Abstract
Cellulose nanomaterials are naturally occurring with unique structural, mechanical and optical properties. While the paper and packaging, automotive, personal care, construction, and textiles industries have recognized cellulose nanomaterials’ potential, we suggest cellulose nanomaterials have great untapped potential in water treatment technologies. In this review, we gather evidence of cellulose nanomaterials’ beneficial role in environmental remediation and membranes for water filtration, including their high surface area-to-volume ratio, low environmental impact, high strength, functionalizability, and sustainability. We make direct comparison between cellulose nanomaterials and carbon nanotubes (CNTs) in terms of physical and chemical properties, production costs, use and disposal in order to show the potential of cellulose nanomaterials as a sustainable replacement for CNTs in water treatment technologies. Finally, we comment on the need for improved communication and collaboration across the myriad industries invested in cellulose nanomaterials production and development to achieve an efficient means to commercialization.
1. INTRODUCTION
Cellulose, the most prevalent natural polymer, has greatly impacted the world and society in broad reaching areas. While the ancient Egyptians recognized the utility of cellulose, it was only within the last century that advanced processing and analytical instrumentation allowed researchers to discover the nanoscale cellulose structures that exhibit extraordinary properties.1–4 These nanostructures are naturally occurring and have sizes ranging from a few nanometers to microns with high strength properties comparable to Kevlar.5 The discovery of these remarkable, naturally occurring nanomaterials has spawned interest from broad markets including paper and packaging,6–11 food additives,8,12 personal care products,8,13,14 lightweight composites for aerospace and automotive,8,15,16 building and construction,8 biomedical8,17–24 and energy.8,25,26 These application areas have been reviewed thoroughly and interested readers are directed to the afore-referenced reviews.
Even with these myriad applications, the commercialization of CNs seems to be hindered by a lack of valuable end-products for these biobased nanomaterials. Interest in developing CN-enabled products spans fields as diverse as forestry, engineering, physics and chemistry.27,28 The forest-product industry is especially interested in harnessing the potential of CN as it already has the infrastructure in place for harvesting and transporting the requisite raw material. With an ever-expanding number of patents filed each year,2 CNs appear to occupy a more prominent position in the patent literature than in the peer-reviewed scientific literature, exemplifying the ongoing race to find success with CN-based materials. There has been an exponential increase in the number of patents filed that include cellulose nanomaterials in a predominant way. To date 475 patents have been filed that include the words nanocellulose (112 patents), cellulose nanocrystals (38 patents), microfibrillated cellulose (125 patents), bacterial nanocellullose (5 patents), cellulose nanowhiskers (5 patents), and cellulose nanofibers (190 patents). One fifth of all the CN patents filed are related to environmental applications including membrane technology, with a total of 97 patents linking the above keywords describing CNs with at least one of the following terms: membrane, adsorbent, environment, and filter. The total number of environmentally relevant CN patents and the keywords they use in their description (eliminating redundant searches) is presented in Figure 1. Patents for CNs have been filed for a wide range of applications. Cellulose nanomaterial uses in membranes (35 patents) and filters (39 patents) are primarily claimed as additives to mechanically reinforce materials, increase their molecular weight, alter their surface chemistry, and act as a binding agent. Patents for CNs in membranes and filters are touted for applications in membranes for fuel cells, membranes used as wound dressings, membranes used in electrode assemblies, water filtration membranes, gas barrier membranes, conductive thin films, paper barriers, and cigarette filters. Interestingly, one of the earliest patents filed in 1999 claimed the use of microfibrillated cellulose for applications in environmentally benign cigarette filters. Patent claims that include environmental terms (20 patents) for CN applications, predominantly mention the environmentally friendly nature of CNs and often claim their material is produced in an environmentally friendly manner as a result of using CNs. These patent applications include environmental protective coatings. Cellulose nanomaterial uses in patents for adsorbents (3 patents) are claimed as environmentally friendly carriers for catalysts and various chemicals. To drive the future commercialization of CNs, applications that are broadly impactful, easily scalable, and result in a needed and valuable end product are necessary.
Figure 1.
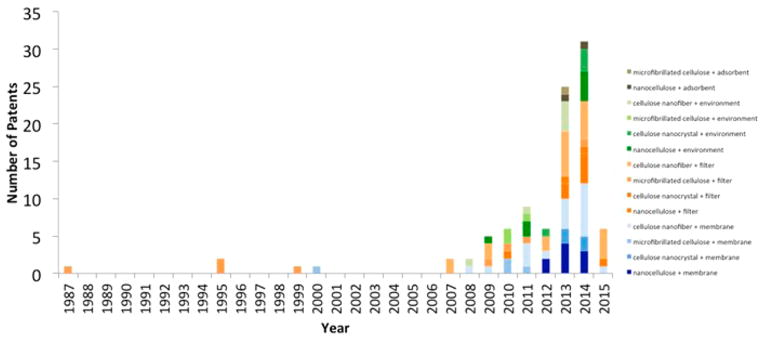
Total number of patents filed for environmental applications of cellulose nanomaterials.
As shown in patent and literature reviews, the use of CNs in environmental engineering applications is much less explored, even though CNs hold great potential.29 Nanomaterials in general have recently garnered much attention for remediation of toxic compounds as their size, high surface area-to-volume ratio, and functionalizable surfaces allow for high reactivity, targeting of species, and sufficient transport for in situ application.30 However, environmental concerns necessitate that the nanoremediation scaffold have no negative impact of its own. Next generation water filtration membranes require higher strength and selectivity, while maintaining water permeability, than current formulations. Cellulose nanomaterials’ inherent fibrous nature and remarkable mechanical properties, coupled with its low cost, biocompatibility and sustainable source, suggest huge potential as a component in water filtration membranes. While other engineering applications of CNs hold great potential, such as membranes for air filtration31–33 and as rheological agents in oil recovery (i.e., fracking),25,26 we have focused this review specifically on water treatment technologies to serve as a guide and inspiration to those considering CN-based materials in these areas. We first consider lessons learned from developments in other more mature applications, then make comparisons between CNs and other high-aspect ratio high strength nanoparticles (i.e., CNTs) previously studied. We compile key results in the areas of remediation and water filtration membranes that are promising contributions to developing CNs in environmental engineering. Lastly, we comment on the future of CNs in environmental applications.
2. CONSIDERATIONS FOR CELLULOSE NANOMATERIAL-BASED DEVELOPMENT IN ENVIRONMENTAL ENGINEERING APPLICATIONS
2.1. Cellulose Nanomaterial Structures and Inconsistencies in Nomenclature
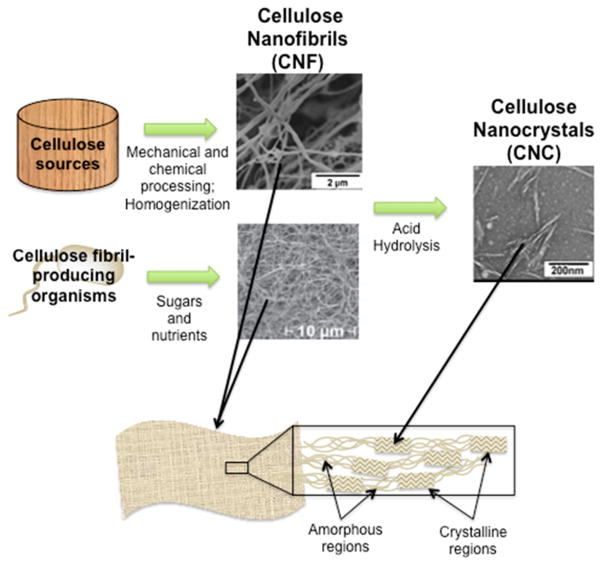
There are numerous types of cellulose nanomaterials isolated through different methods, and the disjointed investigation of these materials by varying research disciplines has resulted in a lack of consistent nomenclature.34 Thus, when researching literature, one must search many different names to gain a full understanding of the state of the art. These names include nanocellulose, microcrystalline cellulose, nanocrystalline cellulose, cellulose nanocrystals, cellulose nanowhiskers, polysaccharide nanocrystals, Avicel, cellulose microfibrils, cellulose nanofibrils, cellulose nanofibers, cellulose microfibers, nanofibrillated cellulose, microfibrillated cellulose, bacterial cellulose, and any combination of the above. A consortium including the USDA Forest Service, the Technical Association of the Pulp and Paper Industry (TAPPI), and interested entities in Canada have proposed that cellulose nano-objects be categorized into two groups: cellulose nanocrystals (CNC) and cellulose nanofibrils (CNF).35 As illustrated in Figure 2, CNFs are isolated either through homogenization of cellulose feedstocks or directly produced by bacteria. Only certain types of bacteria, mainly Gluconacetobacter xylinus, are known to produce CNFs, which are typically characterized by high average molecular weight, higher crystallinity, and different surface chemistry than CNFs isolated through homogenization of wood/plant fibers.36,37 Often, the fibrils produced directly from bacteria have been designated as their own group (i.e., bacterial nanocellulose). However, the consortium places all cellulose nanofibrils in one category regardless of their production method. CNFs are characterized by diameters between 5–60 nm and lengths on the micrometer scale and are comprised of both amorphous and crystalline regions of cellulose. Acid hydrolysis of CNFs to remove the amorphous regions yields CNCs (Figure 2). The dimensions of CNCs range from 5–70 nm in diameter and 100–250 nm in length depending on the source material and isolation method. As the name implies, CNCs are highly crystalline, containing fewer amorphous regions compared to CNFs. Of note, recent work by Su et al. using small-angle X-ray-scattering suggests that CNCs and CNFs exhibit a ribbon-like structure with an oblong cross section, not a circular cross-section as is often depicted.38 In this review, we adhere to the naming standards put forth by the afore-mentioned consortium. Adherence to a consistent naming system would benefit future knowledge transfer, collaboration and more efficient development.
Figure 2.
Cellulose nanomaterials are obtained through mechanical and chemical processing of cellulose feedstocks as well as direct production from living organisms. Acid hydrolysis of cellulose nanofibrils (CNFs) to remove amorphous regions yields cellulose nanocrystals (CNCs). Reprinted in part with permission from Ref. 37 (Copyright 2011 Cambridge University Press) and Ref. 96 (Copyright 2013 Elsevier).
2.2. Comparison to Carbon Nanotubes
The high aspect ratio that accompanies CN’s impressive mechanical properties is reminiscent of carbon nanotubes, yet cellulose nanomaterials are an abundant, naturally occurring, and renewable resource that is both biocompatible and hydrophilic due to the cellulose building blocks. Moreover, CNs can be functionalized extensively with little disruption to their unique crystalline properties.15,39,40 These significant advantages suggest CNs would be a sustainable replacement for carbon nanotubes (CNTs). Especially given the lower EHS concerns regarding CNs vs CNTs, turning from CNTs to CNs for enhancers in water filtration membranes is an obvious progression. Table 1 provides a comparison of CNs to CNTs in terms of material properties as well as life cycle assessment including manufacturing cost, applications, ecotoxicity and end of life disposal. Depending on the preparation (i.e., slurry or dry) CNs range from $1 to $5/g where CNTs can be as low as $8/g to as high as $280/g (calculated from suppliers: University of Maine Process Development Center, Cheap Tubes Inc., Buckyusa, Carbon Solutions Inc., U.S. Research Nanomaterials Inc.). The difference in CN cost is due to the high energy needed to freeze-dry the slurry material. While the cost of CNs may still be too high for use in some large scale applications, such as remediation scaffolds and rheological modifiers for fracking, many entities are interested in CNs as they add value to wood products and can replace those products produced from coal (i.e., CNTs and activated carbon). Given the numerous biodegradation pathways for CNs that exist, the reduced persistence of these materials compared with CNTs suggests that these materials will be of less concern in the context of long-term environmental exposures, bioaccumulation and trophic transfer.41 Though limited, current data suggest that CNs exhibit very good biocompatibility and low toxicity.41,42 There are significant differences between CNs and CNTs that limit its complete substitution. Unlike CNTs, unmodified CNs are not electrically conductive nor photocatalytic. Moreover, while the tensile strength of CNs is elevated, it is more than ten times less than that of CNTs.43 Regardless, the replacement of CNTs with CNs will likely become more prominent with the development and improvement of modification methodologies and CN-based composite design.
Table 1.
Comparison of Cellulose Nanomaterials and Carbon Nanotubes Including Chemical and Physical Properties, Production, Applications and Disposal
| Cellulose Nanomaterials | Carbon Nanotubes | Ref | |||
|---|---|---|---|---|---|
| CNCs | CNFs | SWCNTs | MWCNTs | ||
| Physical Properties | |||||
| Diameter | 5–70 nm | 5–100 nm | 0.4–2 nm | 2–100 nm | 5,44 |
| Length | 100–250 nm | several microns | microns-millimeters | microns-centimeters | |
| Size distribution | Polydisperse | Polydisperse | Mono- or Polydisperse depending on preparation | Mono- or Polydisperse depending on preparation | |
| Tensile Strength | 2–6 GPa | 2–4 GPa | 13–52 GPa | 11–63 GPa | 98–100 |
| Young’s Modulus | 50–143 GPa | 15–150 GPa | 0.32–1.47 TPa | 0.27–0.95 TPa | |
| Conductivity | None | σ= 102–108 S/m | 44, 100–101 | ||
| Optical Properties | Transparent and Iridescent films | Transparent films | None | 102 | |
| Life Cycle Assessment | |||||
| Manufacturing (Energy requirement) | 500–2,300 kWht−1 | 278,000–250,200,000 kWht−1 | 5 103 | ||
| Source | Wood, cotton, hemp, flax, wheat straw, ramie, sugar beet, potato tuber, tunicin, algae, certain bacteria (Gluconacetobacter xylinus) | Fossil fuels | 5 | ||
| Cost | $1/g (dry) | $2/g (dry) | $80–$280/g | $8–$15/g | 41 |
| $5/g (slurry) | $3/g (slurry) | ||||
| Major Use/Application | Paper, packaging, plastic film, cement, automotive components, food products, drug delivery, biomedical implants, wound dressings | Microelectronics, solar cells | Strength enhancers, coatings/films, biosensors, medical devices, drug delivery | 8, 104 | |
| Ecotoxicity | Low toxicity | Low toxicity | Oxidative stress and inflammation; Inhalation and dermal exposure considered largest risk | 43, 105 | |
| Some proinflammatory cytokines | Some pulmonary inflammation | ||||
| Disposal | Biodegradable by organisms with cellulase enzymes | Persistent, non-degradable | 105–106 | ||
Calculated from suppliers: University of Maine Process Development Center, Cheap Tubes Inc., Buckyusa, Carbon Solutions Inc., U.S. Research Nanomaterials Inc.
2.3. Manufacturing of Cellulose Nanomaterials
Although CNs are naturally occurring, the extraction of CNFs requires large energy consumption,6,44 and the chemical processes to obtain CNCs result in large amounts of acidic waste.44,45 Thus, significant efforts are focused on engineering more efficient CN production and modification methods.6 For example, enzymatic degradation can separate the cellulose fibers with a lower energy requirement, but only small enzyme doses can be used to ensure that the degree of polymerization of cellulose nanostructures is not compromised.5 Incorporation of charges into the pulp fibers aids in lowering the energy requirement for homogenization as the charge repulsion lowers fiber–fiber friction and decreases aggregation that can clog the machinery.5 Even with these advances, the minimum energy requirement achieved is still on the order of 500 kWh per ton of cellulose. While this is orders of magnitude smaller than the energy requirements for producing CNTs,41 the need for environmentally responsible and economically viable CN production strategies remains a major goal for the success of CNs.
3. USE OF CELLULOSE NANOMATERIALS IN WATER TREATMENT TECHNOLOGIES
3.1. Cellulose Nanomaterials in Nanoremediation Strategies
Nanotechnologies have been touted as having great potential for reducing costs and improving efficiency in pollution prevention, treatment and cleanup.30 Two applications for CNs in this area that have generated interest are as an active sorbent material for contaminants and as a stabilizer for other active particles.
3.1.1. Cellulose Nanomaterials As Contaminant Adsorbents
Typical strategies for removing heavy metal contaminants from the environment include sorption, chemical precipitation, membrane separation, and electrochemical treatment. Of these, sorption remains one of most effective and efficient processes with activated carbon being the mainstay of sorptive media. Cellulose nanomaterials are a promising alternative adsorbent due to its high surface area-to-volume ratio, low cost, high natural abundance, and inherent environmental inertness. Moreover, CN’s easily functionalizable surface allows for the incorporation of chemical moieties that may increase the binding efficiency of pollutants to the CN. Carboxylation of CNs is by far the most studied method for increasing their sorptive capacity. Yu et al. found that the incorporation of succinic acid groups onto CNCs significantly increased the binding efficiency to Pb2+ and Cd2+ from aqueous solutions.46 The conversion of the carboxylic acid groups to sodiated carboxylates further enhanced their ability to remove these toxic metal ions from solution. The advantage of incorporating carboxylate groups has also been demonstrated on cellulose CNFs. For example, Srivastava et al. exhibited the ability of COO−-modified CNFs to sorb Ni2+ and Cr3+ in addition to Cd2+ and Pb2+ with efficiencies 3–10% higher than unmodified CNFs.47 Ma et al. further supported the remediative utility of CNFs by illustrating their ability to remove radioactive uranylions (UO22+) from solution.48 The UO22+ ions coordinated to the carboxylate groups of TEMPO-oxidized CNFs up to 167 mg/g, which is 2–3 times greater than that achieved with traditional adsorbents (i.e., montmorillonite, polymer particles, silica particles, and hydrogels). Alternatively, incorporation of cysteine provides thiol groups to bind Cr(VI) and Pb(II).49
To target anionic contaminants, CN-based materials can be modified to present positively charged functionalities as well. For example, the inclusion of amine groups onto the surface of CNCs allowed for up to 98% removal of anionic chromate forms containing Cr(VI) at a concentration of 12.5 mg/g.50
Bacteria-derived CNFs have also proven suitable for sorbing heavy metals including Pb2+, Mn2+, and Cr3+.51 Of note, the dead cells and cellular debris that remain in the CNF network provide extra sites for sorption as they are rich in metal-binding functionalities such as carboxyls, phosphoryls, hydroxyls, phosphates, and amines.51
The sorption of organic contaminants has also been demonstrated with modified CN matrices. The inherent hydrophilicity of CNs can be reduced to improve the affinity of the material for hydrophobic compounds. The manipulation of the surface chemistry of CNs can be achieved by inclusion of both organic and inorganic functionalities. For example, Kohrhonen et al. achieved this through atomic layer deposition of titanium dioxide (TiO2) nanoparticles onto the surface of CN aerogels.52 The TiO2 coating created a low-energy surface on the fibers to yield a CN-based material that was both hydrophobic and oleophilic. These materials were able to absorb oil and a variety of organic solvents from the surface of water at a capacity of 20–40 g/g and 80–90% vol/vol. Jiang and Hseih achieved even greater sorption capacities of model organic solvents ranging from 139–345 g/g by vapor depositing triethoxyl(octyl)silane onto CNF aerogels.53 The addition of the hydrophobic silanes to the CN structure rendered the material oleophilic and water-repellant such that it could remove oils that were spread on the surface of water or that were trapped below water. Recently, Zhang et al. demonstrated the ability to silanate CNFs by simply freeze-drying aqueous suspensions in the presence of a methyl-trimethoxysilane sol.54 Similar to the aforementioned silanated CNFs, these materials were able to efficiently absorb oils and organics from the surface of water. Freezing-drying was also used by Wang et al. to prepare hydrogels with graphene oxide trapped within the CN matrix.55 Following reduction under H2 gas, the graphene oxide-CN composites were capable of sorbing 147 and 164 g/g of dimethylformamide and cyclohexane, respectively. While promising on a lab-scale, vapor deposition and freeze-drying large quantities required for field-scale remediation may be prohibitive. Modifications that are easily scalable would be more valuable.
Promising remediation candidates would also have a mechanism for removing the contaminant-loaded CN. For example, the aforementioned UO22+-sorbing CNFs formed a gel upon binding that facilitates separation of the contaminant-laden CNFs from water.48 Magnetite nanoparticles can easily be incorporated into the CN structure to allow for controlled collection via magnetic separation.56 Zhu et al. fabricated CNFs with magnetic particles entrapped within the fibers by including Fe3O4 nanoparticles into the growth media of CNF-producing bacteria during the formation of the fibers.51 While this latter method represents a low energy pathway for modifying CNs, long growth times and difficulty with scale-up may limit commercialization.
Following capture of the contaminant-loaded material, the ability to remove/wash the contaminant from the sorbent and reuse the material is also a major goal in designing sustainable remediation strategies. Recycleable CN-based adsorbent materials, exhibiting 80–90% retention of adsorption capacity has been demonstrated with many of the materials discussed above.46,47,50–52,56 Mechanisms for regeneration are tailored to the functionalization introduced to the CN or the type of contaminant removed. For example, removal of sorbed metallic ions was achieved through washing with acid or salt solutions, whereas the removal of organic compounds from the TiO2-modified CNFs was achieved by submersion in organic solvents or by evaporation. The number of cycles tested typically ranges between 3 and 5,46,47,50,51,56 but Korhonen et al. produced a CN-based aerogel that maintained >80% recovery after 10 cycles.52
3.1.2. Cellulose Nanomaterials as Scaffolds
Cellulose nanomaterials find application in nanoremediation strategies in a passive manner as well where it serves as a scaffold or particle-stabilizer for reactive nanoparticles. Nanoparticles are often modified with polymers to prevent aggregation and to aid in transport; however, these surface-bound stabilizers coat the reactive particle surface and may thereby inhibit the sorption and degradation of target compounds. Aggregation issues plague the use of iron oxide nanoparticles that can be employed as adsorbent materials for arsenic removal.57 Nata et al. designed a simple one-pot solvothermal method for growing aminated iron oxide particles onto a CNF matrix for use in arsenic remediation.58 The robust CNF matrix prevented particle aggregation and allowed for increased modification of the magnetite particles with amines. As a result, these materials exhibited significantly higher arsenic removal (36.49 mg/g As(V)) than any previously published iron oxide-based adsorbents.
The growth of reactive nanoparticles for the photocatalytic degradation of contaminants has also been demonstrated. Titanium dioxide particles, a popular photocatalytic nanoremediation strategy, were grown along CNFs using a controlled surface hydrolysis method.59 Near complete UV degradation of methyl orange, a model organic dye for textile effluents, was observed after 20 min, which was 20% increased rate compared to TiO2 alone. Snyder et al. created hybrid Au/TiO2- and Ag/TiO2–CNF composites that removed methylene blue, a model for organic contaminants, by 75% and 70%, respectively, after 1 h through both adsorption and photocatalytic degradation.60 Of note, the addition of the metallic nanoparticles proved to increase the mechanical strength of the CNF films, making them more robust and recyclable. Yang et al. prepared another type of photocatalyic CN using a hydrothermal reaction to grow cadmium sulfide nanoparticles along CNFs.61 These composite materials were capable of 80% methyl orange degradation after 90 min irradiation with visible light.
While the aforementioned materials have proven effective at sequestering or reacting a wide range of contaminants, the environmental impact of modified CNs must be considered when determining their ultimate success. The nontoxic nature and biodegradability of CNs may be compromised when chemical modifications are made. Thus, complete studies should also include stability experiments and analyses of any new byproducts that might be formed. Since most remediation applications are likely to require large quantities of CNs, the cost, feasibility, and life cycle considerations of manufacturing these materials on a large scale must be considered.62 The current cost of CNs ($1/g) is significantly higher than that of activated carbon ($1.65/kg),63 which is often used as a sorbent in remediation. However, CN has the environmental advantage over charcoal-derived activated carbon. Biochar is similar to activated carbon and derived from plants, but is less functionalizable than CN.63 Transport studies will reveal whether the higher cost of CN will be offset by lower deployment costs.
3.2. Cellulose Nanomaterials in Membranes for Water Filtration
The dimensions of cellulose nano- and microfibers and the strength of the material can be exploited in the fabrication of membranes for water treatment. Membranes have been formed as pristine CN mats64–68 as well as from CNs incorporated into a multitude of polymer matrices including cellulose triacetate,69 poly(vinylidene fluoride) (PVDF),70 poly(ether sulfone) (PES),71 poly(ethylene oxide) (PEO),72 poly(vinyl alcohol) (PVA),68,73,74 poly(acrylonitrile) (PAN),68,75 poly(3-hydroxybutyrate) (PHB),76 and polypyrrole (PPy).77 Polymer-CN composite membranes have been conceived in a range of membrane processes (i.e., micro-filtration, ultrafiltration, hemodialysis, nanofiltration, membrane distillation) and taken together they demonstrate a wide variety of membrane characteristics. Much like with the addition of carbon nanotubes, the inclusion of CNs within polymer matrices distinctly alters the membrane properties even at very low weight percent loadings. The most notable property enhancement is the large increases in membrane tensile strength obtained from small weight additions of CNs. Other beneficial properties include changes to membrane surface hydrophilicity, greater permeability, greater selectivity, and greater resistance to biofouling. Unlike CNTs, however, CN are highly biocompatible and environmentally benign, which is of significance to biomedical and environmental applications of such nanocomposite membranes. Each of these enhanced membrane-properties is expounded upon in the following sections.
3.2.1. Mechanical Property Enhancement
Membranes can greatly benefit from the high tensile strength of CNs. At low loadings of a few weight percent, CNs can increase the tensile strength of polymer membranes by up to 50%70,71 and a reduction in strain-at-break. While the tensile properties of CNs are orders of magnitude lower than those of CNTs, the demonstrated preliminary increases in polymer-CN membrane tensile strength are within the same order of magnitude to those achieved with polymer-CNT membranes at similar loadings.78,79 The large disparity in tensile properties between CN and those of CNTs on an individual basis is not reflected when they are dispersed in bulk polymer matrices.
The increase in polymer-CN membranes’ tensile strength and subsequently, Young’s Modulus, does not scale linearly with CN loading. Currently, optimal CN loading is achieved at low weight percent, as CN aggregation occurs at higher loadings leading to inhomogeneity. The challenges to and limitations of incorporating CNs in membranes are discussed in more depth at the end of this section. Despite these limitations, impressive tensile enhancements have been achieved with both forms of CN – CNCs and CNFs. In most cases, the addition of CNs caused an increase in Young’s Modulus due to an increase in maximum tensile stress and a slight reduction in strain-at-break. The greatest increase in Young’s Modulus was achieved for PVDF membrane distillation membranes. The addition of 2 wt % CNC increased the Young’s Modulus of the PVDF membranes by 45.8%.70 The greatest increase in tensile stress was achieved for PES ultrafiltration membranes where the addition of 1 wt % CNF increased the tensile stress by 42.4%.71 Details of tensile strength and Young’s Modulus for several polymer-CN composites are presented in Table 2.
Table 2.
Summary of the Stress, Strain, Young’S Modulus and Percent Change of Membranes Formed from Various Cellulose Nanomaterial-Polymer Composites
| membrane type | material | stress (MPa) | % change in stress | strain (%) | Young’s Modulus (MPa) | % change in Young’s Modulus | no. of replicates | ref |
|---|---|---|---|---|---|---|---|---|
| MD electrospun fibers | PVDF-HFP | 12.6 | 17.5a | 72 | 3 | 70 | ||
| PVDF-HFP + 2 wt % CNC | 17.2 | 36.5 | 16.4a | 105 | 45.8 | |||
| UF | PES | 5.1 | 9 | 56.7a | NA | 71 | ||
| PES + 1 wt % CNF | 7.3 | 42.2 | 11.6 | 62.5a | 10.2 | |||
| PES + 4 wt % CNF | 5.5 | 7.8 | 10.3 | 53.4a | −5.8 | |||
| NF | PVA | 57 | NA | NA | NA | 5 | 74 | |
| PVA + 5 wt % CNC | 75.2 | 31.9 | ||||||
| CNF | 129 | 10.3a | 1250 | 10 | 76 | |||
| CNF + PHB | 142 | 10.1 | 9.2a | 1550 | 24 | |||
| CNF | 35.9 | 5 | 681.7 | NA | 106 | |||
| CNF + CdSe | 31.9 | −11.1 | 6.2 | 325.9 | −52.2 | |||
| UF | CA | 4.6 | 6 | 77.2 | 3 | 69 | ||
| CA + CNF | 6.1 | 31.5 | 11.4 | 53.8 | −30.3 |
These values were determined from information provided in the published works.
3.2.2. Hydrophilicity, Permeability, and Separation
In all polymer-CN composite membranes, as in polymer-CNT membranes, the addition of CNs caused some morphological and structural differences compared with membranes made of the polymer alone. At low CN loadings (<4 wt %), membranes show increased porosity, larger pore sizes, and greater surface hydrophilicity, all of which lead to greater water permeability, and occasionally lead to slightly higher molecular weight cutoffs. Higher CN loadings yield smaller membrane pore sizes. Qu et al. formed UF membranes composed of poly- (ethersulfone) (PES) blended with CNFs.71 The pure water flux of the membranes increased significantly with the addition of 1 wt % CNFs, as did the mean pore size and the porosity. With higher CNF loading, the mean pore size dropped from a maximum of 70.9–53 nm. This observed increase in surface hydrophilicity and permeability at low loadings and subsequently lower permeability at high loadings is very similar to results obtained from polymer nanocomposite membrane formed with the addition of functionalized CNTs.78,80,81 The smaller pore size may be a result of increased solution viscosity, which reduces pore size during inversion precipitation.69 Reported morphological effects of CNs on polymer membranes are summarized in Table 3.
Table 3.
Effect of Cellulose Nanomaterials on Pore Size and Porosity in Polymer Nanocomposites
| type of membrane | material | max pore size | average pore size | porosity | reference |
|---|---|---|---|---|---|
| MF electrospun fibers | PAN | 780 nm | 660 nm | 80% | 68 |
| PAN + PVAm-CNF | 730 nm | 380 nm | 80% | ||
| MD electrospun fibers | PVDF-HFP | 700 nm | 500 nm | >75% | 70 |
| PVDF-HFP + 1 wt % CNC | 400 nm | 330 nm | 75% | ||
| PVDF-HFP + 4 wt % CNC | 270 nm | 60% | |||
| UF | PES | 50 nm | 88% | 71 | |
| PES + 1 wt % CNF | 70.9 nm | 55% | |||
| PES + 4 wt % CNF | 53 nm | 57% | |||
| UF hemodialysis | CNF | 21 nm | 77 | ||
| CNF + PPy | 53 nm | ||||
| NF | CNF (5 nm) | 1.6 – 2.4 nm | 64 | ||
| CNF (50 nm) | 5 – 5.5 nm |
Cellulose nanomaterial-polymer blends may also be electrospun into fibers. Higher concentrations of CNs appear to increase the diameter of electrospun fibers due to increases in solution viscosity. These larger diameter fibers result in membrane mats with narrower pore size distributions and overall smaller pore sizes, as was observed with PVDF-HFP electrospun fibers modified by CNC.70,71 Similarly, nanopapers produced from pure CNF had smaller pores with greater masses of CNs. This was likely caused by partial pore blocking from overlapping nanofibers.64
While it is anticipated that CNs should increase the hydrophilicity of many polymers when introduced as a composite, there is limited data documenting this effect. In the fabrication of UF membranes from PES, Qu et al. determined that membranes containing CNFs had contact angles of 45.8° compared to 55.8° for pure PES membranes corresponding to an increase in surface energy from 113.7 mN/m2 to 123.5 mN/m2 upon inclusion of CNFs.71
The aforementioned CN-induced increases in membrane pore size directly affected flux as well. PES membranes with 1 wt % CNF, for example, showed an elevated pure water flux (813.3 L/m2/h) and similar BSA rejection (92%) compared to pure PES membranes (340 L/m2/h and 94.6%, respectively).71 At a higher cellulose loading of 4 wt %, pure water flux was 780 L/m2h, with 94.6% BSA rejection. This last case is revealing in comparison to pure PES membranes, as these two membranes had approximately the same pore size and identical rejections, but very different values of permeate flux. The inclusion of CNs directly resulted in a greater porosity, demonstrating one of the great benefits of including CNs into polymer membranes.
The high surface area of the CN structures may yield transient benefits in adsorptive removal in addition to size-based separation. For example, microfiltration membranes composed of two-layered nanoscale polyacrylonitrile (PAN)/microscale polyethylene terephthalate (PET) fibrous scaffold were doped with functional “ultra-fine” CNFs.68,75 These microfiltration membranes were tested for removal of a wide range of contaminants (i.e., E. coli, MS2 viruses, Cr(VI) and Pb(II) heavy metals) using both size exclusion sieving for E. coli and charge-mediated adsorption provided by amine functional groups for MS2 viruses and heavy metals.68 Similar charge-mediated separation and adsorption, supported by the high surface area of CNs, was employed by Ferraz et al. and Razzaq et al. in their hemodialysis experiments for the separation of uremic toxins and DNA, respectively.65,77
The laboratories of Dr. Hsiao and Dr. Chu at SUNY Stony Brook have done significant work developing thin-film nanofibrous composite (TFNC) membranes where CNFs are employed as a barrier layer.75,82–87 These asymmetric membrane structures are composed of a bottom support layer that is a strong, nonwoven material to provide strength and an upper layer(s) with smaller pore sizes and greater functionality to provide selectivity. The use of nanofibers in the barrier layer provide control over fine pore structure due to their small diameter and high surface area-to-volume ratio, yielding membranes with 10-fold higher permeation flux than commercial UF membranes while maintaining >99.5% rejection ratio in water/oil emulsion.82 The pore structure can further be controlled by including a cross-linking polymer within the CNF-based barrier layer.87 Recently, the concept of directed water channels is considered to be the key to achieving high water permeability without sacrificing rejection. The hypothesis is that interconnected pores in the barrier layer are small enough to reject large contaminants, while allowing water to pass through.84 Numerous candidates have been presented for forming these directed water channels, including CNTs, and they are discussed in detail elsewhere.84 A cheaper, lower environmental impact method has been developed by Dr. Hsiao’s and Dr. Chu’s laboratories by relying on the small space between the CNFs and the matrix polymer in the barrier layer to serve as the interconnected water directing channels. Flux and selectivity can be controlled by tuning the gap between the fiber and matrix through physical and chemical properties of the two components.84,86,87
Unlike most polymer membranes, mats formed purely from CNs were shown to be highly stable under exposure to organic solvents suggesting promising applications in the filtration of industrial waste streams. Mautner et al. demonstrated that CNF mats could be used in solvent separation with increasing permeability corresponding to increasing hydrophobicity of the solvent.64
3.2.3. Antibiofouling and Biocompatibility
Many membrane studies have demonstrated that hydrophobic surfaces encourage protein adsorption, which often leads to biofouling and membrane inactivation.88,89 The addition of CNs to polymer membranes has generally been shown to increase membrane hydrophilicity, and some studies have linked the inclusion of CNs in membranes to lower surface protein adsorption. CNF-doped cellulose acetate membranes, for example, showed a 30% increase in flux recovery ratio following hydraulic washing compared to undoped membranes.69
As CNs are naturally derived, unlike CNTs, it is generally assumed that CNs are biocompatible, and several studies have substantiated this assumption. Ferraz et al. developed high surface area CNF-polypyrrole (PPy) composites as electro-actively assisted hemodialysis membranes.90 In a series of passive ultrafiltration (dialysis) experiments, these composites were tested for their biocompatibility. Using complement activation products (i.e., model compounds used to evaluate immune response compatibility), composite cellulose-PPy membranes were shown to be more biocompatible than reference materials, including cellulose acetate, unmodified regenerated cellulose, and polysulfone.
Similarly, PPy was combined with CNFs to form high-capacity conductive paper sheets for electrochemically controlled extraction of DNA oligomers.65 Results indicated that this composite material could be an inexpensive, efficient, and biocompatible material as an ion-exchange membrane for the separation of biomolecules.
3.2.4. Current Challenges and Limitations
One of the biggest challenges in working with polymer-CN composites, as with CNT-polymer composites,91 is achieving uniform and homogeneous dispersion within polymer matrices. Homoaggregation of CNs negatively affects both amorphous and semicrystalline polymers by disrupting the homogeneity of the polymer solutions. Specifically, homoaggregation causes local inhomogeneity within amorphous polymers and disturbs polymer alignment and thus polymer crystallinity in semicrystalline polymers. The studies reviewed here tend to indicate diminishing or reduced benefits to composite properties at higher CN loadings. For example, the fact that tensile strength and Young’s Modulus do not increase monotonically with CN loading in the composite may be attributed to agglomeration of the nanocellulose, which caused inhomogeneity of the polymer material. Regions of inhomogeneity may be focal points for stress in the composite.70 Further it has been suggested that while CNFs theoretically provide better stress support to polymer systems, they are more difficult to disperse than CNCs. For example, homogeneously dispersed CNCs in PEO produced membranes with higher strain-at-failure than those composed of CNF-doped PEO.72
Of particular pertinence to membranes was the observation that the mechanical properties of the CN-polymer composites may be sensitive to changes in humidity. For PEI-CNC films, for example, the Young’s Modulus at relative humidity values of 30%, 42%, and 64% was found to be 16, 12, and 3.5 GPa, respectively.92 Water sorption starts primarily at the hydroxyl groups on the surface of CNs, and therefore reduces some of the cellulose properties, including their mechanical properties. If the bulk polymer is highly hydrophilic, however, such as starch-based polymers, the CNs have been shown to decrease water vapor sorption. CNs may increase the hydrophilicity of the membranes to improve water diffusion, but this may reduce their mechanical properties. Using inherently hydrophilic polymers prevents the reduction of CN’s mechanical properties, but this may also reduce the water diffusion through the membrane. There is a trade-off, therefore, between the increased hydrophilicity and increased mechanical strength associated with incorporating CNs into polymer membranes.36
More study is needed, using standardized conditions to evaluate membrane mechanical properties. Published results on CN-based membranes’ tensile stress are confounded by oftentimes-low numbers of repeated tensile stress tests (<5 repeated measurements), nonstandardized measurement practices (wet vs dry sample tests), opaque stress–strain curve analyses in which distinctions between elastic and inelastic deformation curves are not discussed, and a failure to consider the impacts on tensile stress of morphological changes (i.e., pore size, void space, porosity) caused by CN inclusion into polymer matrices. Standard stress–strain practices must be employed across nanocomposite materials in order to easily compare research and advance the development of enhanced polymer-CN composite membranes.
While the biodegradability of cellulose nanomaterials is a great advantage in many applications, there is some concern that when incorporated into membranes that interact with bacteria, they may degrade rapidly. However, CNs used as additives in membranes might not degrade if they are stably contained within the bulk of the polymers that compose the membranes. The polymers would most likely protect the cellulose from degradation, but this hypothesis has yet to be tested and requires further research.
4. SUMMARY AND OUTLOOK
Cellulose nanomaterials represent a new class of sustainable materials with recognized potential in improving paper and packaging as well as the automotive, construction, personal care and textile industries.8 In this review, we posit that CNs holds great promise in environmental engineering applications as well including environmental remediation and water filtration membranes. With structure and strength properties reminiscent of CNTs, CNs may serve as an inexpensive, renewable and biodegradable alternative to CNTs. While isolation and processing of CNs remain costly and energy intensive, the inputs are far below those required for CNT preparation. As more industries realize the potential of CNs and invest in their development, new efficient methods of production that increase yield and decrease costs will arise. For example, researchers from University of Texas at Austin are engineering blue-green algae to synthesize CNCs in order to create a production method that does not require energy intensive homogenization or harsh chemicals.93 Still others are focused on finding alternative feedstocks for isolating CNs. For example, Blue Goose Biorefineries is processing CNs from biomass with high cellulose content, including pulp, recycled paper, pulp mill screening rejects, and cotton linters.94 Melodea Ltd. is developing a method to extract CNC from the sludge of the paper industry.95 The infrastructure for this highly available source material is already in place and would put value to a waste product.
The anticipated success of CNs is evident in the exponential growth in CN-related patents published over the last 10 years. However, this rush by many different industries to stake claim in the CN market may actually be stunting progress overall. For example, the lack of a standardized naming system is a burden on regulatory and commercialization efforts. With the great benefits of CNs in the area of environmental engineering and beyond, those interested should maintain open and willing communication to secure investment, development, and commercialization of this promising material.
Acknowledgments
This work was partially funded by the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program Center Grant (P42ES010356) and by the National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under NSF Cooperative Agreement EF-0830093 and DBI-1266252, Center for the Environmental Implications of NanoTechnology (CEINT). Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF or the EPA. This work has not been subjected to EPA review and no official endorsement should be inferred.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Herrick FW, Casebier RL, Hamilton JK, Sandberg KR. Microfibrillated cellulose: Morphology accessibility. J Appl Polym Sci: Appl Polym Symp Syracuse, NY, Syracuse, NY. 1983 [Google Scholar]
- 2.Charreau H, Foresti ML, Vazquez A. Nanocellulose patents trends: A comprehensive review on patents on cellulose nanocrystals, microfibrillated and bacterial cellulose. Recent Pat Nanotechnol. 2013;7:56–80. [PubMed] [Google Scholar]
- 3.Lavoine N, Desloges I, Dufresne A, Bras J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr Polym. 2012;90:735–764. doi: 10.1016/j.carbpol.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Mariano M, Kissi NE, Dufresne A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J Polym Sci, Part B: Polym Phys. 2014;52:791–806. [Google Scholar]
- 5.Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A. Nanocelluloses: A new family of nature-based materials. Angew Chem, Int Ed. 2011;50:5438–5466. doi: 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- 6.Khalil HPSA, Davoudpour Y, Islam MN, Mustapha A, Sudesh K, Dungani R, Jawaid M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohyd Polym. 2014;99:649–665. doi: 10.1016/j.carbpol.2013.08.069. [DOI] [PubMed] [Google Scholar]
- 7.Brodin FW, Gregersen OW, Syverud K. Cellulose nanofibrils: Challenges and possibilities as a paper additive or coating material—A review. Nord Pulp Pap Res J. 2014;29:156–166. [Google Scholar]
- 8.Shatkin JA, Wegner TH, Bilek EM, Cowie J. Market projections of cellulose nanomaterial-enable products - part 1. Applications. TAPPI J. 2014;13:9–16. [Google Scholar]
- 9.Reddy MM, Vivekanandhan S, Misra M, Bhatia SK, Mohanty AK. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog Polym Sci. 2013;38:1653–1689. [Google Scholar]
- 10.Paunonen S. Strength and barrier enhancements of composites and packaging boards by nanocelluloses - a literature review. Nord Pulp Pap Res J. 2013;28:165–181. [Google Scholar]
- 11.Johansson C, Bras J, Mondragon I, Nechita P, Plackett D, Simon P, Svetec DG, Virtanen S, Baschetti MG, Breen C, Clegg F, Aucejo S. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. Bioresources. 2012;7:2506–2552. [Google Scholar]
- 12.Shi ZJ, Zhang Y, Phillips GO, Yang G. Utilization of bacterial cellulose in food. Food Hydrocolloids. 2014;35:539–545. [Google Scholar]
- 13.Plackett DV, Letchford K, Jackson JK, Burt HM. A review of nanocellulose as a novel vehicle for drug delivery. Nord Pulp Pap Res J. 2014;29:105–118. [Google Scholar]
- 14.Kolakovic R, Peltonen L, Laaksonen T, Putkisto K, Laukkanen A, Hirvonen J. Spray-dried cellulose nanofibers as novel tablet excipient. Aaps Pharmscitech. 2011;12:1366–1373. doi: 10.1208/s12249-011-9705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalia S, Boufi S, Celli A, Kango S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym Sci. 2014;292:5–31. [Google Scholar]
- 16.Miao CW, Hamad WY. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose. 2013;20:2221–2262. [Google Scholar]
- 17.Jorfi M, Foster EJ. Recent advances in nanocellulose for biomedical applications. J Appl Polym Sci. 2015:132. [Google Scholar]
- 18.Abeer MM, Amin M, Martin C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J Pharm Pharmacol. 2014;66:1047–1061. doi: 10.1111/jphp.12234. [DOI] [PubMed] [Google Scholar]
- 19.Domingues RMA, Gomes ME, Reis RL. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules. 2014;15:2327–2346. doi: 10.1021/bm500524s. [DOI] [PubMed] [Google Scholar]
- 20.Shelke NB, James R, Laurencin CT, Kumbar SG. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polymer Adv Technol. 2014;25:448–460. [Google Scholar]
- 21.Silvestre AJD, Freire CSR, Neto CP. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin Drug Delivery. 2014;11:1113–1124. doi: 10.1517/17425247.2014.920819. [DOI] [PubMed] [Google Scholar]
- 22.Dugan JM, Gough JE, Eichhorn SJ. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine. 2013;8:287–298. doi: 10.2217/nnm.12.211. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes EM, Pires RA, Mano JF, Reis RL. Bionanocomposites from lignocellulosic resources: Properties, applications and future trends for their use in the biomedical field. Prog Polym Sci. 2013;38:1415–1441. [Google Scholar]
- 24.Petersen N, Gatenholm P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl Microbiol Biotechnol. 2011;91:1277–1286. doi: 10.1007/s00253-011-3432-y. [DOI] [PubMed] [Google Scholar]
- 25.Lafitte V, Lee JC, Ali SA, Sullivan PF. U.S. Patent 20130274149. Fluids and Methods Including Nanocellulose. 2013 Apr 5;
- 26.Potapenko DI, Nesterova SV, Lecerf B, Ivanov MG, Fu D, Bulova MN. WO Patent 2013085412. Well Treatment with High Solids Content Fluids. 2013 Jun 13;
- 27.Lin N, Huang J, Dufresne A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale. 2012;4:3274–3294. doi: 10.1039/c2nr30260h. [DOI] [PubMed] [Google Scholar]
- 28.Wegner TH, Ireland S, Jones JPE. Cellulosic nanomaterials: Sustainable materials of choice for the 21st century. In: Postek MT, Moon RJ, Rudie AW, Bilodeau MA, editors. Production and Applications of Cellulose Nanomaterials. TAPPI Press; Peachtree Corners, GA: 2012. [Google Scholar]
- 29.Wei H, Rodriguez K, Renneckar S, Vikesland PJ. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ Sci : Nano. 2014;1:302–316. [Google Scholar]
- 30.Karn B, Kuiken T, Otto M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ Health Perspect. 2009;17:1823–1831. doi: 10.1289/ehp.0900793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balamurugan R, Sundarrajan S, Ramakrishna S. Recent trends in nanofibrous membranes and their suitability for air and water filtrations. Membranes. 2011;1:232–248. doi: 10.3390/membranes1030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebald C, Wurzbacher JA, Borgschulte A, Zimmermann T, Steinfeld A. Single-component and binary co2 and h2o adsorption of amine-functionalized cellulose. Environ Sci Technol. 2014;48 doi: 10.1021/es404430g. [DOI] [PubMed] [Google Scholar]
- 33.Gebald C, Wurzbacher JA, Tingaut P, Steinfeld A. Stability of amine-functionalized cellulose during temperature-vacuum-swing cycling for co2 capture from air. Environ Sci Technol. 2013;47:10063–10070. doi: 10.1021/es401731p. [DOI] [PubMed] [Google Scholar]
- 34.Hansen F, Brun V, Keller E, Nieh W, Wegner T, Meador M, Friedersdorf L. USDA Forest Service, N. N, I, editor. Cellulose nanomaterials- a path towards commercialization worshop report. Washington D.C: 2014. [Google Scholar]
- 35.Nieh WL-S. Current international standards development activities for cellulose nanomaterials. In: Postek MT, Moon RJ, Rudie AW, Bilodeau MA, editors. Production and Applications of Cellulose Nanomaterials. TAPPI Press; Peachtree Corners, GA: 2013. pp. 213–214. [Google Scholar]
- 36.Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941–3994. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- 37.Gatenholm P, Klemm D. Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull. 2010;35:208–213. [Google Scholar]
- 38.Su Y, Burger C, Hsiao BS, Chu B. Characterization of tempo-oxidized cellulose nanofibers in aqueous suspension by small-angle x-ray scattering. J Appl Crystallogr. 2014;47:788–798. [Google Scholar]
- 39.Habibi Y. Key advances in the chemical modification of nanocelluloses. Chem Soc Rev. 2014;43:1519–1542. doi: 10.1039/c3cs60204d. [DOI] [PubMed] [Google Scholar]
- 40.Hubbe MA, Rojas OJ, Lucia LA, Sain M. Cellulosic nanocomposites: A review. Bioresources. 2008;3:929–980. [Google Scholar]
- 41.Li QQ, McGinnis S, Sydnor C, Wong A, Renneckar S. Nanocellulose life cycle assessment. ACS Sustainable Chem Eng. 2013;1:919–928. [Google Scholar]
- 42.Lin N, Dufresne A. Nanocellulose in biomedicine: Current status and future prospect. Eur Polym J. 2014;59:302–325. [Google Scholar]
- 43.Fecht HJ, Bruhne K. Carbon-Based Nanomaterials and Hybrids. Taylor & Francis Group; Boca Raton, FL: 2014. [Google Scholar]
- 44.Chirayil CJ, Mathew L, Thomas S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev Adv Mater Sci. 2014;37:20–28. [Google Scholar]
- 45.Brinchi L, Cotana F, Fortunati E, Kenny JM. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr Polym. 2013;94:154–169. doi: 10.1016/j.carbpol.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J Environ Sci. 2013;25:933–943. doi: 10.1016/s1001-0742(12)60145-4. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava S, Kardam A, Raj KR. Nanotech reinforcement onto cellulose fibers: Green remediation of toxic metals. Int J Green Nanotechnol. 2012;4:46–53. [Google Scholar]
- 48.Ma H, Hsiao BS, Chu B. Ultrafine cellulose nanofibers as efficient adsorbents for removal of uo22+ in water. ACS Macro Lett. 2012;1:213–216. doi: 10.1021/mz200047q. [DOI] [PubMed] [Google Scholar]
- 49.Yang R, Aubrecht KB, Ma HY, Wang R, Grubbs RB, Hsiao BS, Chu B. Thiol-modified cellulose nanofibrous composite membranes for chromium(vi) and lead(ii) adsorption. Polymer. 2014;55:1167–1176. [Google Scholar]
- 50.Singh K, Arora JK, Sinha TJM, Srivastava S. Functionalization of nanocrystalline cellulose for decontamination of cr(iii) and cr(vi) from aqueous system: Computational modeling approach. Clean Techn Environ Policy. 2014;16 [Google Scholar]
- 51.Zhu H, Jia S, Wan T, Jia Y, Yang H, Li J, Yan L, Zhong C. Biosynthesis of spherical fe3o4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohyd Polym. 2001;86:1558–1564. [Google Scholar]
- 52.Korhonen JT, Kettunen M, Ras RHA, Ikkala O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl Mater Interfaces. 2011;3:1813–1816. doi: 10.1021/am200475b. [DOI] [PubMed] [Google Scholar]
- 53.Jiang F, Hsieh YL. Amphiphilic superabsorbent cellulose nanofibril aerogels. J Mater Chem A. 2014;2:6337–6342. [Google Scholar]
- 54.Zhang Z, Sèbe G, Rentsch D, Zimmermann T, Tingaut P. Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem Mater. 2014;26:2659–2668. [Google Scholar]
- 55.Wang Y, Yadav S, Heinlein T, Konjik V, Breitzke H, Buntkowsky G, Schneider JJ, Zhang K. Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids. RSC Adv. 2014;4:21553–21558. [Google Scholar]
- 56.Zhou Y, Fu S, Zhang L, Zhan H, Levit MV. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for pb(ii) Carbohyd Polym. 2014;101:75–82. doi: 10.1016/j.carbpol.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 57.Yu X, Tong S, Ge M, Zuo J, Cao C, Song W. One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J Mater Chem A. 2013;1:959–965. [Google Scholar]
- 58.Nata IF, Sureshkumar M, Lee CK. One-pot preparation of amine-rich magnetite/bacterial cellulose nanocomposite and its application for arsenate removal. RSC Adv. 2011;1:625–631. [Google Scholar]
- 59.Sun D, Yang J, Wang X. Bacterial cellulose/tio2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale. 2010;2:287–292. doi: 10.1039/b9nr00158a. [DOI] [PubMed] [Google Scholar]
- 60.Snyder A, Bo ZY, Moon R, Rochet JC, Stanciu L. Reusable photocatalytic titanium dioxide-cellulose nanofiber films. J Colloid Interface Sci. 2013;399:92–98. doi: 10.1016/j.jcis.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Yu J, Fan J, Sun D, Tang W, Yang X. Biotemplated preparation of cds nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J Hazard Mater. 2011;189:377–383. doi: 10.1016/j.jhazmat.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 62.Shatkin JA, Wegner TH, Neih W. Incorporating life-cycle thinking into risk assessment for nanoscale materials: Case study of nanocellulose. In: Postek MT, Moon RJ, Rudie AW, Bilodeau MA, editors. Production and Applications of Cellulose Nanomaterials. TAPPI PRESS; Peachtree Corners, GA: 2013. [Google Scholar]
- 63.Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- 64.Mautner A, Lee K-Y, Lahtinen P, Hakalahti M, Tammelin T, Li K, Bismarck A. Nanopapers for organic solvent nanofiltration. Chem Commun. 2014;50 doi: 10.1039/c4cc00467a. [DOI] [PubMed] [Google Scholar]
- 65.Razaq A, Nyström G, Strømme M, Mihranyan A, Nyholm L. High capacity conductive nanocellulose paper sheets for electrochemical controlled extraction of DNA oligomers. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Q, Meng Y, Concha K, Wang S, Li Y, Ma L, Fu S. Influence of temperature and humidity on nano-mechanical properties of cellulose nanocrystal films made from switchgrass and cotton. Ind Crops Prod. 2013;48 [Google Scholar]
- 67.Fernandes SCM, Sadocco P, Aonso-Varona A, Palomares T, Eceiza A, Silvestre AJD, Mondragon I, Freire CSR. Bioinspired antimicrobial and biocompativle bacterial cellulose membranes obtained by surface functionalization with aminoalkyl groups. ACS Appl Mater Interfaces. 2013;5:3290–3297. doi: 10.1021/am400338n. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, Guan S, Sato A, Wang X, Wang Z, Yang R, Hsiao BS, Chu B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J Membr Sci. 2013;446:376–382. [Google Scholar]
- 69.Kong L, Zhang DM, Shao Z, Han B, Lv Y, Gao K, Peng X. Superior effect of tempo-oxidized cellulose nanofibrils (tocns) on the performance of cellulose triacetate (cta) ultrafiltration membrane. Desalination. 2014;332:117–125. [Google Scholar]
- 70.Lalia BS, Guillen E, Arafat HA, Hashaikeh Nanocrystalline cellulose reinforced pvdf-hfp membranes for membrane distillation application. Desalination. 2013;332 [Google Scholar]
- 71.Qu P, Tang H, Gao Y, Zhang L, Wang S. Polyethersulfone composite membrane blended with cellulose fibrils. BioRes. 2010;5:2323–2336. [Google Scholar]
- 72.Xu X, Liu F, Jiang L, Zhu JY, Haagenson D, Wiesenborn DP. Cellulose nanocrystals vs cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl Mater Interfaces. 2013;5:2999–3009. doi: 10.1021/am302624t. [DOI] [PubMed] [Google Scholar]
- 73.Pakzad A, Simonsen J, Yassar RS. Elastic properties of thin poly(vinyl alcohol)-cellulose nanocrystal membranes. Nanotechnology. 2012;23:1–9. doi: 10.1088/0957-4484/23/8/085706. [DOI] [PubMed] [Google Scholar]
- 74.Xu X, Yang YQ, Xing YY, Yang JF, Wang SF. Properties of novel polyvinyl alcohol/cellulose nanocrystals/silver nanoparticles blend membranes. Carbohyd Polym. 2013;98:1573–1577. doi: 10.1016/j.carbpol.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 75.Ma HY, Burger C, Hsiao BS, Chu B. Nanofibrous microfiltration membrane based on cellulose nanowhiskers. Biomacromolecules. 2012;13:180–186. doi: 10.1021/bm201421g. [DOI] [PubMed] [Google Scholar]
- 76.Barud HS, Souza JL, Santos DB, Crespi MS, Ribeiro CA, Messaddeq Y, Ribeiro SJL. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr Polym. 2011;83:1279–1284. [Google Scholar]
- 77.Ferraz N, Leschinskaya A, Toomadj F, Fellström B, Strømme M, Mihranyan A. Membrane characterization and solute diffusion in porous composite nanocellulose membranes for hemodialysis. Cellulose. 2013;20 [Google Scholar]
- 78.de Lannoy CF, Soyer E, Wiesner MR. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J Membr Sci. 2013;447:395–402. [Google Scholar]
- 79.Shawky HA, Chae SR, Lin S, Wiesner MR. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination. 2011;272:46–50. [Google Scholar]
- 80.Celik E, Park H, Choi H, Choi H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2010;45:274–282. doi: 10.1016/j.watres.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 81.Choi JH, Jegal J, Kim WN. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J Membr Sci. 2006;284:406–415. [Google Scholar]
- 82.Ma H, Burger C, Hsiao BS, Chu B. Ultra-fine polysaccharide nanofibrous membranes for water purification. Biomacromolecules. 2011;12:970–976. doi: 10.1021/bm1013316. [DOI] [PubMed] [Google Scholar]
- 83.Ma H, Burger C, Hsiao BS, Chu B. Ultra-fine cellulose nanofibers: New nano-scale materials for water purification. J Mater Chem. 2011;21:7501–7510. [Google Scholar]
- 84.Ma H, Burger C, Hsiao BS, Chu B. Highly permeable polymer membranes containing directed channels for water purification. ACS Macro Lett. 2012;1:723–726. doi: 10.1021/mz300163h. [DOI] [PubMed] [Google Scholar]
- 85.Ma H, Burger C, Hsiao BS, Chu B. Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J Membr Sci. 2014;454:272–282. [Google Scholar]
- 86.Wang X, Yeh TM, Wang Z, Yang R, Wang R, Ma H, Hsiao BS, Chu B. Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer. 2014;55:1358–1366. [Google Scholar]
- 87.Wang Z, Ma H, Hsiao BS, Chu B. Nanofibrous ultrafiltration membranes containing cross-linked poly(ethylene glycol) and cellulose nanofiber composite barrier layer. Polymer. 2014;55:366–372. [Google Scholar]
- 88.Mansouri J, Harrisson S, Chen V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J Mater Chem. 2010;20:4567–4586. [Google Scholar]
- 89.Rana D, Matsuura T. Surface modifications for antifouling membranes. Chem Rev. 2010;110:2448–2471. doi: 10.1021/cr800208y. [DOI] [PubMed] [Google Scholar]
- 90.Ferraz N, Carlsson DO, Hong J, Larsson R, Fellström B, Nyholm L, Strømme M, Mihranyan A. Haemocompatibility and ion exchange capability of nanocellulose polypyrrole membranes intended for blood purification. J R Soc Interface. 2012:1–13. doi: 10.1098/rsif.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie X-L, Mai Y-W, Zhou X-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater Sci Eng. 2005;49 [Google Scholar]
- 92.Cranston ED, Eita M, Johansson E, Netrval J, Salajková M, Arwin H, Wågberg L. Determination of young’s modulus for nanofibrillated cellulose multilayer thin films using buckling mechanics. Biomacromolecules. 2011;12:961–969. doi: 10.1021/bm101330w. [DOI] [PubMed] [Google Scholar]
- 93.Czaja W, Romanovicz D, Brown RM. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose. 2004;11:403–411. [Google Scholar]
- 94.Blue Goose Biorefineries Inc. http://bluegoosebiorefineries.com.
- 95.Melodea Bio Based Solutions. http://www.melodea.eu.
- 96.Jiang F, Hsieh YL. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohyd Polym. 2013;95:32–40. doi: 10.1016/j.carbpol.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 97.Wu X, Moon RJ, Martini A. Tensile strength of iβ crystalline cellulose predicted by molecular dynamics simulation. Cellulose. 2014;21:2233–2245. [Google Scholar]
- 98.Saito T, Kuramae R, Wohlert J, Berglund LA, Isogai A. An ultrastrong nanofibrillar biomaterial: The strength of single cellulose nanofibrils revealed via sonication-induced fragmentation. Biomacromolecules. 2013;14:248–253. doi: 10.1021/bm301674e. [DOI] [PubMed] [Google Scholar]
- 99.Dresselhaus MS, Dresselhaus G, Charlier JC, Hernandez E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos Trans R Soc A. 2004;362:2065–2098. doi: 10.1098/rsta.2004.1430. [DOI] [PubMed] [Google Scholar]
- 100.Ebbesen TW, Lezec HJ, Hiura H, Bennett JW, et al. Electrical conductivity of individual carbon nanotubes. Nature. 1996;382:54–56. [Google Scholar]
- 101.Postek MT, Moon RJ, Rudie AW, Bilodeau MA. Production and Applications of Cellulose Nanomaterials. TAPPI PRESS; Peachtree Corners, GA: 2013. [Google Scholar]
- 102.Kim HC, Fthenakis V. Life cycle energy and climate change implications of nanotechnologies. J Ind Ecol. 2012;17:528–541. [Google Scholar]
- 103.De Volder MFL, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: Present and future commercial applications. Science. 2013;339 doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 104.Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SAK, Tran CL, Christensen FM. Review of carbon nanotubes toxicity and exposure-appraisal of human health risk assessment based on open literature. Crit Rev Toxicol. 2010;40:759–790. doi: 10.3109/10408444.2010.506638. [DOI] [PubMed] [Google Scholar]
- 105.Vikman M, Vartiainen J, Tsitko I, Korhonen P. Biodegradability and compostability of nanofibrillar cellulose-based products. J Polym Environ. 2014 doi: 10.1007/s10924-014-0694-3. [DOI] [Google Scholar]
- 106.Yang Z, Chen S, Hu W, Yin N, Zhang W, Xiang C, Wang H. Flexible luminescent cdse/bacterial cellulose nanocomposite membranes. Carbohyd Polym. 2012;88:173–178. [Google Scholar]