Abstract
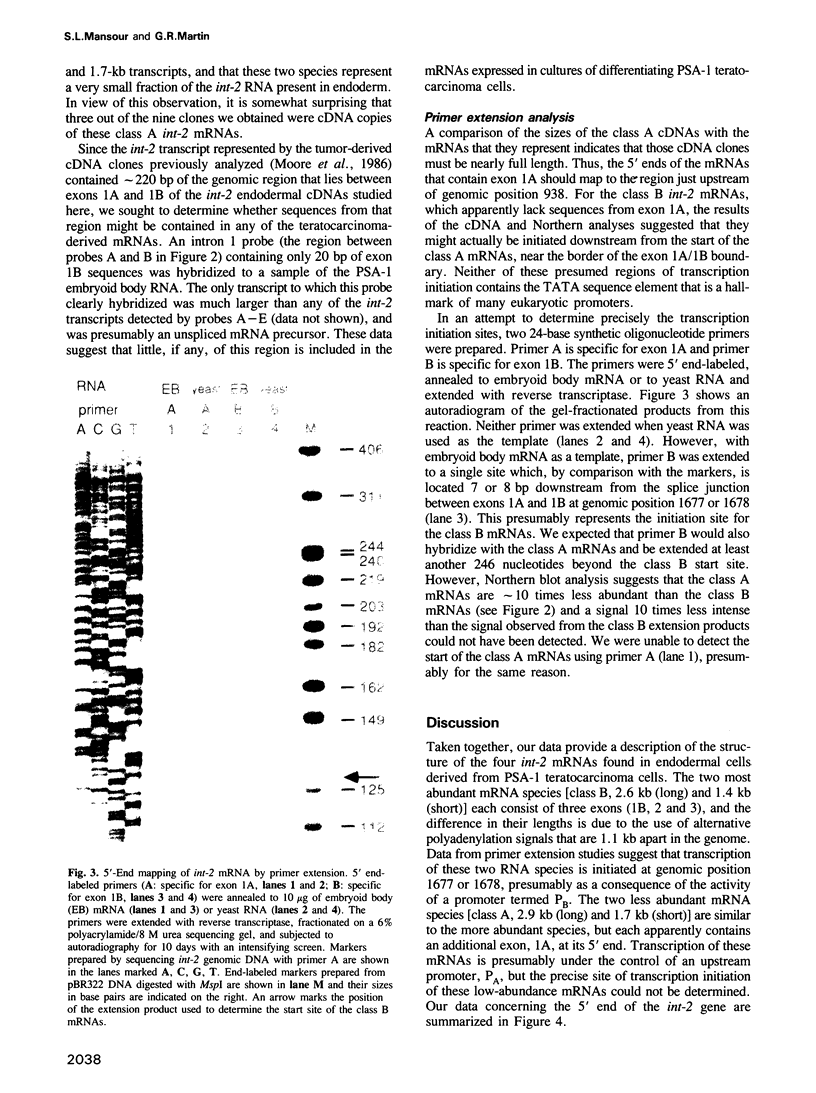
Mouse embryos at 7.5 days of gestation and endodermal cells derived from embryonal carcinoma cells each express four int-2 mRNAs of similar size and relative abundance. To determine their structure and coding potential, we prepared a cDNA library from endoderm mRNA and isolated several int-2 cDNAs. Structural analysis of these cDNAs combined with Northern blot hybridization and primer extension analyses of int-2 mRNA revealed that the differences between the mRNAs are generated through the use of two alternate transcriptional start sites and two alternate polyadenylation sites. All four mRNAs share a common core sequence that encodes a protein with amino acid similarity to fibroblast growth factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Grabel L. B., Casanova J. E. The outgrowth of parietal endoderm from mouse teratocarcinoma stem-cell embryoid bodies. Differentiation. 1986;32(1):67–73. doi: 10.1111/j.1432-0436.1986.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Grabel L. B., Martin G. R. Tunicamycin reversibly inhibits the terminal differentiation of teratocarcinoma stem cells to endoderm. Dev Biol. 1983 Jan;95(1):115–125. doi: 10.1016/0012-1606(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Shackleford G. M., Varmus H. E., Martin G. R. Two proto-oncogenes implicated in mammary carcinogenesis, int-1 and int-2, are independently regulated during mouse development. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7806–7810. doi: 10.1073/pnas.83.20.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Kornberg T., Coleman K. G., Cox D. R., Martin G. R. Expression during embryogenesis of a mouse gene with sequence homology to the Drosophila engrailed gene. Cell. 1985 Nov;43(1):29–37. doi: 10.1016/0092-8674(85)90009-1. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Wiley L. M., Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977 Dec;61(2):230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Concerted activation of two potential proto-oncogenes in carcinomas induced by mouse mammary tumour virus. Nature. 1986 Apr 17;320(6063):628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith R., Peters G., Dickson C. Multiple RNAs expressed from the int-2 gene in mouse embryonal carcinoma cell lines encode a protein with homology to fibroblast growth factors. EMBO J. 1988 Apr;7(4):1013–1022. doi: 10.1002/j.1460-2075.1988.tb02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]