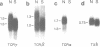Abstract
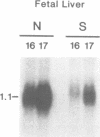
The scid mouse mutant is severely deficient in lymphocytes; cells of the B or T lymphocyte lineage cannot be detected by either serological or functional assays. However, as shown here, germ-line transcripts of B cell immunoglobulin (Ig) constant and variable region genes and of T cell receptor (TCR) genes are detectable in lymphopoietic tissues of scid mice, as well as B and T lineage-specific lambda 5 and T3 delta transcripts. We conclude that B and T lineage-committed cells do arise in scid mice and that their Ig and TCR genes are accessible to enzymes involved in their recombination. This suggests that scid impairs lymphopoiesis at the stage at which antigen receptor genes normally undergo rearrangement.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Yagüe J., Palmer E., Kappler J., Marrack P. Rearrangement of T-cell receptor beta-chain genes during T-cell development. Proc Natl Acad Sci U S A. 1985 May;82(9):2925–2929. doi: 10.1073/pnas.82.9.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Yoshikai Y., Taylor S., Siu G., Hood L., Mak T. W. Identification of a diversity segment of human T-cell receptor beta-chain, and comparison with the analogous murine element. 1984 Sep 27-Oct 3Nature. 311(5984):387–389. doi: 10.1038/311387a0. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Balaton A. M. T-cell receptor and immunoglobulin genes are rearranged together in Abelson virus-transformed pre-B and pre-T cells. Mol Cell Biol. 1987 Jan;7(1):266–272. doi: 10.1128/mcb.7.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Haars R., Kronenberg M., Gallatin W. M., Weissman I. L., Owen F. L., Hood L. Rearrangement and expression of T cell antigen receptor and gamma genes during thymic development. J Exp Med. 1986 Jul 1;164(1):1–24. doi: 10.1084/jem.164.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S., Kimura M., Katsuki M., Hioki K., Nomura T. Correlation of T cell receptor gene rearrangements to T cell surface antigen expression and to serum immunoglobulin level in scid mice. Eur J Immunol. 1987 Oct;17(10):1467–1471. doi: 10.1002/eji.1830171013. [DOI] [PubMed] [Google Scholar]
- Hirayoshi K., Nishikawa S., Kina T., Hatanaka M., Habu S., Nomura T., Katsura Y. Immunoglobulin heavy chain gene diversification in the long-term bone marrow culture of normal mice and mice with severe combined immunodeficiency. Eur J Immunol. 1987 Jul;17(7):1051–1057. doi: 10.1002/eji.1830170723. [DOI] [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Pollok B. A., Atchison M. L., Perry R. P. The coupling between enhancer activity and hypomethylation of kappa immunoglobulin genes is developmentally regulated. Mol Cell Biol. 1988 Feb;8(2):930–937. doi: 10.1128/mcb.8.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Adams J. M. Transcripts of the immunoglobulin C mu gene vary in structure and splicing during lymphoid development. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7400–7404. doi: 10.1073/pnas.77.12.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. 1985 Dec 5;318(6045):475–478. doi: 10.1038/318475a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene. 1980 Jul;10(2):113–129. doi: 10.1016/0378-1119(80)90129-8. [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Haimovich J., Perry R. P. Characterization of productive and sterile transcripts from the immunoglobulin heavy-chain locus: processing of micron and muS mRNA. Mol Cell Biol. 1983 Jul;3(7):1317–1332. doi: 10.1128/mcb.3.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. J., Kelley D. E., Perry R. P. Inducible transcription of the unrearranged kappa constant region locus is a common feature of pre-B cells and does not require DNA or protein synthesis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5305–5309. doi: 10.1073/pnas.82.16.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Berger C. N., Melchers F. Isolation of a cDNA copy of an RNA species expressed in murine pre-B cells. EMBO J. 1986 Sep;5(9):2139–2147. doi: 10.1002/j.1460-2075.1986.tb04477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Siden E., Alt F. W., Shinefeld L., Sato V., Baltimore D. Synthesis of immunoglobulin mu chain gene products precedes synthesis of light chains during B-lymphocyte development. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1823–1827. doi: 10.1073/pnas.78.3.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Weigert M., Coleclough C., Mather E. L., Kelley D. E., Perry R. P. Transcription of the unrearranged mouse C kappa locus: sequence of the initiation region and comparison of activity with a rearranged V kappa-C kappa gene. Cell. 1981 Dec;27(3 Pt 2):593–602. doi: 10.1016/0092-8674(81)90401-3. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Witte P. L., Burrows P. D., Kincade P. W., Cooper M. D. Characterization of B lymphocyte lineage progenitor cells from mice with severe combined immune deficiency disease (SCID) made possible by long term culture. J Immunol. 1987 Apr 15;138(8):2698–2705. [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Borst J., Coligan J. E., Markham A. F., Orkin S., Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. 1984 Nov 29-Dec 5Nature. 312(5993):413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Cho M., Terhorst C. Isolation and characterization of a cDNA clone encoding the murine homologue of the human 20K T3/T-cell receptor glycoprotein. Nature. 1985 Apr 11;314(6011):542–544. doi: 10.1038/314542a0. [DOI] [PubMed] [Google Scholar]