Abstract
Hemoperitoneum due to nontraumatic liver rupture is rare. The most common cause of nontraumatic rupture of the liver is hepatocellular carcinoma (HCC). The other causes of nontraumatic liver ruptures are peliosis hepatis, polyarteritis nodosa, systemic lupus erythematosus, preeclampsia, metastatic carcinoma, and other primary liver tumors. In this report, we present the computed tomography findings of spontaneous liver rupture in a 52-year-old male patient due to multifocal HCC, with the diagnosis proven by surgical specimen.
Keywords: computed tomography, hemoperitoneum, liver, nontraumatic liver rupture
Introduction
The incidence of hepatocellular carcinoma (HCC) is increasing throughout the world in parallel with the increase in the incidence of hepatitis B and hepatitis C. HCC has the fifth most common incidence of all cancers. The rupture incidence in HCC is reduced by early diagnosis.1,2 Spontaneous rupture has been reported in patients with HCC between 3% and 26% and the mortality rate due to ruptured HCC is between 32% and 66%.3 The lack of initial diagnosis such as cirrhosis and HCC leads to difficulty in the diagnosis of ruptured HCC in emergency conditions. The most common symptom is the sudden onset of abdominal pain; signs of shock may also be seen. Ultrasound (US) and computed tomography (CT) are the useful imaging tools in primary diagnosis. Active bleeding is rarely shown in CT. Surgery, arterial embolization, and/or conservative therapy are the treatment options in ruptured HCC patients and are performed according to the status of the disease.1–3 In this report, we present the clinical and CT findings of hemoperitoneum due to spontaneous HCC rupture in a 52-year-old patient first diagnosed in the emergency radiology department. Consent was obtained from the patient for publication of this article.
Case report
A 52-year-old male patient arrived in the accident and emergency department after suddenly collapsing during his daily work. His mental functions were normal; he was conscious and cooperative. He had abdominal pain of 1 month’s duration and had extreme pruritus in his body for 4 months. The history of the patient was insignificant. Physical examination revealed hepatomegaly and upper-quadrant repletion. There was widespread abdominal tenderness. His blood pressure was 80/50 mmHg and his heart rate was 128 bpm. Laboratory parameters were as follows: hemoglobin: 8 g/dL (normal range: 12.2–18.1), leukocytes 14.4 10^3/uL (4.3–10.3), alanine aminotransferase 87 U/L (0–40), aspartate aminotransferase 221 U/L (0–41), direct bilirubin 0.30 mg/dL (0.0–0.20). The other parameters were in the normal range.
After these findings were determined, the patient went to the emergency radiology department. Abdominal US revealed multiple liver masses with a maximum axis diameter of 13 cm. Several of the lesions constituted markedly irregular liver contour lobulations. There were also copious free fluids in the abdomen. The peritoneal aspiration material was bloody. The patient was directed to abdominal dynamic CT.
CT revealed multiple nodular liver lesions with a long axis of a maximum of 133 mm. The lesions were heterogeneous and had cystic areas. Varied masses had contact with the liver contour and several of these lesions resulted in being unable to select contour integrity. The liver lesions had heterogeneous contrast enhancement in the arterial phase. Contrast material output leakage in the arterial delayed phase images was demonstrated outside of the liver in the neighborhood of these areas. Delayed phase images revealed focal contrast material accumulation in these lesions and several of the lesions demonstrated contrast material washout (Figures 1 and 2). We diagnosed the patient with hemoperitoneum secondary to liver lesion rupture. The contrast enhancement and washout characteristics of the liver lesions were compatible with multifocal HCC (noninvasive tissue characterization).
Figure 1.
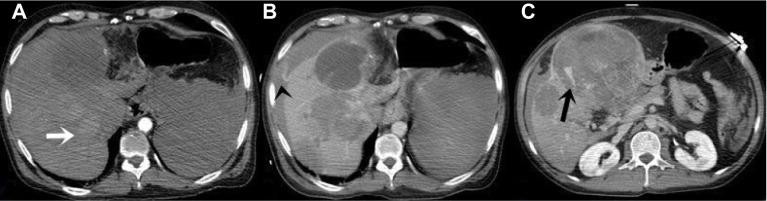
CT images of 52 year old male.
Notes: (A) Arterial phase image. The lesion in segment 7–8 has increased contrast enhancement according to liver parenchyma (white arrow). (B) Portal phase image. The lesion appears hypodense. The lesion in the left lobe has focal contrast material accumulation pooling and contrast output leakage outside the liver is visible (black arrowhead). (C) Venous phase image. Delayed phase reveals focal contrast material accumulation pooling in the large lesion of the left lobe (black arrow).
Abbreviation: CT, computed tomography.
Figure 2.
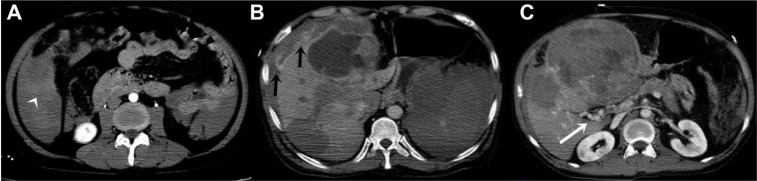
CT images of 52 year old male.
Notes: (A) Arterial phase image. The contrast accumulation in peritoneal cavity is prominent (white arrowhead) (B) Delayed phase image. The contrast accumulation outside of the liver is prominent (black arrows). (C) Portal phase image. Collateral vascular structures are visible at the hilus of the liver (white arrow).
Abbreviation: CT, computed tomography.
The patient underwent surgery and the pathological result was concordant with HCC in a non-cirrhotic liver. Cellular differentiation of HCC was G1. The serology findings were negative for hepatitis B virus (HBV) and hepatitis C virus (HCV).
Discussion
Ruptured HCC has a mortality rate of between 32% and 66%.3 The incidence of spontaneous rupture in HCC has been reported as being between 3% and 26%,3 although the rate of spontaneous rupture without prior diagnosis is between 41% and 75% in HCC cases.4 The symptoms of ruptured HCC are abdominal pain, distension, and signs of shock.5 Our patient had abdominal tenderness and upper-right-quadrant abdominal tenderness, but no signs of shock.
The incidence of spontaneous rupture in HCC is higher in Asia and Africa than in Europe.7 The cause of spontaneous rupture remains unclear. The main causes of spontaneous rupture are thought to be minor trauma of surface localized tumors, a fragile feeder artery, tumor size, superficial location, increased intra-tumoral pressure secondary to hepatic tumor invasion, portal hypertension, invasion, and tumor necrosis.6,7 Our patient had no trauma history.
HCC typically demonstrates arterial early phase contrast material enhancement on dynamic CT. In venous and delayed phases, the lesion shows contrast attenuation. However, this typical result may not always be visible.8 In our patient, most of the lesions showed contrast enhancement in the arterial phase and contrast weakening in the delayed phase, although the large lesions demonstrated intra-tumoral focal contrast material pooling and these findings gave rise to the thought of vascular damage in the tumor.
The main risk factors for HCC are alcoholism, cirrhosis, HBV and HCV, aflatoxin intoxication, a family history of malignant liver tumor, diabetes and obesity, metabolic diseases of the liver, and hormonal therapies. HCC appears more frequently in males than in females. The majority of HCC is due to cirrhosis, although it may also develop without cirrhosis in nonalcoholic fatty liver disease. The other risk factor for HCC in pregnant women is HBV infection; there is a higher incidence of HCC in pregnant women with HBV than in pregnant women without HBV.9,10 We did not find any etiological factor in our patient.
Rarely, hemoperitoneum in a cirrhotic patient may shine the light on HCC after a sudden increase in alkaline phosphatase and aspartate transaminase/alanine transaminase levels.9 Hemoperitoneum, environmental hematoma, active contrast material leakage outside of the liver parenchyma, tumor protrusions through the liver contour, interruption of hepatic surface, and enucleation are the main CT signs in ruptured HCC. Our patient had most of these signs. It should be keep in mind that contrast material leakage in spontaneous rupture of HCC is not always visible in CT or digital subtraction angiography. Evaluation of CT with maximum intensity projection images may help to show contrast material extravasation.7,8 Surgery and/or transarterial embolization techniques are the treatments of choice for ruptured HCC.7
Conclusion
Spontaneous rupture in HCC patients without initial diagnosis leads to difficulties in emergency conditions. HCC should be considered as a primary differential diagnosis in spontaneous hemoperitoneum in patients with a liver lesion or lesions.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Chedid AD, Klein PW, Tiburi MF, Villwock MM, Bassani LE, Chedid MF. Spontaneous rupture of hepatocellular carcinoma with haemoperitoneum: a rare condition in Western countries. HPB (Oxford) 2001;3(3):227–230. doi: 10.1080/136518201753242262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141(2):191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol. 2012;18(48):7302–7307. doi: 10.3748/wjg.v18.i48.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CL, Fan ST, Lo CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19(17):3725–3732. doi: 10.1200/JCO.2001.19.17.3725. [DOI] [PubMed] [Google Scholar]
- 5.Kirikoshi H, Saito S, Yoneda M, et al. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. 2009;30(9):29. doi: 10.1186/1471-230X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HC, Yang DM, Jin W, Park SJ. The various manifestations of ruptured hepatocellular carcinoma: CT imaging findings. Abdom Imaging. 2008;33(6):633–642. doi: 10.1007/s00261-007-9353-7. [DOI] [PubMed] [Google Scholar]
- 7.Rossetto A, Adani GL, Risaliti A, et al. Combined approach for spontaneous rupture of hepatocellular carcinoma. World J Hepatol. 2010;2(1):49–51. doi: 10.4254/wjh.v2.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolog N, Andreisek G, Oancea I, Mangrau A. CT and MR imaging of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2011;20(2):181–189. [PubMed] [Google Scholar]
- 9.Rao PN. Nodule in Liver: Investigations, Differential Diagnosis and Follow-up. J Clin Exp Hepatol. 2014;4(Suppl 3):S57–S62. doi: 10.1016/j.jceh.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Xie L, Yang WS, et al. Risk factors of hepatocellular carcinoma – current status and perspectives. Asian Pac J Cancer Prev. 2012;13(3):743–752. doi: 10.7314/apjcp.2012.13.3.743. [DOI] [PubMed] [Google Scholar]


