (Jackson Laboratories, Bar Harbor, ME, USA).
EIIa-Cre transgenic mice (Jackson Laboratories, Bar Harbor, ME, USA).
Rosa26CreERT2 line driving ubiquitous expression of tamoxifen inducible CreERT2 (gift from A. Joyner, NYU).
Tamoxifen (20 mg/ml in corn oil).
3. Methods
3.1. Generation of Vegfr-1 germline null allele
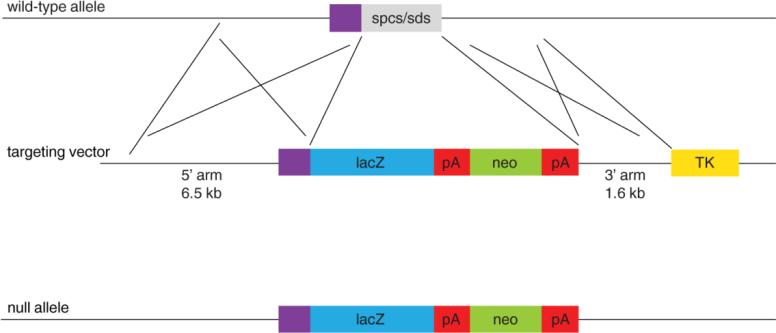
The Vegfr-1 targeting vector was generated by replacing the signal peptide coding sequence in exon 1 with the E. coli lacZ gene (Figure 1). This replacement resulted in the disruption of translational initiation and led to a reading-frame shift in downstream sequences.
The targeting vector was introduced into R1 ES cells by electroporation, and plated out in ES cell media on a layer of neomycin resistance MEFs. Approximately 36 hours later, selection for Geneticin (150 μg/ml) and Ganciclovior (2 μM) resistance was started.
At 7 to 8 days of selection in Geneticin and Ganciclovior containing ES cell media, single colonies were picked, expanded, and duplicated.
DNA was purified from ES cells, digested by PvuII, and analyzed by Southern blotting according to Joyner et al (27). The probe hybridized to a genomic region immediately downstream from the 3’ end the 3’ homology region.
Targeted ES cell clones were identified based on Southern blotting data, and recovered from frozen duplicate of ES cells.
DNA was isolated from recovered cultures, and digested by EcoRV.
Southern blotting was performed using a probe hybridizing to a region immediately upstream to the 5’ end of the 5’ homology arm.
Correctly targeted ES cells were used to generate chimeras by in vitro aggregation with CD1 morula embryos (27).
Germline transmission of the targeted Vegfr-1 allele was achieved by crossing chimeric male with CD1 females.
Figure 1. Flt-1/VEGFR-1 targeting strategy.
A Flt-1/VEGFR-1 targeting vector containing the lacZ (blue box) and neo (green box) genes was used to replace a small fragment (gray box) containing the spcs (signal peptide coding sequence) and the sds (splicing donor site). The targeting vector contained a 6.5 kb 5’ homology arm and 1.6 kb 3’ homology arm. Purple box, 5’ untranslated region; red box, polyadenylation signal (pA); yellow box, thymidine kinase negative selection gene (TK). The resulting targeted allele is shown at the bottom.
3.2. Generation of Vegfr-1 floxed allele
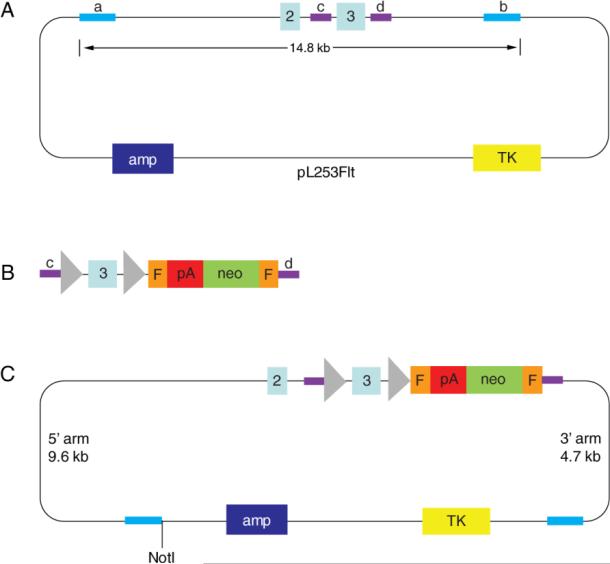
To construct the targeting vector, a 14.8 kb fragment encompassing exon 3 and flanking areas was transferred from FltBac20 into pL253 by γ-Red mediated recombineering (26), resulting in pL253Flt as shown in Figure 2A. Subsequently, a recombination cassette containing floxed exon 3 and Frt-flanked neomycin (Figure 2B) was integrated into pL253Flt to replace exon 3, resulting in the targeting vector as shown in Figure 2C.
The targeting vector and plasmids generated at intermediate steps were sequenced using big dye sequencing through the Molecular Core facility at UConn Health Center, Farmington, CT, USA.
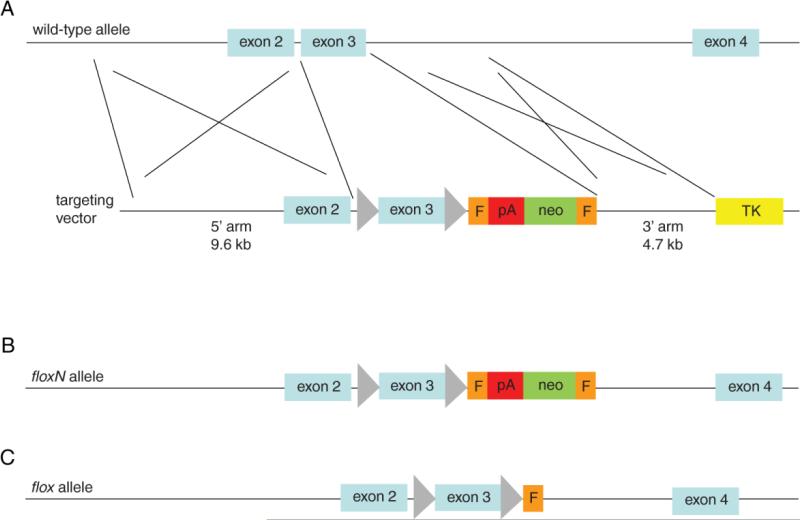
The targeting vector was introduced into G4 ES cells (28) (29) by electroporation to allow homologous recombination as shown in Figure 3A. Methods of ES cell culture and screening are essentially the same as steps 2-7 in section 3.1, except that ES cell DNA was digested by HindIII for Southern blotting with 3’ probe.
ES cells carrying a targeted allele (Figure 3B) were transfected with pCAGGSFLPe, plated out on MEF at several thousand cells per 10 cm plate.
A small fraction of plated cells survived and formed single colonies, which were picked and cultured in 96 well plates, and subsequently duplicated in two 96 well plates, one of which contained Geneticin (100 μg/ml).
ES clones whose Frt flanked neo cassette was deleted by Flpe (Figure 3C) were expanded, and used for in vitro aggregation with wild-type morula embryos. 7. Resultant chimeric mice were crossed with CD1 mice for germline transmission of the floxed allele.
Figure 2. Construction of VEGFR-1 conditional targeting vector by γ-Red-mediated homologous recombination (recombineering).
A. pL253Flt plasmid, consisting of a pL253 plasmid backbone and a 14.8 kb fragment of the Vegfr-1 gene encompassing exons 2 and 3 (labeled light blue boxes) and associated intronic sequences. Navy blue box, ampicillin resistance gene (amp); yellow box, thymidine kinase negative selection marker (TK, ganciclovir resistance); bright blue bars (a and b) represent the short homology sequences where homologous recombination occurred between FltBac20 and pL253-based retrieval vector, with their external ends (relative to exons) marking the 5’ and 3’ ends of the retrieved fragment. Purple bars represent intronic fragments (c, intron 2; d, intron 3) used as homology arms for recombineering with pL253Flt. B. Recombination cassette containing the following elements in the 5’ to 3’ direction: an intron 2 fragment (purple bar, c ), loxP (triangle), ~150 bp sequence from the end of intron 2, exon 3 (light blue box), ~ 150 bp from the beginning of intron 3, a second loxP site, neomycin resistance cassette (green box) with polyadenylation signal (pA, red box) at its end and flanked by a pair of Frt sites (orange boxes), and an intron 3 fragment (purple, d). Purple bars labeled c and d mediate integration into pL253Flt by homologous recombination. C. The VEGFR-1 conditional targeting vector generated by homologous recombination between pL253Flt (shown in A) and the recombination cassette (shown in B). Not I, site of linearization before electroporation into ES cells.
Figure 3. Flt-1/VEGFR-1 conditional knockout targeting strategy.
A. The conditional VEGFR-1 targeting vector contained a 9.6 kb 5’ homology arm, floxed exon 3, an Frt (F, orange boxes) flanked neomycin resistance cassette (green box with a polyadenylation sequence (red box) at its 3’ end), a 4.7 kb 3’ homology arm, thymidine kinase cassette (TK, yellow rectangle), and the vector backbone (not shown). B. floxN allele which is the product of homologous recombination as shown in A. C. flox allele, generated by in vitro removal of the neomycin cassette by transfecting targeted ES cell clones with a Flpe expression vector.
3.3. In vivo validation of the floxed allele
To determine if deletion of floxed exon 3 inactivates the Vegfr-1 gene, Vegfr-1+/flox mice were crossed to EIIaCre mice, resulting in Vegfr-1 +/Δ germline where Δ is an exon 3-deleted allele.
Vegfr-1 +/Δ mice were then crossed with Vegfr-1+/- mice. Embryos were dissected at E8.5, fixed in glutaraldehyde fixation solution, rinsed in embryo washing solution for 3 times (~10 minutes each), and incubated in X-Gal staining solution for up to 4 hours at 37°C. Stained specimens were photographed on Leica dissection microscope outfitted with a CoolSnap digital camera.
X-gal stained embryos and yolk sacs were dehydrated through an ethanol gradient followed by toluene, and then embedded in paraffin. Sections were cut at 5 μm, counterstained with nuclear fast red, and imaged on a Zeiss Axioskop microscope.
3.4. Analysis of angiogenesis in neonatal mice
Vegfr-1flox/flox/Rosa26CreERT2/+ mice were generated by successive rounds of crosses between floxed Vegfr-1 and Rosa26CreERT2/+ mice.
Vegfr-1flox/flox/Rosa26CreERT2/+ and Vegfr-1flox/flox mice were treated with tamoxifen by oral gavage (40 mg/kg body weight).
Retinas were isolated from tamoxifen-treated neonatal mice as follows: Eyes were removed with curved forceps from euthanized mice, rinsed with ice cold phosphate buffered saline (PBS) and then fixed with 4% paraformaldehyde (PFA) for 35 minutes on ice. After rinsing three times with PBS, whole retina cups were isolated, and incubated for 1 hour in blocking solution.
To visualize retinal blood vessels, retinas were stained with IB4-Alexa-594 or IB4-Alexa Fluor®-488 (1:200 dilution in the blocking solution supplemented with 0.5 mM MgCl2 and 0.5 mM CaCl2).
Retinas were also analyzed by immunofluorescence staining with anti-α-SMA conjugated to FITC (1:250) to monitor the recruitment of vascular smooth muscle cells.
To visualize pericytes covering microvessels, retinas were stained with anti-NG2 (1:300) followed by anti-rabbit IgG- Alexa Fluor®-488 (1:300).
Stained retinas were flat-mounted onto glass slides by 4 incomplete radial incisions, placed in mounting media under cover slips, and sealed with nail polish. Mounted retinas were imaged using either Zeiss Pascal or Zeiss 510 confocal microscope system.
Angiogenesis in other tissues such as the heart and liver were evaluated by anti-PECAM-1 (CD31) immunohistochemical staining or IB4-Alexa-594 (1:200). IHC stained slides were counterstained with Haematoxylin QS, and imaged on a Zeiss Axioskop microscope. Immunofluorescence image were taken on a Zeiss Pascal microscope system.
Angiogenic activity was quantified by evaluating the number of branching points located within a defined field of view as well as by calculating percent areas occupied by vascular structures.
3.5. Analysis of angiogenesis in adult tissues
At 6 weeks of age, Vegfr-1flox/flox/Rosa26CreERT2/+ mice and their Vegfr-1flox/flox controls were treated with tamoxifen by oral gavage (40 mg/kg).
Vascular structures were identified by anti-CD31 IHC (for non-retinal tissues) or IB4- Alexa-594 (for retinas) staining, and quantified by counting branching points and percent area occupied by vascular structures.
Vascularization was also evaluated in ischemic myocardium following the ligation of the left anterior descending coronary artery (LAD)(30).
3.6. Mechanisms of angiogenesis
Increased angiogenesis was observed following Rosa26CreERT2-mediated deletion of exon 3 in both neonatal and adult tissues. The mechanisms of increased angiogenesis were investigated by two methods, described in 3.6.2 and 3.6.3.
VEGFR-2 selective inhibitor, SU1498, was injected intraperitoneally, approximately 30mg/kg on a daily basis at alternating injection sites for 14 days between P7 and P20.
A single Vegfr-2– allele was introduced into Vegfr-1flox/flox/Rosa26CreERT2/+ background, and angiogenesis was quantified after tamoxifen-induced Vegfr-1 disruption.
The number of tip cells was compared between floxed controls and mice deficient for VEGFR-1. Tip cells in P5 retinal tissues were identified as IB4 positive cells located at the vascular front, with extensive filopodia, and containing TOPRO3-stained cell nuclei. The observation of tip cells was facilitated by z-stack confocal imaging.
The mechanism of increased tip cell formation was investigated by injecting anti-VEGF-A neutralizing antibody into the vitreous cavity.
Proliferating endothelial cells in P5 retinas were identified by 5’-bromo-2’-deoxyuridine (BrdU) incorporation assay. Neonatal mice were injected with BrdU intraperitoneally at 0.12 g/kg, and euthanized after 90 minutes for retina dissection. Double staining for endothelial cells (IB4-Alexa-594) and proliferating cells (anti-BrdU) was performed as described previously (24). Double positive cells were visualized by confocal imaging.
4. Summary
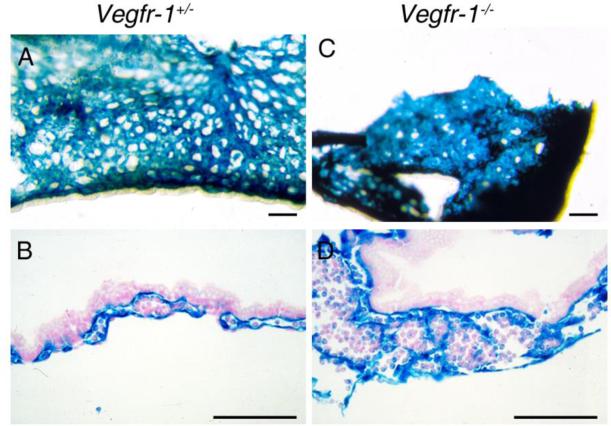
While vascular structures are highly disorganized in germline Vegfr-1-/- or Vegfr-1 -/Δ embryos (Figure 4)(23), somatic knockout of Vegfr-1 in neonatal and adult mice led to elevated angiogenesis (Figure 5), characterized by increased vascular branching points, tip cell formation and endothelial cell proliferation (23). Notably, blood vessels resulting from somatic Vegfr-1 knockout were properly associated with vascular smooth muscle cells and pericytes, maintained normal baseline vascular permeability, and fully supported circulatory function, although vascular permeability was increased in response to excessively high levels of exogenous VEGF-A (24).
Figure 4. X-gal stained Vegfr-1+/- and Vegfr-1-/- yolk sacs.
A and B. Whole mount (A) and histological section (B) of Vegfr-1+/- yolk sac specimens. C and D. Vegfr-1-/- yolk sac images. Note that in Vegfr-1-/- mice, discrete vascular structures are difficult to identify. All scale bars are 100 μm.
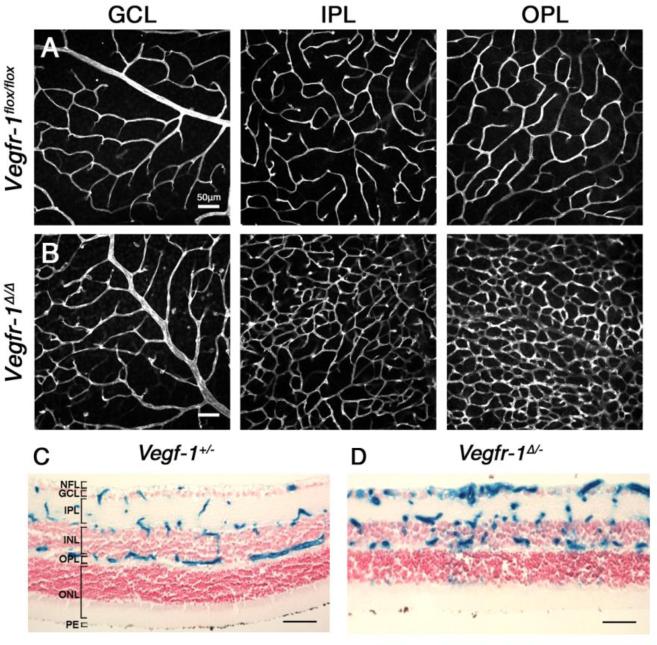
Figure 5. Effects of somatic Vegfr-1 knockout on retinal angiogenesis.
Rows A and B. Vegfr-1flox/flox and Vegfr-1flox/flox/Rosa26CreERT2/+ mice were treated with tamoxifen by oral gavage for 3 days between P1 and P3, and retinal angiogenesis was examined at P21 by IB4-Alexa 594 staining of flat mounted retinas. Vegfr-1Δ/Δ represents tamoxifen-treated Vegfr-1flox/flox/Rosa26CreERT2/+ mice. GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer. C and D. Retinal cross sections from Vegfr-1+/- and Vegfr-1Δ/- neonatal mice, the latter of which was generated by tamoxifen treatment of Vegfr-1flox/-/Rosa26CreERT2/+ mice. Note more abundant presence of X-gal positive vascular structures in D. All scale bars are 50 μm.
In spite of apparently contrasting morphological phenotypes between germline and somatic Vegfr-1 knockout mice, a common feature is increased number of endothelial cells. We hypothesize that different morphological appearances might be a manifestation of distinct tissue contexts in embryonic and postnatal tissues, rather than fundamental differences in molecular and cellular mechanisms.
Molecularly, increased angiogenic activities in somatic Vegfr-1 knockout mice were accompanied by elevated VEGFR-2 protein level and tyrosine phosphorylation without apparent increases in VEGFR-2 mRNA. The hyperactive angiogenesis could be reverted back towards normal levels by manipulations that diminish VEGFR-2 expression and signaling or suppress VEGF-A bioactivity, including reduction of Vegfr-2 gene dosage, injection of VEGFR-2 selective kinase inhibitor, or VEGF-A neutralization by anti-VEGF-A. These findings suggest that increased angiogenesis was at least partially caused by loss of VEGF-A sink function rather than loss of VEGFR-1 kinase signaling activity.
References
- 1.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 2.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 3.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- 4.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 6.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 7.Watanabe Y, Lee SW, Detmar M, Ajioka I, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene. 1997;14:2025–2032. doi: 10.1038/sj.onc.1201033. [DOI] [PubMed] [Google Scholar]
- 8.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 9.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 10.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 11.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 14.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 15.Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 16.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 18.Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 20.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiratsuka S, Nakao K, Nakamura K, Katsuki M, Maru Y, Shibuya M. Membrane fixation of vascular endothelial growth factor receptor 1 ligand-binding domain is important for vasculogenesis and angiogenesis in mice. Mol Cell Biol. 2005;25:346–354. doi: 10.1128/MCB.25.1.346-354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 23.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 24.Ho VC, Duan LJ, Cronin C, Liang BT, Fong GH. Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation. 2012;126:741–752. doi: 10.1161/CIRCULATIONAHA.112.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shizuya H, Birren B, Kim UJ, Mancino V, Slepak T, Tachiiri Y, Simon M. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci U S A. 1992;89:8794–8797. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyner AL. Gene targeting : a practical approach. xviii. Oxford University Press; Oxford ; New York: 2000. p. 293. [Google Scholar]
- 28.George SH, Gertsenstein M, Vintersten K, Korets-Smith E, Murphy J, Stevens ME, Haigh JJ, Nagy A. Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:4455–4460. doi: 10.1073/pnas.0609277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, Nagy A. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 30.Sonin D, Zhou SY, Cronin C, Sonina T, Wu J, Jacobson KA, Pappano A, Liang BT. Role of P2X purinergic receptors in the rescue of ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1191–H1197. doi: 10.1152/ajpheart.00577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 32.Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002;129:1881–1892. doi: 10.1242/dev.129.8.1881. [DOI] [PubMed] [Google Scholar]
- 33.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012;151:384–399. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 38.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 39.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 41.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 42.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 43.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 44.Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, et al. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- 45.Walz A, Rodriguez I, Mombaerts P. Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J Neurosci. 2002;22:4025–4035. doi: 10.1523/JNEUROSCI.22-10-04025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 47.Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25:2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]