Summary
The human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) establish long-term latent infections associated with diverse human cancers. Viral oncogenesis depends on the ability of the latent viral genome to persist in host nuclei as episomes that express a restricted, yet dynamic pattern of viral genes. Multiple epigenetic events control viral episome generation and maintenance. This Review highlights some of the recent findings on the role of chromatin assembly, histone and DNA modifications, and higher-order chromosome structures that enable gammaherpesvirus to establish stable latent infections that mediate viral pathogenesis.
Introduction
Epstein-Barr Virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) are distinguished among the herpesvirus family for their causal association with several human cancers1, 2. The viral genomes and gene products can be detected in tumour cells of various tissue origins, including lymphomas, carcinomas and sarcomas. In general, gammaherpesvirus oncogenesis correlates with the capacity of viral genomes to persist in dividing cells and to express a limited set of viral genes that drive host cell proliferation and survival3, 4.
Like other herpesviruses, EBV and KSHV can establish latent, non-productive infections (that is, infections during which viral genomes exist in host cells without the production of infectious viral particles). In contrast to other herpesviruses, gammaherpesviruses are particularly adept at establishing stable latent infections in proliferating host cells. During gammaherpesvirus latency, the viral genomes are maintained in the host nuclear compartment as multicopy, non-integrated circular genomes with chromatin structure similar to that of the host chromosome. These latent genomes, referred to as episomes or minichromosomes, have features similar to the host cell chromosome, that allow them to be transcribed and replicated by the host cell machinery. In addition, the viral genome is epigenetically modified, which allows the virus to fine tune its gene expression patterns in response to changes in the host cell environment. Moreover, epigenetic features provide the heritable memory required to maintain a consistent gene expression pattern during multiple divisions of proliferating host cells5, 6.
This Review focuses on recent work highlighting the importance of chromatin assembly, epigenetic modifications and chromatin-organizing factors that control the establishment of gammaherpesvirus latency. The Review highlights some of the key steps of primary viral infection, and considers how each stage of infection may contribute to the building of the latent viral episome. The major events include the assembly of viral chromatin, the patterning of histone post-translational modifications and DNA methylation, and the formation of higher-order chromosome conformations that coordinate gene expression programmes and maintain epigenetic memory during cell division.
Early epigenome establishment
Gammaherpesviruses enter the host cell nucleus as naked linear DNA genomes protected by a viral encoded protein capsid that is delivered to the nuclear compartment (Fig. 1). These early events, including receptor engagement and capsid transport, are likely to set the stage for viral gene expression in the nucleus (Box 1). How the naked, unmodified viral DNA is assembled in the nucleus into a functional circular minichromosome that is competent for programmed gene expression and DNA replication remains poorly understood. The processes of genome circularization and chromatinization are thought to be key regulatory events that are crucial for the establishment of latent infection.
Figure 1. Early events that regulate the establishment of a latent gammaherpesvirus minichromosome.
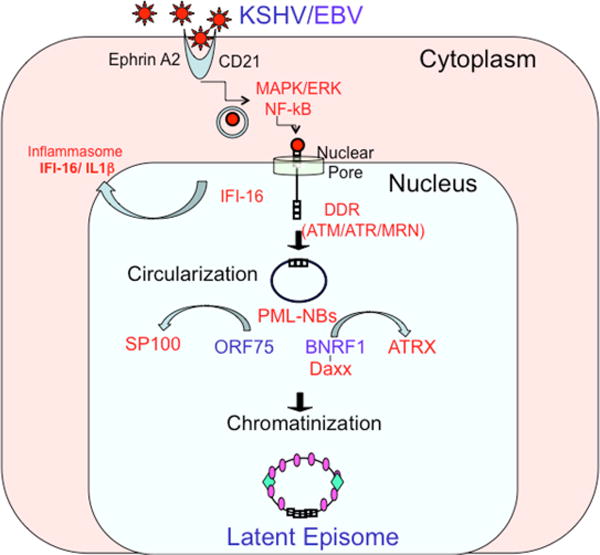
Depiction of the early events affecting the fate of gammaherpesvirus genomes. Receptor-mediated signaling sets the stage for viral gene expression programmes. Entry of the viral DNA into the nucleus activates IFI16, leading to inflammasome activation (in the case of KSHV) and may trigger the DNA damage response (DDR) following recognition by ATM kinase. The linear genome then undergoes circularization and chromatinization, which results in the formation of a stable episome. The viral tegument proteins ORF75 (for KSHV) and BNRF1 (for EBV) have a key role in promoting chromatinization, by disrupting proteins in PML-NBs, for example Daxx and ATRX.
Box 1 | Viral entry and recognition.
The earliest event that can influence the outcome of gammaherpesvirus infection is the interaction of the viral particle with host cell surface receptors (Fig. 1). EBV binding to CD21181 and KSHV binding to ephrin A2182, 183 initiate cell signals, such as PI3K and AKT activation, that can modify nuclear factors which are important for viral gene expression. The viral genomes are then transported to the nuclear pore through a protective viral capsid such that viral DNA is never exposed to cytoplasmic DNA sensors 184. Interaction of the viral capsid with the nuclear pore results in the injection of a single naked viral genome into the nucleus185. How these cytoplasmic events influence the outcome of viral DNA in the nucleus is not yet known, but they likely to be important for the establishment of successful latent infection
In the case of KSHV, one of the earliest nuclear events is the interaction of viral DNA with IFI16186,187, an AIM2-like receptor (ALR) that recognizes double-stranded DNA through its HIN200 domain188. Binding to viral DNA activates the inflammasome, through a mechanism involving the release of nuclear ASC and procaspase 1 into the cytoplasm189; similar IFI16 recognition mechanisms have been described during the early stages of HSV infection190,191. IFI16 has also been shown to associate with the EBV genome at all stages of latent infection, resulting in constitutive induction of the inflammasome192. It is not known how nuclear IFI16 interacts with the viral DNA nor how IFI16 activation of the inflammatory response alters the fate of viral infection. During early HSV infection, IFI16 is degraded by the viral tegument protein ICP0, resulting in a block to this innate immune pathway193. One possibility is that the failure to inactivate IFI16 during KSHV and EBV infection may suggest that IFI16 is important for directing gammaherpesvirus to latency, rather than lytic cycle gene expression.
Viral genome circularization
Genome circularization is likely to be important for protecting viral DNA ends and establishing a genome structure capable of completing the gammaherpesvirus life cycle7. For EBV, circularization is required to generate the templates for the terminal repeat transcripts encoding LMP2a and LMP2b, which promote B cell proliferation and suppress viral lytic cycle reactivation8. For KSHV, circularization is necessary to generate an intact episome maintenance element consisting of multiple tandem copies of the viral terminal repeats (TRs)9. Circularization is thought to be necessary for rolling-circle DNA replication, which is thought to be the conserved mechanism of all herpesvirus lytic cycle DNA replication10. Lytic cycle DNA replication may also be required for the amplification of the viral genome prior to the establishment of latency11. This is consistent with observations that viral lytic gene products are produced transiently during the early stage of primary infection11.
Circularization is an inefficient process and can be detected for only a subset of genomes at 24 hours post infection12. Circularization of the gammaherpesvirus genomes is thought to involve some form of DNA end-processing and homologous recombination followed by ligation. In support of a requirement for DNA end processing is the finding that linear EBV virion DNA has asymmetric end structures - one blunt and one with a single base overhang13 – suggesting that additional end-processing is required for ligation. A role for homologous recombination is based on the finding that terminal repeat copy number changes upon circularization during primary EBV infection14. Genomes that fail to circularize can integrate into the host chromosome, but these integrated genomes frequently lose essential genetic information and viral function12, 15.
Genome circularization might also be necessary for proper chromatinization (see below). Linear genomes are known to activate the host DNA damage response (DDR), which can function as a potent antiviral defense16. The host DNA damage response is tightly coordinated with chromatinization, as it is well established that double-strand breaks are subject to histone modifications, especially phosphorylation of histone H2AX mediated by ataxia-telengiectasia (ATM) kinase to generate γH2AX17. The ATM pathway has been implicated as a control point for gammaherpesvirus primary infection18. Primary EBV infection activates ATM, and inhibitors of ATM increase the number of cells that become immortalized by EBV latent infection. Upon detection of damage, ATM phosphorylates histone H2AX to generate γH2AX and initiate the DNA damage response19. For the mouse gammaherpesvirus MHV68, ATM kinase activity and γH2AX formation support chronic infection20, 21. For KSHV, γH2AX is enriched at the terminal repeats and contributes to episome maintenance20, 22–23. Together, these findings suggest that genome circularization is coordinated with host DDR-dependent histone modifications and chromatin assembly to promote the establishment of latency.
Viral genome chromatinization
Cellular DNA is packaged with proteins known as histones to form chromatin. This condensed configuration, which is mediated by several host factors, ensures that the genome is protected from DNA damage and offers tight regulation of gene expression24. Chromatin assembly after primary infection is likely to be a crucial determinant of gammaherpesvirus fate. Failure to establish the appropriate nucleosome positions and epigenetic modifications may account for variability in viral genome expression. Most infecting gammaherpesvirus linear genomes fail to establish stable episomal infections, and it is possible that improper circularization or chromatinization may account for this high failure rate25, 26.
For alphaherpesviruses, specific histone chaperones (for example, ASF1) and histone variants (for example, H3.3) have been implicated as cofactors in lytic infection8, 27, 28. The role of histone loading factors in gammaherpesvirus infection has not been addressed directly, but may be inferred from the interactions with some viral tegument proteins. All gammaherpesviruses encode a class of tegument protein that targets cellular factors involved in chromatinization. These include the EBV BNRF1, KSHV ORF75, murine herpesvirus 68 (MHV68) ORF75c, and herpesvirus saimiri (HVS) ORF3. Each of these proteins targets components of PML nuclear bodies (PML-NBs, also known as ND10) (Fig. 1), which consist of cellular proteins (PML, SP100, DAXX and ATR) that regulate chromatin assembly and have a central role in the intrinsic resistance to viral nuclear infection29–31. For example, DAXX and ATRX form a protein complex that loads histone variant H3.3 to suppress transcription at repetitive DNA elements32–34. DAXX alone can mediate transcriptional repression through its interaction with histone deacetylases35, 36. Other components of PML NBs, including SP100, have also been implicated in transcriptional regulation of viral genomes37, 38.
Interestingly, the gammaherpesvirus tegument proteins target different components of the PML-NBs through non-conserved regions (despite substantial evolutionary homology elsewhere in the proteins). EBV BNRF1 interacts with DAXX and prevents ATRX localization to PML-NBs39. MHV68 ORF75c and HVS ORF3 interacts with and degrade PML and SP100, respectively40. Destruction of the PML-NBs is thought to be necessary for the completion of herpesvirus lytic cycle gene expression and DNA replication41. However, BNRF1 does not degrade any component of the PML-NBs, but merely prevents ATRX localization and binding. It is therefore tempting to speculate that BNRF1 remodels, but does not destroy PML-NBs to permit latent cycle viral gene expression, but still restrict lytic gene expression.
Control of latent cycle transcription
Human gammaherpesvirus primary infection typically results in a slow, abortive lytic cycle that competes with a more robust latent cycle gene expression programme. Chromatin and other epigenetic features have important roles in regulating the balance between different gene expression programmes. Below I discuss the gene expression programme of EBV and KSHV and consider how chromatin organization is a common regulatory mechanism for each virus.
EBV transcription regulation during latency
EBV can establish at least four distinct latency-associated transcription patterns, referred to as latency types42, 43. The most restrictive transcription programme is latency type 0 (observed in non-cycling, resting memory B cells), during which no viral gene products are synthesized. Type I latency (observed in proliferating memory B cells and Burkitt lymphoma cells) involves the expression of EBNA1 and several non-coding RNAs. Type II latency is defined by the expression of one or several latency membrane proteins (LMP1, LMP2a or LMP2b), in addition to the type I gene products, and this type of latency is observed in Hodgkin’s lymphomas and epithelial cell carcinomas. Type III latency (observed in highly proliferating B cells and immortalized cell lines) is the most permissive for transcription, during which all of the viral genes associated with latency are detected, including EBNA1, EBNA-LP, EBNA2, EBNA3a, EBNA3b, EBNA3c, LMP1, LMP2a and LMP2b.
During the early phase of EBV infection of naive B cells, the first latent viral gene detected is an EBNA2 mRNA, transcription of which initiates at the W repeat promoter (Wp) (Fig 2). EBNA2 is essential for the transcriptional activation of host and viral genes required for B cell proliferation. This is achieved through interactions with the host transcription factors RBP-jK (also known as CBF1 and CSL) and PU.144. As EBNA2 accumulates, it associates with RBP-jK and PU.1 sites on viral promoters. This causes transcription to shift from the Wp to an upstream promoter known as Cp. Transcription that initiates from Cp gives rise to a much larger (~100kB) polycistronic and alternatively spliced transcript that generates EBNA-LP, EBNA2, EBNA3A, EBNA3B, EBNA3C and EBNA1. EBNA2 is also required for transcriptional activation of the LMP1 and the LMP2 promoters, through interactions with RBP-jK or Pu.145, 46. Additional host factors involved in this process include PAX5, which is required for early activation of Wp47, 48, and OCT2, which has been implicated in the activation of Cp49.
Fig. 2. Establishment of the EBV epigenome.
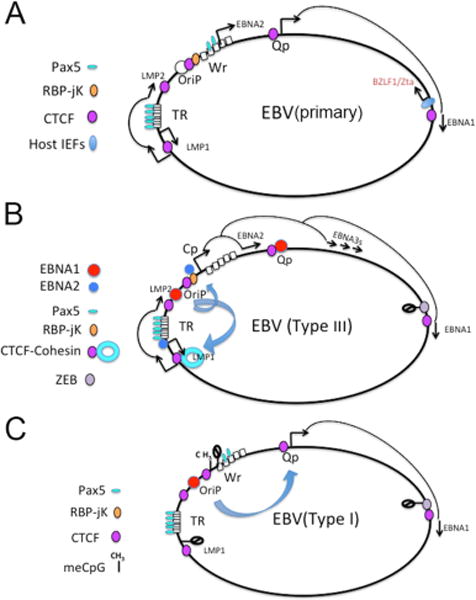
(A) EBV primary infection initiates with transcription from the latent W promoter (Wp) and the lytic Zta promoter. Lower levels of Qp and LMP1 transcripts are also detected. PAX5 and host immediate early factors have an important role in early transcription programming of viral gene expression. (B) Type III latency is established when EBNA2 expression is sufficient to stabilize Cp and LMP1 and LMP2 promoter initiation. EBNA2 binds RBP-jK at Cp and PU.1 at LMP1 promoter. Silencing of the Zta promoter is mediated by repressors, like ZEB. Loop formation is detected between OriP and Cp, and OriP and LMP1/LMP2 region. (C) Type I latency occurs when Cp is silenced by DNA methylation. OriP forms an exclusive loop with Qp, but not Cp
EBV early gene expression is more complicated since genetic studies indicate that EBNA1 is also required for the transcriptional activation of Cp during primary infection of B cells50. This suggests that EBNA1 must be expressed prior to the switch to Cp-initiated transcription50. EBNA1 mRNA is generated from Cp at later times in type III latency, but it is not clear how EBNA1 is generated during the earliest stages of B-cell infection. In type I latency, EBNA1 is expressed from a constitutive promoter, termed Qp. It not known, but tempting to speculate that EBNA1 transcripts during early phase infection may originate from Qp, rather than Wp51–53. Qp has two high-affinity EBNA1 binding sites that result in auto-inhibition by EBNA1 protein, so it is possible that this inhibition of EBNA1 correlates with and may facilitate the switch to Cp-initiated transcription.
The temporal order of transcription events during primary viral infection is therefore not completely clear, and it is possible that Qp-driven EBNA1 and Wp-driven EBNA2 are generated simultaneously and cooperate to reinforce the type III latency programme52. How type III latency evolves into the more restrictive type I or type II latency is also not entirely clear. It is known that in type I latency, Cp-driven transcription is repressed, and only EBNA1 is transcribed by Qp. This promoter switch correlates with epigenetic changes at the Cp and LMP1 promoter, including DNA methylation of critical transcription factor binding sites54, 55. Factors that inhibit EBNA2-mediated transcriptional activation might also have a role in the switch from type III to type I latency.
EBV can also show partial lytic cycle gene activation during de novo infection of primary B cells; this has been referred to as the pre-latency phase56. In the process of establishing a latent infection, EBV transiently expresses some early phase lytic cycle gene products, including the immediate early protein Zta (also known as BZLF1; a DNA-binding protein that activates the transcription of most lytic cycle genes), but virion production is minimal11. Similarly, early, but not late lytic cycle gene transcripts are detected in transcriptome analyses of latently infected lymphoblastoid cell lines (LCLs), suggesting partial lytic cycle gene activity occurs in these cell population57. These findings suggest that partial lytic cycle gene expression is permitted during the establishment phase of latency and in some latently infected cells. Importantly, completion of the lytic cycle can be restricted at multiple levels, including inhibition of viral gene expression58 and protein function59–61(see below).
KSHV transcriptional regulation
KSHV seems to show less variation than EBV with regard to latency transcription, although heterogenous gene expression has been reported in some latency models and tumour-derived cells62. Like EBV, KSHV primary infection typically results in an abortive lytic infection, with infected cells producing low levels of viral particles and primarily expressing the latent transcriptional programme. The major latency-associated transcript consists of a multicistronic message encoding LANA, vCyclin and vFLIP. LANA is the KSHV orthologue of EBNA1, and can bind directly to the terminal repeat DNA to promote DNA replication and episome maintenance during latency63, 64; vCyclin and vFLIP are required for host cell cycle proliferation and survival65. A strong initiator element has been identified at the core promoter for these latency transcripts66, 67. Alternative downstream promoters can initiate a transcript for vFLIP, K12 and a cluster of viral miRNAs68, 69. In addition, the latency proteins vIL6 and vIRF3 are also detected in most models of KSHV latency and in KSHV-infected tumour cells70, 71.
However, some viral genes associated with the lytic cycle can be detected in the context of tumours. For example, in tumour isolates from primary effusion lymphoma, the lytic cycle gene vGPCR (also known as ORF74) can be detected along with latency transcripts72, and sustained vGPCR expression is thought to be crucial for B cell tumogenesis73–76. As vGPCR is typically considered a lytic viral gene product, its expression in tumour cells may reflect an aberrant control of latent infection75.
More recent studies suggest that KSHV can adopt different transcription patterns depending on the host cell type62. In human lymphatic endothelial cells (LECs), the KSHV lytic cycle immediate early genes ORF45 and ORF50 are transcribed along with canonical latency genes (LANA, vCyclin and vFLIP), but other lytic genes are not detected. One phenotypic consequence of this different gene expression programme is that LECs are more sensitive to treatment with rapamycin (an immunosuppressant that activates the growth control protein mammalian target of rapamycin (mTOR), as the KSHV ORF45 protein induces chronic activation of mTOR 62. These findings suggest that KSHV may have different latency types similar to that of EBV, and that both viruses may express some lytic genes without full commitment to lytic cycle DNA replication and viral production.
Ensuring expression of the viral epigenome
As mentioned above, EBV and KSHV can establish stable and distinct transcription programmes during latent infection, which in the case of EBV reflect the different latency types77–80. In many cases, these different latency types have been shown to have correspondingly different epigenetic modification patterns, referred to as ‘epigenotypes’55, 80.
Epigenetic stabilization of latency programmes
DNA methylation patterns have been shown to have a key role in regulating both KSHV81 and EBV latency types 54, 56, 78. DNA methylation, which typically represses gene expression, occurs gradually after primary infection. For EBV, the slow rate of DNA methylation restricts lytic cycle gene activation, as DNA methylation is required for transcription activation of some viral genes by Zta 56, 82–89. Zta is unusual in that it can bind selectively to DNA with methylated cytosine86; in fact, methylation of some viral promoters is necessary for Zta-dependent binding and transcription activation and lytic gene expression89. Thus, the lack of DNA methylation provides a paradoxical restriction to EBV lytic cycle gene expression56, 82. In the case of KSHV, DNA methylation does not occur at constitutively active latency promoters, like the LANA promoter, but instead at several transcriptionally inactive regions. Similarly, in EBV DNA methylation is spared at transcriptionally active latency promoters, as well as other protected sites such as OriP and Qp, which constitutively bind the episome maintenance protein EBNA190. However, DNA methylation has been shown to repress Cp in type I latency, resulting in EBNA2 and EBNA3 silencing54. The mechanisms that determine DNA methylation patterns are not yet understood, although it is possible that some sites are methylated owing to a lack of transcriptional activity (‘methylation by neglect’), whereas others are spared DNA methylation owing to the protective effects of some DNA binding proteins, like EBNA1.
Histone modifications also have a central role in regulating EBV and KSHV latency. Many studies have shown that gammaherpesvirus latency could be disrupted with histone deacetylase inhibitors91. Transcriptional activation of both latent and lytic genes correlate with changes in histone tail modifications at active promoter regions92, 93. These modifications include the well-established histone marks associated with eukaryotic gene activation, namely hyperacetylation of histone H3 and H4 N-terminal tails, and trimethylation of H3 at lysine 4 (H3K4me3)92, 93. More recent genome-wide studies have indicated that EBV and KSHV have complex histone modification patterns during latent infection57, 77, 81, 94–97. The epigenetic landscape of KSHV latent genomes has been examined in several cell types81 and compared with reactivating genomes96. These studies revealed that the promoter region upstream of lytic immediate early gene ORF50 (encoding the lytic activator Rta) is enriched with both activating (H3K4me3) and repressing (H3K27me3) histone modifications81, 96. This ‘bivalent’ control of gene expression is also found at promoters of cellular genes that remain poised for activation during developmental switches98. The small molecule inhibitor of the H3K27me3 methylase EZH2, DZNep, was shown to stimulate KSHV lytic cycle gene activation96, suggesting a role for H3K27me3 in promoting latency. The transcriptional repressive effects of H3K27me3 are known to be mediated by the chromatin modulator Polycomb99, suggesting that these proteins have a central role in restricting the lytic cycle gene programme and chromatin structure of KSHV during latency.
Much of the data collected for the EBV epigenome has been derived from metadata analyses of the ENCODE ChIP-Seq data collection on LCLs containing the EBV B95.8 genome57. The study indicated that type III latency EBV in LCLs has a complex organization of histone modifications, with high enrichment of H3K4me3 at the active promoters for Cp, LMP2A, LMP2 and at the RPMS1/BART promoter regions. In contrast to KSHV, these studies did not show a high level of repressive histone marks at lytic promoters, suggesting that EBV latency is regulated by other mechanisms96.
Chromatin-organizing factors: CTCF and cohesins
Organization of histone modifications and nucleosome positioning is a key regulatory feature of eukaryotic chromosomes100, 101. How this process occurs de novo on newly infecting viral genomes, and how these patterns are maintained during multiple cell divisions is of great relevance to understanding the epigenetic control of gammaherpesvirus latency. At least some of the nucleosome positions and histone tail modifications (see above) are directed by sequence-specific transcription factors and their cofactors. In addition, specialized factors such as CCCTC-binding factor (CTCF) are known to function as chromatin-organizing factors102–104.
CTCF can prevent the spread of repressive or active chromatin from one regulatory domain into another, and can prevent enhancer communication with a specific promoter (acting as an insulator). CTCF can also function in DNA-loop formation, and it is possible that these structural loops serve as the molecular basis for other functions in chromatin boundary and enhancer insulator function105–107. DNA loop formation by CTCF commonly involves another cellular protein complex, termed cohesin. Cohesin is a multiprotein complex that was originally characterized for its role in sister-chromatid cohesion, which keeps newly synthesized chromosomes in close contact in early mitosis and is necessary for faithful chromosome segregation during cell division102, 108, 109. Cohesin has been found to colocalize with CTCF at many cellular chromosomal positions and supports DNA-loop structures, which are important for gene regulation102, 108. Co-occupancy of CTCF and cohesin has also been observed on the gammaherpesvirus episomes during latent infections57, 97, 110.
In the KSHV episome, a cluster of three CTCF binding sites within the first intron of the major latency transcript show strong colocalization with cohesin110. The function of CTCF and cohesin binding within this position seems to be multifactorial. Genetic disruption of the CTCF sites in KSHV bacmids destabilizes episomal maintenance in some cell lines and reduces the efficiency of de novo infection110. CTCF-site mutant genomes show defects in latency transcript regulation, with a shift towards the unspliced form of the multicistronic transcript111. Mutation of CTCF binding sites also disrupts cohesin and RNA polymerase II binding111. The complexity of regulatory events surrounding CTCF binding sites suggests that they have a fundamental structural and organization role in the viral chromosome (Fig. 3).
Fig. 3. Establishment of the KSHV epigenome.
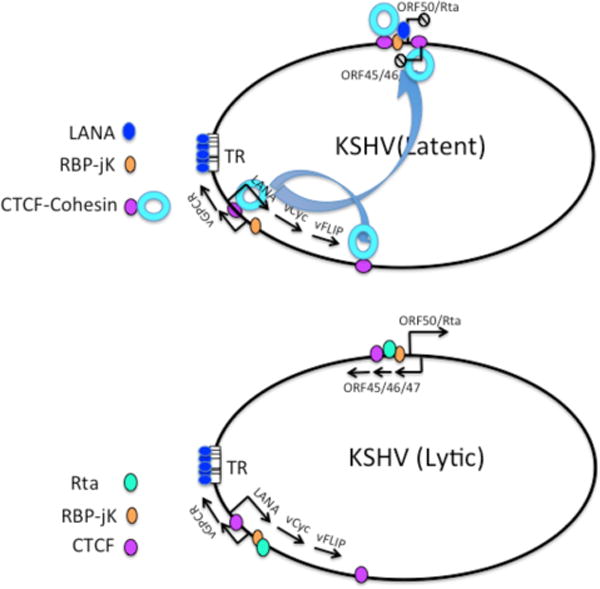
(A) Primary infection of KSHV results in combination of both lytic and latent transcription. (B) Latency is stabilized by a CTCFI-cohesin loop between immediate early promoter region (ORF50–ORF45–46–47) and latency control region (LANA-vCyc-vFLIP). RBP-jK functions as a scaffold for activation by Rta (encoded by ORF50) during lytic and for repression by LANA during latency.
One potential mechanism by which CTCF organizes chromatin may be by disrupting the normal positioning of nucleosomes. At the KSHV latency promoter, the cluster of three CTCF sites prevented the positioning of a second nucleosome downstream of the latency transcript initiation site111. As the first nucleosome downstream of the transcription start site is commonly enriched in modifications associated with RNA polymerase II function (for example, H3K4me3 and H3K9ac)112, it is possible that CTCF-binding sites prevents the processive spreading of these modifications and thereby modulate transcription elongation and mRNA splicing of the complex latency transcripts.
A chromatin boundary function for CTCF has been observed at two regulatory regions in the EBV episome. The CTCF-binding site upstream of Qp protects this core promoter from DNA methylation and consequent transcriptional silencing. This site also blocks the spread of the repressive histone modification H3K9me3 that appears just upstream of Qp90, 113. A CTCF-binding site located between the Cp and the EBNA1-binding sites at OriP can regulate EBNA1-mediated transcription activation of Cp114, 115. Specifically, CTCF binding between OriP and Cp might prevent the unwarranted spread of euchromatic H3K4me3 (which is enriched around OriP in most latency types) into Cp, especially in more restrictive latency types I and II where Cp is repressed.
CTCF-binding sites may also provide chromatin boundary functions at the lytic control region of the KSHV episome, which, as mentioned above, has bivalent histone modifications77, 81. As CTCF-binding sites have been identified at the boundaries of lytic promoter regions, it is possible that CTCF, together with cohesin, might protect this bivalent chromatin organization116. Chromatin-immunoprecipitation (ChiP) assays also suggest that CTCF also functions to retain RNA polymerase II at the lytic promoter in a conformation poised for rapid response to reactivation signals116. Phosphorylated RNA polymerase II (associated with transcription initiation, but not yet competent for elongation) is enriched at the KSHV lytic control region116, so the presence of CTCF might provide a boundary for trapping poised, but not elongating RNA pol II, at this location.
Chromatin conformations regulating viral gene expression
Higher-order chromosome structures, such as promoter-enhancer DNA loop interactions, contribute to the coordinate control of eukaryotic gene expression100. For gammaherpesviruses, it is not know how latent and lytic promoters located tens of kilobases away may coordinately regulate their transcription programmes. DNA loops between transcriptional regulatory elements have been identified in both EBV and KSHV latent genomes. In EBV type III latency, OriP functions as a transcriptional enhancer for both Cp and LMP1 promoters57, 117. In both cases, the physical interaction involves the formation of DNA loops, which are dependent on CTCF-binding sites and cohesin57. Cohesin colocalizes with both CTCF sites, and shRNA-mediated depletion of cohesin subunits disrupts loop formation and deregulates transcription from both promoters. Interestingly, in type I latency, when both Cp and LMP1 and LMP2 promoters are repressed, OriP forms a DNA loop with the active Qp117. This suggests that OriP functions as a transcriptional enhancer that selectively loops with the active promoters for each latency type (Fig. 2).
DNA loops involving OriP may be mediated, in part, by EBNA1, which is known to bind multiple sites within OriP and can form a short DNA loop between these sites within OriP118, 119. The EBNA1 amino-terminal domain is known to have transcription enhancer function, as point mutations in the EBNA1 N-terminal unique region 1 (UR1) disrupt transcriptional activation of Cp50. UR1 was shown to coordinate Zn through a pair of cysteine residues that are essential for EBNA1 homotypic interactions and transcriptional enhancer function120. Furthermore, Zn binding and homotypic interactions were shown to be redox sensitive, suggesting that EBNA1 mediated loop formations are regulated by oxidative stress120. These findings support a model whereby EBNA1 forms a Zn-hook like structure121 that may regulate long-distance interactions required for OriP loop formation and transcription enhancer function.
DNA regulatory loops have also been described for the KSHV episome122. Chromatin conformation capture (3C) studies revealed that the CTCF-cohesin site in the latency control region forms two loops: a short ~10kb loop between the CTCF cluster in the first intron of LANA and the region 3′ of K12, encompassing the major latency transcripts; and a larger loop between the CTCF-cohesin sites and the control region for lytic transcripts of ORF50 and ORF45–46–47122. As noted above, the lytic control region is bracketed by CTCF-binding sites with a poised RNA polymerase II. shRNA-mediated depletion of cohesin subunits leads to a loss of DNA loop structures (as measured by 3C analysis) and a robust stimulation of lytic transcription122, which suggests that the CTCF-cohesin bound to the latency control region function as a repressor of lytic transcription. Thus, CTCF-cohesin mediated loops can function in both activation, as well as repression, of viral transcription.
DNA replication and episome maintenance
Gammaherpesviruses are unique in their ability to maintain a stable copy number of viral episomes in proliferating host cells. This is accomplished through the coordination of viral gene expression, DNA replication and genome segregation. Episome maintenance requires binding of the virus proteins EBNA1 (for EBV) or LANA (for KSHV) to repetitive sequences in either OriP (for EBV) and the terminal repeats (TR; for KSHV)123; thus, these proteins and DNA regions are referred to as episome maintenance elements.
Tethering to host chromosomes
A key feature of episome maintenance is the ability to tether the viral genome to the host metaphase chromosome (Fig. 4A), to retain viral genomes in the nuclear and chromosomal domains during host cell division. Thus, tethering allows gammaherpesviruses to ‘hitch a ride’ on the host chromosome and maintain a stable copy number after host cell division. Both EBNA1 and LANA have peptide motifs that confer metaphase chromosome tethering. EBNA1 tethering is mediated by RGG-like motifs in the EBNA1 N-terminal domain, which interacts with the host cell protein EBP2124–126, with AT-rich DNA127 and with G-quadruplex RNA128. For KSHV, attachment to the metaphase chromosome is mediated by binding of the LANA N-terminal domain to core histones H2A and H2B129, and of its C-terminal domain to host cell chromatin proteins, including the bromodomain proteins BRD2 and BRD4130, 131, which recognize acetylated histones. Together, these observations suggest that in EBV and KSHV tethering is mediated through multiple interactions with host chromatin.
Fig. 4. Episome Maintenance and Viral Chromosome Structure.
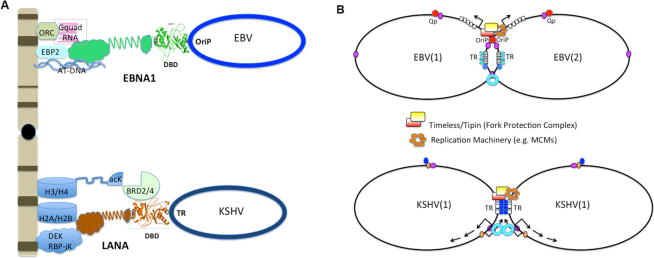
(A) Both EBNA1 and LANA tether the viral episomes to metaphase chromosomes. (Top) The tethering domains of EBNA1 are RGG-like and capable of binding to G-quadruplex RNA and ORC, EBP2, and AT-rich DNA. (Bottom) The N-terminal tethering domain of LANA interacts with histones H2A/H2B. LANA can also interact with other candidate chromatin mediators, including DEK, RBP-jK, and BRD2/4 proteins capable of binding acetylated lysines in the H3 or H4 N-terminal tails. (B) Both EBV and KSHV form sister chromatid catenations that depend on replication fork stalling or terminaton at the episome maintenance elements. EBV OriP and KSHV TR function as a replication fork blocks that recruit Timeless-Tipin replication fork protection complex and promote sister-chromatid linkages (catenations). Timeless and Tipin are essential for episome maintenance, and may be required for the establishment of stable epigenomes.
Promoting DNA replication
The gammaherpesvirus episome maintenance elements can also function as origins of DNA replication. Both EBNA1 and LANA can interact with the host replication factors, including the origin recognition complex (ORC) and replication protein A (RPA) to facilitate efficient replication origin formation. The precise mechanism of how EBNA1 and LANA stimulate origin formation remains poorly understood. EBNA1 and LANA can interact with ORC subunits, and the BAH domain of ORC1 has been implicated in EBNA1 binding132. The EBNA1 RGG-motifs are required for replication function, and this correlates with ORC recruitment. In addition, G-quadruplex RNA has been shown to stimulate the interaction between EBNA1 RGG-motifs and ORC133.
Although viral episome maintenance elements such as EBV OriP and KSHV TR can act as efficient replication origins, their function depends on poorly understood epigenetic features134. Histone acetylation and enrichment of H3K4 trimethylation are known to influence origin selection on host chromosomes135,136. Similar modifications are observed at gammaherpesvirus episome maintenance elements in cells with established latent infections137, 138, but this may not be sufficient for replication initiation, as single molecule studies indicate that DNA replication can initiate at sites outside of these episome maintenance elements139–143. Thus, replication initiation may not be a primary essential function of episome maintenance elements.
Partitioning the viral genome during cell division
The precise mechanism through which gammaherpesviruses partition their genomes to maintain a stable copy number is not yet known. Studies have shown that partitioning is non-random and coupled to DNA replication144. One potential mechanism for active partitioning may involve the formation of DNA catenations between newly replicated DNA molecules145–148. Two-dimensional agarose gel analyses reveal that DNA catenation-like structures form specifically at OriP (in EBV) and TR (in KSHV)145, 146, 146. DNA catenations are thought to provide a mechanism to attach newly replicated sister chromosomes to each other, similar to sister chromatid cohesion for cellular chromosomes. This mechanism has been shown to be important for plasmid partitioning in yeast and bacteria, and has been referred to as ‘chromosome kissing’150. Both EBNA1 and LANA may induce DNA catenation formation at OriP and TR by perturbing DNA polymerase and replication fork structure145–148. Evidence to support this model is provided by studies that show that host replication fork protection proteins, Timeless and Tipin, colocalize with OriP and provide essential functions in EBV and KSHV episome maintenance145, 146. These studies suggest that gammaherpesvirus episome maintenance elements can form replication-dependent catenated structures that are required for faithful segregation of viral episomes during cell division. (Fig. 4B).
Reactivation and virus production
Reactivation from latency is required for the completion of the gammaherpesvirus life cycle and to produce new infectious viral particles. Reactivation can be stimulated by various stress responses, ranging from the unfolded protein response to hypoxia, as well as cell differentiation signals. These pathways typically converge by activating transcription of the immediate early genes, which can recruit numerous host cell regulators that modulate viral chromatin structure and function (for reviews see151–156).
Disruption of repressive chromatin may be an essential pathway for gammaherpesvirus reactivation. The viral immediate early proteins Zta (for EBV) and Rta (for both EBV and KSHV) are required for transcription activation of lytic cycle genes157. Zta and Rta can associate with histone acetyltransferases and chromatin remodeling proteins to stimulate transcription of chromatinized viral episomes158,159. KSHV Rta can function as a E3 ubiquitin ligase to degrade transcriptional repressor complexes, like K-RBP and Hey1, that block KSHV lytic cycle transcription160, 161. In addition to these viral immediate early proteins, recent studies have revealed that viral non-coding RNAs can also regulate the lytic switch. The KSHV polyadenylated nuclear non-coding RNA PAN can facilitate lytic cycle gene expression by disrupting polycomb-mediated mediated chromatin repression. PAN RNA was found to bind and recruit the histone H3K27 demethylases UTX and JMJD3 to reverse the polycomb-mediated repression of KSHV immediate early transcripts162. Interestingly, polycomb repression is also the target of the EBV-encoded EBNA3C, but in this case, EBNA3C promotes polycomb repression on host tumor suppressor genes163, 164. It is not yet known whether EBNA3C recruits H3K27 methylases and polycomb to repress viral lytic genes, and thus stabilize latency.
One of the emerging themes in the regulation of gammaherpesvirus reactivation is that factors that promote latency can also repress lytic reactivation. For example, loss of EBNA1 or LANA increases viral lytic cycle gene expression, suggesting that these latency maintenance proteins also repress lytic gene expression165–167. LANA functions as a transcriptional repressor that can interact with RBP-jK sites at lytic promoters, including the promoters for immediate early gene transcripts168, 169. EBNA1 can also repress lytic gene transcription during latency, since its depletion leads to lytic cycle activation170. However, the mechanism for EBNA1 transcriptional repression of EBV lytic gene transcription is not yet understood. (Fig. 5)
Fig. 5. Chromosome Control of Lytic Reactivation.
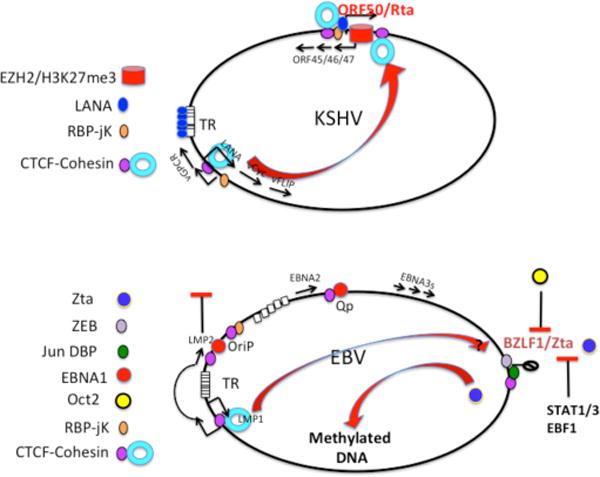
(A) Factors that restrict lytic replication of KSHV include the histone H3K27 trimethylase EZH2 and polycomb repression complex that prevents the transcription of the ORF50/Rta immediate early gene. Additional controls include the CTCF-cohesin binding sites upstream and downstream of the ORF50 IE promoter control region, and the cohesin linkage between IE and lytic control regions. (B) EBV lytic control is provided by host-cell factors that repress the BZLF1/Zta IE control region, including ZEB, Jun dimer binding protein (Jun DBP), and CTCF. A cohesin loop between the Zta IE region and the LMP1/2 control region may repress lytic, similar to that observed in KSHV. Additional restrictions to lytic activation include the repressive interaction of Zta with Oct2, and the requirement for methylated DNA at Zta responsive promoters. Stat1/2 and EBF1 have also been implicated in restricting lytic cycle reactivation.
Many other factors contribute to the balance between latent and lytic gene expression. Since gammaherpesviruses encode numerous miRNAs, it is not suprising that one of the functions of these non-coding RNAs is to stabilize latency by maintaining repressive epigenetic marks. For example, the KSHV miRNA K12–5 was shown to prevent lytic cycle gene expression by increasing global viral and cellular DNA methylation levels171. This was achieved through downregulation of the host protein RBL2, which represses the DNA methyltransferase DNMT3b171..
Heterogeneity of genomes and host cells is also an important consideration in gammaherpesvirus gene regulation. It is well known that gammaherpesvirus reactivation from latency is stochastic and multifactorial172, as only a subset of cells and genomes may respond to an activation signal. Global epigenetic regulators such as HDAC inhibitors and demethylating agents and histone methylase inhibitors can stimulate partial lytic reactivation, and the extent of reactivation varies among cell and latency types. The refractory nature of viral latency in some cell types has been difficult to explain, and remains a challenge for lytic therapies, during which some cells fail to respond to a reactivation signal. In one study the refractory cells showed high levels of STAT3 expression173, 174, whereas in another study the block to reactivation correlated with increased levels of EBF1175. Several additional host proteins, including the repressor ZEB58, 176, 177, Jun dimerization protein178 and OCT260, have been shown to block lytic reactivation. In addition to these, viral immediate early proteins can be inhibited by post-translational modifications59 and by epigenetic modifications of the viral genome179. Taken together, these studies suggest that combinatorial control and epigenetic variations of the viral genome may explain the sporadic and stochastic process of reactivation from latency.
Conclusions
Gammaherepesvirus latency is a complex and sophisticated form of genetic parasitism that involves the formation of a stable mini-chromosome that is highly responsive to the host cell environment and developmental status.
The establishment of latency involves a competition between lytic gene expression and a more dominating class of latency gene products. Stable latent infection depends on the acquisition of several epigenetic features, including circularization, chromatinization and post-translational modifications of histones and DNA. In addition, higher-order chromatin structures, such as DNA loops and catenations, may have a role in gene regulation and episome maintenance. These epigenetic features are necessary for stable gene expression programmes and faithful transmission of viral genomes to daughter host cells.
Despite the enormous wealth of information on gammaherpesvirus latency, there are considerable gaps in our knowledge of how latency is established and maintained. For instance, it is not yet known what host cell factors are primarily responsible for the restriction of gammaherpesvirus lytic gene expression during primary infection. We also do not know what epigenetic events are principle drivers of viral latency. Although we know that the formation of a stable viral episome involves nucleosome assembly and histone modifications, it remains unclear how nucleosome position and histone modification patterns are established on the newly infecting viral genomes, or how these patterns of chromatin organization are maintained over cell division cycles.
It will also be important to determine how higher-order chromosome conformations are established and how these structures facilitate interactions between enhancers, like OriP, and the appropriate promoter elements selected for transcription activation, like Cp or Qp.
How the viral episomes are replicated and segregated during each cell cycle may also be subject to important epigenetic control, including the formation of DNA catenations that promote sister chromatid cohesion after DNA replication. Whether these epigenetic factors allow the gammaherpesvirus genomes to survive as stable episomes and maintain a stable copy number in proliferating cells is an important unanswered question.
Finally, the mechanism of gammaherpesvirus persistence in cancer cells may be different from that in normal cells180. Abberations in the prototypical epigenetic programmes may account for the rare incidence of virus-associated tumour formation. At present, we do not know whether specific epigenetic modifications correlate with cancer cells and whether these are inherently different than latency associated with normal, non-malignant cells. Understanding the detailed mechanisms of each of these processes discussed in this Review, and their potential aberrations in virus-associated cancers may provide insights into the oncogenic potential of gammaherpesvirus latency, and may provide novel strategies for therapeutic interventions that target latent infection and viral carcinogenesis.
OnLine Summary.
-Gammaherpesvirus latency is a programmed event with multiple epigenetic steps
-During latency, the viral genome establishes a meta-stable epigenetic pattern of histone modifications and DNA methylation.
-Epigenetic patterns and chromosome conformations are stabilized by host chromatin boundary factors CTCF and cohesin.
-Lytic reactivation is a stochastic process controlled by multiple epigenetic barriers.
Glossary
- histone deacetylase
Histone deacetylases (HDAC) are a family of enzymes that remove an acetyl group from lysines on histone tails. HDACs typically promote “closed” or repressive chromatin, and reverse the action of histone acetylases that promote “open” chromatin, Small molecule inhibitors of HDACs, like sodium butyrate, trichostatin A, and valproic acid, are commonly used to reactivate latent gammaherpesviruses
- Bacmids
Recombinant gammaherpesvirus genomes can be propagated as large bacterial plasmids referred to as Bacmids. Bacmids have antibiotic selection resistance and fluorescent protein markers that greatly facilitate the engineering of site-directed mutations in gammaherpersvirus genomes. Mutations are made when Bacmids are grown in recombinogenic strains of E. coli, and then passaged into mammalian host cells where infectious recombinant virus can be generated
- Euchromatic
Euchromatic refers to regions of “open” chromatin that are more accessible to DNA binding proteins and transcription initiation. Euchromatic chromatin (euchromatin) tends to be enriched in acetylated histones and di and tri-methylation of histone H3 lysine 4 (H3K4me2 and H3K4me3). In contrast, heterochromatin refers to “closed” chromatin that is less accessible to transcription factors. Heterochromatin tends to be enriched in trimethylation of histone H3K9me3 (constitutive heterochromatin) and H3K27me3 (facultative heterochromatin)
- Zn hook
Some proteins, like EBNA1, can bind to Zn through an interaction with two amino acids, like cysteine and histidine. A Zn hook occurs when Zn mediates the interaction between two different molecules of the same protein through homotypic interactions, such that four amino acids (two from each protein monomer) form a cage to coordinate with a Zn atom at its center
- Chromatin conformation capture (3C)
Chromatin conformation capture is a method developed by Job Dekker to measure the interaction between different DNA sites on the same or different chromosomes in vivo. The method involves cross-linking, restriction enzyme digestion, dilution, and relegation of cross-linked molecules. The method determines whether two different DNA regions are in close proximity to each other in vivo, and is used to show that promoters and enhancers form interactions through DNA looping
- G-quadruplex
G-quadruplex (or G-quartets or G4-DNA) are DNA or RNA structures that can form in guanine rich stretches where four guanine molecules can form a planar structure that can be stacked to form higher-order stable structures. G-quadruplexes can form between one (intramolecular) or more (intermolecular) DNA or RNA molecules. Repetitive G-rich sequences like telomere DNA and RNA (TERRA) can form G-quadruplex structures. EBNA1 RGG-motif can bind selectively to G-quadruplex RNA
- DNA catenations
DNA catenations can form when DNA strands are entangled, as occurs at when two replication forks collide to terminate DNA replication. Most DNA catenations can be decatenated by topoisomerases. Some catenations, including hemi-catenanes, form when the newly-replicated DNA strands are entangled. Catenated, and possibly hemicatenated, structures have been observed at the EBV and KSHV episome maintenance elements
- unfolded protein response
The unfolded protein response (UPR) is a stress response that occurs in the endoplasmic reticulum (ER) when numerous proteins are misfolded or the ER is overwhelmed, as occurs during viral infection and during immunoglobulin production in plasma B cells. The XPB-1 protein is a central regulator of the UPR and has been implicated in the reactivation of both EBV and KSHV from latency
- Hypoxia
Hypoxia refers to a condition of reduced oxygen which can typically occur in tumor cells lacking sufficient oxygenation. Hypoxia is sensed by the hypoxia-inducible factor 1 (HIF1) that can activate transcription in response to low oxygen due to chemical modification of proline residues in HIF1. HIF1 has been shown to play important roles in both EBV and KSHV reactivation from latency
- TOC blurb
EBV and KSHV establish latent infections, during which the viral genomes are maintained in the host cell as viral episomes. As discussed by Lieberman, latency depends on numerous factors, including viral genome chromatinization and epigenetic modification, as well as tight control of the latency transcription programme
Biography
Paul M. Lieberman is Professor in the Gene Expression and Regulation Program at the Wistar Institute. He is also the Director of the Center for Chemical Biology and Translational Medicine at the Wistar Institute. His recent work focuses on the chromosome biology of EBV and KSHV latency, and the development of small molecule regulators of latent virus infection.
Footnotes
Note: We apologize to our many colleagues for not citing numerous related studies due to manuscript space limitations.
References
- 1.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nature reviews Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Wen KW, Damania B. Kaposi sarcoma-associated herpesvirus (KSHV): Molecular biology and oncogenesis. Cancer letters. 2010;289:140–150. doi: 10.1016/j.canlet.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorley-Lawson DA, Hawkins JB, Tracy SI, Shapiro M. The pathogenesis of Epstein-Barr virus persistent infection. Current opinion in virology. 2013 doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nature reviews Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner BM. Epigenetic responses to environmental change and their evolutionary implications. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2009;364:3403–3418. doi: 10.1098/rstb.2009.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Deng Z, Wang Z, Lieberman PM. Telomeres and viruses: common themes of genome maintenance. Frontiers in oncology. 2012;2:201. doi: 10.3389/fonc.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Placek BJ, et al. The histone variant H3.3 regulates gene expression during lytic infection with herpes simplex virus type 1. Journal of virology. 2009;83:1416–1421. doi: 10.1128/JVI.01276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 10.Strang BL, Stow ND. Circularization of the herpes simplex virus type 1 genome upon lytic infection. Journal of virology. 2005;79:12487–12494. doi: 10.1128/JVI.79.19.12487-12494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalla M, Hammerschmidt W. Human B cells on their route to latent infection–early but transient expression of lytic genes of Epstein-Barr virus. European journal of cell’ biology. 2011;91:65–69. doi: 10.1016/j.ejcb.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Hurley EA, et al. When Epstein-Barr virus persistently infects B-cell lines, it frequently integrates. Journal of virology. 1991;65:1245–1254. doi: 10.1128/jvi.65.3.1245-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann J, Hammerschmidt W. Structure and role of the terminal repeats of Epstein-Barr virus in processing and packaging of virion DNA. Journal of virology. 1995;69:3147–3155. doi: 10.1128/jvi.69.5.3147-3155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kintner CR, Sugden B. The structure of the termini of the DNA of Epstein-Barr virus. Cell. 1979;17:661–671. doi: 10.1016/0092-8674(79)90273-3. [DOI] [PubMed] [Google Scholar]
- 15.Delecluse HJ, Kohls S, Bullerdiek J, Bornkamm GW. Integration of EBV in Burkitt’s lymphoma cells. Current topics in microbiology and immunology. 1992;182:367–373. doi: 10.1007/978-3-642-77633-5_47. [DOI] [PubMed] [Google Scholar]
- 16.Weitzman MD, Lilley CE, Chaurushiya MS. Genomes in conflict: maintaining genome integrity during virus infection. Annu Rev Microbiol. 2010;64:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- 17.Smeenk G, van Attikum H. The chromatin response to DNA breaks: leaving a mark on genome integrity. Annual review of biochemistry. 2013;82:55–80. doi: 10.1146/annurev-biochem-061809-174504. [DOI] [PubMed] [Google Scholar]
- 18.Nikitin PA, et al. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell host & microbe. 2010;8:510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nature reviews Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 20.Kulinski JM, et al. Ataxia telangiectasia mutated kinase controls chronic gammaherpesvirus infection. Journal of virology. 2012;86:12826–12837. doi: 10.1128/JVI.00917-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mounce BC, et al. Gammaherpesvirus gene expression and DNA synthesis are facilitated by viral protein kinase and histone variant H2AX. Virology. 2011;420:73–81. doi: 10.1016/j.virol.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mounce BC, Tsan FC, Kohler S, Cirillo LA, Tarakanova VL. Dynamic association of gammaherpesvirus DNA with core histone during de novo lytic infection of primary cells. Virology. 2011;421:167–172. doi: 10.1016/j.virol.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jha HC, et al. H2AX phosphorylation is important for LANA mediated KSHV episome persistence. Journal of virology. 2013 doi: 10.1128/JVI.03575-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nature structural & molecular biology. 2013;20:259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 25.Hurley EA, Thorley-Lawson DA. B cell activation and the establishment of Epstein-Barr virus latency. The Journal of experimental medicine. 1988;168:2059–2075. doi: 10.1084/jem.168.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darst RP, Haecker I, Pardo CE, Renne R, Kladde MP. Epigenetic diversity of Kaposi’s sarcoma-associated herpesvirus. Nucleic acids research. 2013 doi: 10.1093/nar/gkt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng H, Nogueira ML, Vogel JL, Kristie TM. Transcriptional coactivator HCF-1 couples the histone chaperone Asf1b to HSV-1 DNA replication components. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2461–2466. doi: 10.1073/pnas.0911128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh J, Ruskoski N, Fraser NW. Chromatin assembly on herpes simplex virus 1 DNA early during a lytic infection is Asf1a dependent. Journal of virology. 2012;86:12313–12321. doi: 10.1128/JVI.01570-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maul GG, Negorev D, Bell P, Ishov AM. Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J Struct Biol. 2000;129:278–287. doi: 10.1006/jsbi.2000.4239. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay CR, Morozov VM, Ishov AM. PML NBs (ND10) and Daxx: from nuclear structure to protein function. Frontiers in bioscience : a journal and virtual library. 2008;13:7132–7142. doi: 10.2741/3216. [DOI] [PubMed] [Google Scholar]
- 31.Tavalai N, Stamminger T. Interplay between Herpesvirus Infection and Host Defense by PML Nuclear Bodies. Viruses. 2009;1:1240–1264. doi: 10.3390/v1031240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14075–14080. doi: 10.1073/pnas.1008850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg AD, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes & development. 2010;24:1253–1265. doi: 10.1101/gad.566910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Q, Maul GG. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. Journal of virology. 2003;77:1357–1367. doi: 10.1128/JVI.77.2.1357-1367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greger JG, Katz RA, Ishov AM, Maul GG, Skalka AM. The cellular protein daxx interacts with avian sarcoma virus integrase and viral DNA to repress viral transcription. Journal of virology. 2005;79:4610–4618. doi: 10.1128/JVI.79.8.4610-4618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling PD, et al. Mediation of Epstein-Barr virus EBNA-LP transcriptional coactivation by Sp100. The EMBO journal. 2005;24:3565–3575. doi: 10.1038/sj.emboj.7600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negorev DG, Vladimirova OV, Ivanov A, Rauscher F, 3rd, Maul GG. Differential role of Sp100 isoforms in interferon-mediated repression of herpes simplex virus type 1 immediate-early protein expression. Journal of virology. 2006;80:8019–8029. doi: 10.1128/JVI.02164-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS pathogens. 2011;7:e1002376. doi: 10.1371/journal.ppat.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling PD, Tan J, Sewatanon J, Peng R. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. Journal of virology. 2008;82:8000–8012. doi: 10.1128/JVI.02752-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everett RD, Chelbi-Alix MK. PML and PML nuclear bodies: implications in antiviral defence. Biochimie. 2007;89:819–830. doi: 10.1016/j.biochi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Rowe M, Rowe DT, Gregory CD, Rickinson AB, et al. Differences in B-cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 44.Zimber-Strobl U, Strobl LJ. EBNA2 and Notch signalling in Epstein-Barr virus mediated immortalization of B lymphocytes. Seminars in cancer biology. 2001;11:423–434. doi: 10.1006/scbi.2001.0409. [DOI] [PubMed] [Google Scholar]
- 45.Sjoblom A, et al. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. The Journal of general virology. 1995;76(Pt 11):2679–2692. doi: 10.1099/0022-1317-76-11-2679. [DOI] [PubMed] [Google Scholar]
- 46.Laux G, Adam B, Strobl LJ, Moreau-Gachelin F. The Spi-1/PU.1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-J kappa interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. The EMBO journal. 1994;13:5624–5632. doi: 10.1002/j.1460-2075.1994.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tierney R, Kirby H, Nagra J, Rickinson A, Bell A. The Epstein-Barr virus promoter initiating B-cell transformation is activated by RFX proteins and the B-cell-specific activator protein BSAP/Pax5. Journal of virology. 2000;74:10458–10467. doi: 10.1128/jvi.74.22.10458-10467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tierney R, et al. Epstein-Barr virus exploits BSAP/Pax5 to achieve the B-cell specificity of its growth-transforming program. Journal of virology. 2007;81:10092–10100. doi: 10.1128/JVI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borestrom C, Forsman A, Ruetschi U, Rymo L. E2F1, ARID3A/Bright and Oct-2 factors bind to the Epstein-Barr virus C promoter, EBNA1 and oriP, participating in long-distance promoter-enhancer interactions. The Journal of general virology. 2012;93:1065–1075. doi: 10.1099/vir.0.038752-0. [DOI] [PubMed] [Google Scholar]
- 50.Altmann M, et al. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV’s transforming genes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14188–14193. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai CN, Liu ST, Chang YS. Identification of a novel promoter located within the Bam HI Q region of the Epstein-Barr virus genome for the EBNA 1 gene. DNA Cell Biol. 1995;14:767–776. doi: 10.1089/dna.1995.14.767. [DOI] [PubMed] [Google Scholar]
- 52.Schlager S, Speck SH, Woisetschlager M. Transcription of the Epstein-Barr virus nuclear antigen 1 (EBNA1) gene occurs before induction of the BCR2 (Cp) EBNA gene promoter during the initial stages of infection in B cells. Journal of virology. 1996;70:3561–3570. doi: 10.1128/jvi.70.6.3561-3570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nonkwelo C, Ruf IK, Sample J. The Epstein-Barr virus EBNA-1 promoter Qp requires an initiator-like element. Journal of virology. 1997;71:354–361. doi: 10.1128/jvi.71.1.354-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambinder RF, Robertson KD, Tao Q. DNA methylation and the Epstein-Barr virus. Seminars in cancer biology. 1999;9:369–375. doi: 10.1006/scbi.1999.0137. [DOI] [PubMed] [Google Scholar]
- 55.Minarovits J. Epigenotypes of latent herpesvirus genomes. Current topics in microbiology and immunology. 2006;310:61–80. doi: 10.1007/3-540-31181-5_5. [DOI] [PubMed] [Google Scholar]
- 56.Woellmer A, Hammerschmidt W. Epstein-Barr virus and host cell methylation: regulation of latency, replication and virus reactivation. Current opinion in virology. 2013;3:260–265. doi: 10.1016/j.coviro.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arvey A, et al. An atlas of the epstein-barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell host & microbe. 2012;12:233–245. doi: 10.1016/j.chom.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraus RJ, Perrigoue JG, Mertz JE. ZEB negatively regulates the lytic-switch BZLF1 gene promoter of Epstein-Barr virus. Journal of virology. 2003;77:199–207. doi: 10.1128/JVI.77.1.199-207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagemeier SR, et al. Sumoylation of the Epstein-Barr virus BZLF1 protein inhibits its transcriptional activity and is regulated by the virus-encoded protein kinase. Journal of virology. 2010;84:4383–4394. doi: 10.1128/JVI.02369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson AR, Kwek SS, Kenney SC. The B-cell specific transcription factor, Oct-2, promotes Epstein-Barr virus latency by inhibiting the viral immediate-early protein, BZLF1. PLoS pathogens. 2012;8:e1002516. doi: 10.1371/journal.ppat.1002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raver RM, Panfil AR, Hagemeier SR, Kenney SC. The B-Cell Specific Transcription Factor and Master Regulator, Pax5, Promotes EBV Latency by Negatively Regulating the Viral Immediate Early Protein, BZLF1. Journal of virology. 2013 doi: 10.1128/JVI.00546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang HH, Ganem D. A Unique Herpesviral Transcriptional Program in KSHV-Infected Lymphatic Endothelial Cells Leads to mTORC1 Activation and Rapamycin Sensitivity. Cell host & microbe. 2013;13:429–440. doi: 10.1016/j.chom.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ballestas ME, Kaye KM. The latency-associated nuclear antigen, a multifunctional protein central to Kaposi’s sarcoma-associated herpesvirus latency. Future microbiology. 2011;6:1399–1413. doi: 10.2217/fmb.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma SC, Lan K, Robertson E. Structure and function of latency-associated nuclear antigen. Current topics in microbiology and immunology. 2007;312:101–136. doi: 10.1007/978-3-540-34344-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leidal AM, Pringle ES, McCormick C. Evasion of oncogene-induced senescence by gammaherpesviruses. Current opinion in virology. 2012;2:748–754. doi: 10.1016/j.coviro.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Jeong J, Papin J, Dittmer D. Differential regulation of the overlapping Kaposi’s sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. Journal of virology. 2001;75:1798–1807. doi: 10.1128/JVI.75.4.1798-1807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong JH, et al. Regulation and autoregulation of the promoter for the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. The Journal of biological chemistry. 2004;279:16822–16831. doi: 10.1074/jbc.M312801200. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Komatsu T, Dezube BJ, Kaye KM. The Kaposi’s sarcoma-associated herpesvirus K12 transcript from a primary effusion lymphoma contains complex repeat elements, is spliced, and initiates from a novel promoter. Journal of virology. 2002;76:11880–11888. doi: 10.1128/JVI.76.23.11880-11888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearce M, Matsumura S, Wilson AC. Transcripts encoding K12, v-FLIP, v-cyclin, and the microRNA cluster of Kaposi’s sarcoma-associated herpesvirus originate from a common promoter. Journal of virology. 2005;79:14457–14464. doi: 10.1128/JVI.79.22.14457-14464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fakhari FD, Dittmer DP. Charting latency transcripts in Kaposi’s sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. Journal of virology. 2002;76:6213–6223. doi: 10.1128/JVI.76.12.6213-6223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dittmer DP. Transcription profile of Kaposi’s sarcoma-associated herpesvirus in primary Kaposi’s sarcoma lesions as determined by real-time PCR arrays. Cancer research. 2003;63:2010–2015. [PubMed] [Google Scholar]
- 72.Nador RG, et al. Expression of Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor monocistronic and bicistronic transcripts in primary effusion lymphomas. Virology. 2001;287:62–70. doi: 10.1006/viro.2001.1016. [DOI] [PubMed] [Google Scholar]
- 73.Hayward GS. Initiation of angiogenic Kaposi’s sarcoma lesions. Cancer cell. 2003;3:1–3. doi: 10.1016/s1535-6108(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 74.Montaner S, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 75.Sodhi A, Montaner S, Gutkind JS. Does dysregulated expression of a deregulated viral GPCR trigger Kaposi’s sarcomagenesis? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:422–427. doi: 10.1096/fj.03-1035hyp. [DOI] [PubMed] [Google Scholar]
- 76.Mutlu AD, et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi’s sarcoma. Cancer cell. 2007;11:245–258. doi: 10.1016/j.ccr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toth Z, Brulois K, Jung JU. The chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. Viruses. 2013;5:1346–1373. doi: 10.3390/v5051346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murata T, Tsurumi T. Epigenetic modification of the Epstein-Barr virus BZLF1 promoter regulates viral reactivation from latency. Frontiers in genetics. 2013;4:53. doi: 10.3389/fgene.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein G, Klein E, Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochemical and biophysical research communications. 2010;396:67–73. doi: 10.1016/j.bbrc.2010.02.146. [DOI] [PubMed] [Google Scholar]
- 80.Takacs M, et al. Epigenetic regulation of latent Epstein-Barr virus promoters. Biochimica et biophysica acta. 2010;1799:228–235. doi: 10.1016/j.bbagrm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Gunther T, Grundhoff A. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS pathogens. 2010;6:e1000935. doi: 10.1371/journal.ppat.1000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karlsson QH, Schelcher C, Verrall E, Petosa C, Sinclair AJ. The reversal of epigenetic silencing of the EBV genome is regulated by viral bZIP protein. Biochemical Society transactions. 2008;36:637–639. doi: 10.1042/BST0360637. [DOI] [PubMed] [Google Scholar]
- 83.Karlsson QH, Schelcher C, Verrall E, Petosa C, Sinclair AJ. Methylated DNA recognition during the reversal of epigenetic silencing is regulated by cysteine and serine residues in the Epstein-Barr virus lytic switch protein. PLoS pathogens. 2008;4:e1000005. doi: 10.1371/journal.ppat.1000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramasubramanyan S, Osborn K, Flower K, Sinclair AJ. Dynamic chromatin environment of key lytic cycle regulatory regions of the Epstein-Barr virus genome. Journal of virology. 2011;86:1809–1819. doi: 10.1128/JVI.06334-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramasubramanyan S, et al. Genome-Wide Analyses of Zta Binding to the Epstein-Barr Virus Genome Reveals Interactions in both Early and Late Lytic Cycles and an Epigenetic Switch Leading to an Altered Binding Profile. Journal of virology. 2012;86:12494–12502. doi: 10.1128/JVI.01705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhende PM, Seaman WT, Delecluse HJ, Kenney SC. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nature genetics. 2004;36:1099–1104. doi: 10.1038/ng1424. [DOI] [PubMed] [Google Scholar]
- 87.Bhende PM, Seaman WT, Delecluse HJ, Kenney SC. BZLF1 activation of the methylated form of the BRLF1 immediate-early promoter is regulated by BZLF1 residue 186. Journal of virology. 2005;79:7338–7348. doi: 10.1128/JVI.79.12.7338-7348.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bergbauer M, et al. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS pathogens. 2010;6:e1001114. doi: 10.1371/journal.ppat.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalla M, Gobel C, Hammerschmidt W. The lytic phase of Epstein-Barr virus requires a viral genome with 5-methylcytosine residues in CpG sites. Journal of virology. 2011 doi: 10.1128/JVI.06314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fejer G, et al. Latency type-specific distribution of epigenetic marks at the alternative promoters Cp and Qp of Epstein-Barr virus. The Journal of general virology. 2008;89:1364–1370. doi: 10.1099/vir.0.83594-0. [DOI] [PubMed] [Google Scholar]
- 91.Miller G, et al. Antibodies to butyrate-inducible antigens of Kaposi’s sarcoma-associated herpesvirus in patients with HIV-1 infection. The New England journal of medicine. 1996;334:1292–1297. doi: 10.1056/NEJM199605163342003. [DOI] [PubMed] [Google Scholar]
- 92.Alazard N, Gruffat H, Hiriart E, Sergeant A, Manet E. Differential hyperacetylation of histones H3 and H4 upon promoter-specific recruitment of EBNA2 in Epstein-Barr virus chromatin. Journal of virology. 2003;77:8166–8172. doi: 10.1128/JVI.77.14.8166-8172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu F, et al. Chromatin remodeling of the Kaposi’s sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. Journal of virology. 2003;77:11425–11435. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Day L, et al. Chromatin profiling of Epstein-Barr virus latency control region. Journal of virology. 2007;81:6389–6401. doi: 10.1128/JVI.02172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arvey A, Tempera I, Lieberman PM. Interpreting the Epstein-Barr Virus (EBV) epigenome using high-throughput data. Viruses. 2013;5:1042–1054. doi: 10.3390/v5041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toth Z, et al. Epigenetic analysis of KSHV latent and lytic genomes. PLoS pathogens. 2010;6:e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holdorf MM, Cooper SB, Yamamoto KR, Miranda JJ. Occupancy of chromatin organizers in the Epstein-Barr virus genome. Virology. 2011;415:1–5. doi: 10.1016/j.virol.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 99.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Current opinion in genetics & development. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Van Bortle K, Corces VG. Spinning the web of cell fate. Cell. 2013;152:1213–1217. doi: 10.1016/j.cell.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Bortle K, Corces VG. The role of chromatin insulators in nuclear architecture and genome function. Current opinion in genetics & development. 2013;23:212–218. doi: 10.1016/j.gde.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dorsett D. Cohesin: genomic insights into controlling gene transcription and development. Current opinion in genetics & development. 2011;21:199–206. doi: 10.1016/j.gde.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herold M, Bartkuhn M, Renkawitz R. CTCF: insights into insulator function during development. Development. 2012;139:1045–1057. doi: 10.1242/dev.065268. [DOI] [PubMed] [Google Scholar]
- 104.Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends in genetics : TIG. 2001;17:520–527. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- 105.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohlsson R, Lobanenkov V, Klenova E. Does CTCF mediate between nuclear organization and gene expression? BioEssays : news and reviews in molecular, cellular and developmental biology. 2010;32:37–50. doi: 10.1002/bies.200900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weth O, Renkawitz R. CTCF function is modulated by neighboring DNA binding factors. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2011;89:459–468. doi: 10.1139/o11-033. [DOI] [PubMed] [Google Scholar]
- 108.Chien R, Zeng W, Ball AR, Yokomori K. Cohesin: a critical chromatin organizer in mammalian gene regulation. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2011;89:445–458. doi: 10.1139/o11-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Losada A. Cohesin regulation: fashionable ways to wear a ring. Chromosoma. 2007;116:321–329. doi: 10.1007/s00412-007-0104-x. [DOI] [PubMed] [Google Scholar]
- 110.Stedman W, et al. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. The EMBO journal. 2008;27:654–666. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang H, Cho H, Sung GH, Lieberman PM. CTCF regulates Kaposi’s sarcoma-associated herpesvirus latency transcription by nucleosome displacement and RNA polymerase programming. Journal of virology. 2013;87:1789–1799. doi: 10.1128/JVI.02283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smolle M, Workman JL. Transcription-associated histone modifications and cryptic transcription. Biochimica et biophysica acta. 2013;1829:84–97. doi: 10.1016/j.bbagrm.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tempera I, Wiedmer A, Dheekollu J, Lieberman PM. CTCF prevents the epigenetic drift of EBV latency promoter Qp. PLoS pathogens. 2010;6 doi: 10.1371/journal.ppat.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chau CM, Lieberman PM. Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus. Journal of virology. 2004;78:12308–12319. doi: 10.1128/JVI.78.22.12308-12319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salamon D, et al. Binding of CCCTC-binding factor in vivo to the region located between Rep* and the C promoter of Epstein-Barr virus is unaffected by CpG methylation and does not correlate with Cp activity. The Journal of general virology. 2009;90:1183–1189. doi: 10.1099/vir.0.007344-0. [DOI] [PubMed] [Google Scholar]
- 116.Chen HS, Wikramasinghe P, Showe L, Lieberman PM. Cohesins repress Kaposi’s sarcoma-associated herpesvirus immediate early gene transcription during latency. Journal of virology. 2012;86:9454–9464. doi: 10.1128/JVI.00787-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tempera I, Klichinsky M, Lieberman PM. EBV latency types adopt alternative chromatin conformations. PLoS pathogens. 2011;7:e1002180. doi: 10.1371/journal.ppat.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Avolio-Hunter TM, Frappier L. Mechanistic studies on the DNA linking activity of Epstein-Barr nuclear antigen 1. Nucleic acids research. 1998;26:4462–4470. doi: 10.1093/nar/26.19.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Su W, Middleton T, Sugden B, Echols H. DNA looping between the origin of replication of Epstein-Barr virus and its enhancer site: stabilization of an origin complex with Epstein-Barr nuclear antigen 1. Proc Natl Acad Sci USA. 1991;88:10870–10874. doi: 10.1073/pnas.88.23.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aras S, Singh G, Johnston K, Foster T, Aiyar A. Zinc coordination is required for and regulates transcription activation by Epstein-Barr nuclear antigen 1. PLoS pathogens. 2009;5:e1000469. doi: 10.1371/journal.ppat.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hopfner KP, et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- 122.Kang H, Wiedmer A, Yuan Y, Robertson E, Lieberman PM. Coordination of KSHV latent and lytic gene control by CTCF-cohesin mediated chromosome conformation. PLoS pathogens. 2011;7:e1002140. doi: 10.1371/journal.ppat.1002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lieberman PM, Hu J, Renne R. Maintenance and replication during latency. 2007 [PubMed] [Google Scholar]
- 124.Kapoor P, Lavoie BD, Frappier L. EBP2 plays a key role in Epstein-Barr virus mitotic segregation and is regulated by aurora family kinases. Molecular and cellular biology. 2005;25:4934–4945. doi: 10.1128/MCB.25.12.4934-4945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nayyar VK, Shire K, Frappier L. Mitotic chromosome interactions of Epstein-Barr nuclear antigen 1 (EBNA1) and human EBNA1-binding protein 2 (EBP2) Journal of cell science. 2009;122:4341–4350. doi: 10.1242/jcs.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shire K, Ceccarelli DF, Avolio-Hunter TM, Frappier L. EBP2, a human protein that interacts with sequences of the Epstein-Barr virus nuclear antigen 1 important for plasmid maintenance. J Virol. 1999;73:2587–2595. doi: 10.1128/jvi.73.4.2587-2595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sears J, et al. The amino terminus of Epstein-Barr Virus (EBV) nuclear antigen 1 contains AT hooks that facilitate the replication and partitioning of latent EBV genomes by tethering them to cellular chromosomes. Journal of virology. 2004;78:11487–11505. doi: 10.1128/JVI.78.21.11487-11505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Norseen J, Johnson FB, Lieberman PM. Role for G-quadruplex RNA binding by Epstein-Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. Journal of virology. 2009;83:10336–10346. doi: 10.1128/JVI.00747-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barbera AJ, et al. The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science. 2006;311:856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 130.Viejo-Borbolla A, et al. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi’s Sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. Journal of virology. 2005;79:13618–13629. doi: 10.1128/JVI.79.21.13618-13629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.You J, et al. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. Journal of virology. 2006;80:8909–8919. doi: 10.1128/JVI.00502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noguchi K, Vassilev A, Ghosh S, Yates JL, DePamphilis ML. The BAH domain facilitates the ability of human Orc1 protein to activate replication origins in vivo. The EMBO journal. 2006;25:5372–5382. doi: 10.1038/sj.emboj.7601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Norseen J, et al. RNA-dependent recruitment of the origin recognition complex. The EMBO journal. 2008;27:3024–3035. doi: 10.1038/emboj.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leight ER, Sugden B. Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol Cell Biol. 2001;21:4149–4161. doi: 10.1128/MCB.21.13.4149-4161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aggarwal BD, Calvi BR. Chromatin regulates origin activity in Drosophila follicle cells. Nature. 2004;430:372–376. doi: 10.1038/nature02694. [DOI] [PubMed] [Google Scholar]
- 136.Karnani N, Taylor CM, Malhotra A, Dutta A. Genomic study of replication initiation in human chromosomes reveals the influence of transcription regulation and chromatin structure on origin selection. Mol Biol Cell. 2010;21:393–404. doi: 10.1091/mbc.E09-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stedman W, Deng Z, Lu F, Lieberman PM. ORC, MCM, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. Journal of virology. 2004;78:12566–12575. doi: 10.1128/JVI.78.22.12566-12575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou J, et al. Cell cycle regulation of chromatin at an origin of DNA replication. The EMBO journal. 2005;24:1406–1417. doi: 10.1038/sj.emboj.7600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Norio P, Schildkraut CL. Visualization of DNA replication on individual Epstein-Barr virus episomes. Science. 2001;294:2361–2364. doi: 10.1126/science.1064603. [DOI] [PubMed] [Google Scholar]
- 140.Norio P, Schildkraut CL. Plasticity of DNA replication initiation in Epstein-Barr virus episomes. PLoS biology. 2004;2:e152. doi: 10.1371/journal.pbio.0020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Norio P, Schildkraut CL, Yates JL. Initiation of DNA replication within oriP is dispensable for stable replication of the latent Epstein-Barr virus chromosome after infection of established cell lines. J Virol. 2000;74:8563–8574. doi: 10.1128/jvi.74.18.8563-8574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ott E, Norio P, Ritzi M, Schildkraut C, Schepers A. The dyad symmetry element of Epstein-Barr virus is a dominant but dispensable replication origin. PloS one. 2011;6:e18609. doi: 10.1371/journal.pone.0018609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Verma SC, et al. Single molecule analysis of replicated DNA reveals the usage of multiple KSHV genome regions for latent replication. PLoS pathogens. 2011;7:e1002365. doi: 10.1371/journal.ppat.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. The EMBO journal. 2007 doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dheekollu J, Chen HS, Kaye KM, Lieberman PM. Timeless-dependent DNA Replication-coupled Recombination Promote KSHV Episome Maintenance and Terminal Repeat Stability. Journal of virology. 2013 doi: 10.1128/JVI.02211-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dheekollu J, Lieberman PM. The replisome pausing factor Timeless is required for episomal maintenance of latent Epstein-Barr virus. Journal of virology. 2011;85:5853–5863. doi: 10.1128/JVI.02425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dhar V, Schildkraut CL. Role of EBNA-1 in arresting replication forks at the Epstein-Barr virus oriP family of tandem repeats. Molecular and cellular biology. 1991;11:6268–6278. doi: 10.1128/mcb.11.12.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ermakova OV, Frappier L, Schildkraut CL. Role of the EBNA-1 protein in pausing of replication forks in the Epstein-Barr virus genome. The Journal of biological chemistry. 1996;271:33009–33017. doi: 10.1074/jbc.271.51.33009. [DOI] [PubMed] [Google Scholar]
- 149.Dheekollu J, Deng Z, Wiedmer A, Weitzman MD, Lieberman PM. A role for MRE11, NBS1, and recombination junctions in replication and stable maintenance of EBV episomes. PloS one. 2007;2:e1257. doi: 10.1371/journal.pone.0001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bastia D, Singh SK. “Chromosome kissing” and modulation of replication termination. Bioarchitecture. 2011;1:24–28. doi: 10.4161/bioa.1.1.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amon W, Farrell PJ. Reactivation of Epstein-Barr virus from latency. Reviews in medical virology. 2005;15:149–156. doi: 10.1002/rmv.456. [DOI] [PubMed] [Google Scholar]
- 152.Guito J, Lukac DM. KSHV Rta Promoter Specification and Viral Reactivation. Frontiers in microbiology. 2012;3:30. doi: 10.3389/fmicb.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. Lytic cycle switches of oncogenic human gammaherpesviruses. Advances in cancer research. 2007;97:81–109. doi: 10.1016/S0065-230X(06)97004-3. [DOI] [PubMed] [Google Scholar]
- 154.Sinclair AJ. bZIP proteins of human gammaherpesviruses. The Journal of general virology. 2003;84:1941–1949. doi: 10.1099/vir.0.19112-0. [DOI] [PubMed] [Google Scholar]
- 155.Sinclair AJ. Unexpected structure of Epstein-Barr virus lytic cycle activator Zta. Trends in microbiology. 2006;14:289–291. doi: 10.1016/j.tim.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Staudt MR, Dittmer DP. The Rta/Orf50 transactivator proteins of the gamma herpesviridae. Current topics in microbiology and immunology. 2007;312:71–100. doi: 10.1007/978-3-540-34344-8_3. [DOI] [PubMed] [Google Scholar]
- 157.Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. Lytic cycle switches of oncogenic human gammaherpesviruses (1) Advances in cancer research. 2007;97:81–109. doi: 10.1016/S0065-230X(06)97004-3. [DOI] [PubMed] [Google Scholar]
- 158.Deng Z, et al. The CBP bromodomain and nucleosome targeting are required for Zta-directed nucleosome acetylation and transcription activation. Molecular and cellular biology. 2003;23:2633–2644. doi: 10.1128/MCB.23.8.2633-2644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ellison TJ, Izumiya Y, Izumiya C, Luciw PA, Kung HJ. A comprehensive analysis of recruitment and transactivation potential of K-Rta and K-bZIP during reactivation of Kaposi’s sarcoma-associated herpesvirus. Virology. 2009;387:76–88. doi: 10.1016/j.virol.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Z, Yan Z, Wood C. Kaposi’s sarcoma-associated herpesvirus transactivator RTA promotes degradation of the repressors to regulate viral lytic replication. Journal of virology. 2008;82:3590–3603. doi: 10.1128/JVI.02229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gould F, Harrison SM, Hewitt EW, Whitehouse A. Kaposi’s sarcoma-associated herpesvirus RTA promotes degradation of the Hey1 repressor protein through the ubiquitin proteasome pathway. Journal of virology. 2009;83:6727–6738. doi: 10.1128/JVI.00351-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rossetto CC, Pari G. KSHV PAN RNA associates with demethylases UTX and JMJD3 to activate lytic replication through a physical interaction with the virus genome. PLoS pathogens. 2012;8:e1002680. doi: 10.1371/journal.ppat.1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skalska L, White RE, Franz M, Ruhmann M, Allday MJ. Epigenetic repression of p16(INK4A) by latent Epstein-Barr virus requires the interaction of EBNA3A and EBNA3C with CtBP. PLoS pathogens. 2010;6:e1000951. doi: 10.1371/journal.ppat.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paschos K, Parker GA, Watanatanasup E, White RE, Allday MJ. BIM promoter directly targeted by EBNA3C in polycomb-mediated repression by EBV. Nucleic acids research. 2012;40:7233–7246. doi: 10.1093/nar/gks391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu F, Day L, Gao SJ, Lieberman PM. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi’s sarcoma-associated herpesvirus lytic transcription. Journal of virology. 2006;80:5273–5282. doi: 10.1128/JVI.02541-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ye FC, et al. Disruption of Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. Journal of virology. 2004;78:11121–11129. doi: 10.1128/JVI.78.20.11121-11129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sivachandran N, et al. Contributions of the Epstein-Barr virus EBNA1 protein to gastric carcinoma. Journal of virology. 2012;86:60–68. doi: 10.1128/JVI.05623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jin Y, et al. LANA Carboxyl Terminal Amino Acids 1052 to 1082 Interact with RBP-Jkappa and are Responsible for LANA-Mediated RTA Repression. Journal of virology. 2012 doi: 10.1128/JVI.06788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu J, et al. The RBP-Jkappa binding sites within the RTA promoter regulate KSHV latent infection and cell proliferation. PLoS pathogens. 2012;8:e1002479. doi: 10.1371/journal.ppat.1002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sivachandran N, Wang X, Frappier L. Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection. Journal of virology. 2012;86:6146–6158. doi: 10.1128/JVI.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic regulation of Kaposi’s sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. Journal of virology. 2010;84:2697–2706. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao M, Zhang J, Phatnani H, Scheu S, Maniatis T. Stochastic expression of the interferon-beta gene. PLoS biology. 2012;10:e1001249. doi: 10.1371/journal.pbio.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Daigle D, et al. Valproic acid antagonizes the capacity of other histone deacetylase inhibitors to activate the Epstein-barr virus lytic cycle. Journal of virology. 2011;85:5628–5643. doi: 10.1128/JVI.02659-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Daigle D, et al. Upregulation of STAT3 marks Burkitt lymphoma cells refractory to Epstein-Barr virus lytic cycle induction by HDAC inhibitors. Journal of virology. 2010;84:993–1004. doi: 10.1128/JVI.01745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Davies ML, et al. Cellular factors associated with latency and spontaneous Epstein-Barr virus reactivation in B-lymphoblastoid cell lines. Virology. 2010;400:53–67. doi: 10.1016/j.virol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 176.Yu X, Wang Z, Mertz JE. ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus. PLoS pathogens. 2007;3:e194. doi: 10.1371/journal.ppat.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ellis AL, Wang Z, Yu X, Mertz JE. Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner. Journal of virology. 2010;84:6139–6152. doi: 10.1128/JVI.02706-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Murata T, et al. Involvement of Jun dimerization protein 2 (JDP2) in the maintenance of Epstein-Barr virus latency. The Journal of biological chemistry. 2011;286:22007–22016. doi: 10.1074/jbc.M110.199836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wille CK, et al. Viral genome methylation differentially affects the ability of BZLF1 versus BRLF1 to activate Epstein-Barr virus lytic gene expression and viral replication. Journal of virology. 2013;87:935–950. doi: 10.1128/JVI.01790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature reviews Cancer. 2010;10:878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.D’Addario M, Libermann TA, Xu J, Ahmad A, Menezes J. Epstein-Barr Virus and its glycoprotein-350 upregulate IL-6 in human B-lymphocytes via CD21, involving activation of NF-kappaB and different signaling pathways. Journal of molecular biology. 2001;308:501–514. doi: 10.1006/jmbi.2001.4589. [DOI] [PubMed] [Google Scholar]
- 182.Chakraborty S, Veettil MV, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1163–1172. doi: 10.1073/pnas.1119592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hahn AS, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus. Nature medicine. 2012;18:961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chandran B. Early events in Kaposi’s sarcoma-associated herpesvirus infection of target cells. Journal of virology. 2010;84:2188–2199. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liashkovich I, Hafezi W, Kuhn JM, Oberleithner H, Shahin V. Nuclear delivery mechanism of herpes simplex virus type 1 genome. J Mol Recognit. 2011;24:414–421. doi: 10.1002/jmr.1120. [DOI] [PubMed] [Google Scholar]
- 186.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell host & microbe. 2012;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Singh VV, et al. Kaposi’s Sarcoma-Associated Herpesvirus Latency in Endothelial and B cells Activates Interferon Gamma-Inducible Protein 16 (IFI16) Mediated Inflammasomes. Journal of virology. 2013 doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rathinam VA, Fitzgerald KA. Innate immune sensing of DNA viruses. Virology. 2011;411:153–162. doi: 10.1016/j.virol.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell host & microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Johnson KE, Chikoti L, Chandran B. HSV-1 Infection Induces Activation and Subsequent Inhibition of the IFI16 and NLRP3 Inflammasomes. Journal of virology. 2013 doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ansari MA, et al. Constitutive Interferon-Inducible Protein 16-Inflammasome Activation during Epstein-Barr Virus Latency I, II, and III in B and Epithelial Cells. Journal of virology. 2013;87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Orzalli MH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3008–3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]


