Abstract
Background and Aims
The advantages of endoscopic ultrasound (EUS) and computed tomography-positron emission tomography (CT-PET) in relation to survival in esophageal cancer (EC) patients are unclear. This study aimed to assess the effect of EUS, CT-PET and its combination on overall survival relative to cases not receiving these procedures.
Methods
Patients aged ≥ 66 years diagnosed with EC were identified in the Surveillance, Epidemiology, and End Results-Medicare linked database. Cases were split into four analytic groups: EUS only (n=318), CT-PET only (853), EUS+CT-PET (189) and “no EUS or CT-PET” (2,439). Survival times were estimated by Kaplan-Meier method and compared by using log-rank test for each group versus the “no EUS or CT-PET” group. Multivariable Cox proportional hazards models were used to compare 1, 3 and 5-year survival rates.
Results
Kaplan-Meier analyses showed that patients undergoing EUS, CT-PET and EUS+CT-PET had improved survival for all stages, all compared with “no EUS or CT-PET”, with the exception of stage 0 disease. Receipt of EUS increased the likelihood of receiving endoscopic therapies, esophagectomy and chemoradiation. Multivariable Cox proportional hazards models showed that receipt of EUS was a significant predictor for improved 1-year (HR 0.49, 95% CI 0.39–0.59, p<0.0001), 3-year (HR 0.57, 95% CI 0.48–0.66, p<0.0001) and 5-year (HR 0.59, 95% CI 0.50–0.68) survival. Similar results were noted when results were stratified based on histology, as well as for CT-PET and EUS+CT-PET groups.
Conclusions
Receipt of either EUS or CT-PET alone in EC patients is associated with improved 1, 3 and 5-year survival. Future studies should identify barriers to dissemination of these staging modalities.
Keywords: Esophageal cancer, endoscopic ultrasonography, positron emission tomography and computed tomography, survival, Staging
INTRODUCTION
The incidence of esophageal cancer continues to increase faster than any other cancer in the Western World.1 Most recent estimates in the United States suggest that 17,460 people will be diagnosed annually with esophageal cancer and 15,070 people will die from the disease.2 Patients with esophageal cancer continue to have dismal chances of surviving beyond 5-years (<20% overall survival), despite recent advances in diagnostic and treatment modalities.2
Tumor stage is considered to be the most important prognostic determinant in patients with esophageal cancer.3 Accurate staging is therefore of paramount importance for guiding treatment and preventing futile surgical explorations. Esophagectomy performed on patients with inaccurately staged disease can have negative impacts on quality of life.4, 5 Radical surgery with curative intent is only possible if distant metastasis and infiltration of the primary tumor into adjacent vital structures are absent. In addition, several studies including meta-analyses have demonstrated an improved survival rate in patients with locally advanced disease treated with neoadjuvant chemoradiation followed by esophagectomy.6
Currently, endoscopic ultrasonography (EUS) has been established as the most accurate modality for preoperative locoregional staging (T and N staging) of esophageal cancer and superior to other imaging modalities such as computed tomography (CT), positron emission tomography (PET) and magnetic resonance imaging.5,7,8 Whole body positron emission tomography with 18-Fluorodeoxyglucose (FDG-PET) or CT-PET is widely used to detect distant nodal and systemic metastases. It has been suggested that a combination of EUS and CT-PET improves preoperative staging of esophageal cancer.9,10
A previous study that compared health care costs and effectiveness of multiple staging options for patients with esophageal cancer that included CT, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), PET, thoracoscopy/laparoscopy and combination of these recommended that PET + EUS-FNA should be the recommended staging procedure for patients with esophageal cancer.11 However, few studies have reported on the potential impact of EUS and CT-PET on management and survival in esophageal cancer. Therefore we conducted a case-only study of esophageal cancer using the Surveillance, Epidemiology, and End Results (SEER) - Medicare linked database. We assessed the effect of staging procedures (EUS, CT-PET, and a combination of these) on overall survival relative to cases not receiving these procedures.
METHODS
SEER-Medicare Database
For this study, data from the SEER Program linked to Medicare data was used. SEER-Medicare is a collaborative effort between the National Cancer Institute and the Centers for Medicare and Medicaid Services. The SEER Program collects population-based cancer incidence and survival data on newly diagnosed cancer patients residing in geographically defined areas (http://www.seer.cancer.gov) that covers approximately 28% of the US population.
SEER data include variables such as month and year of diagnosis, cancer site, histology, extent of disease (stage and grade), initial treatment, and socio-demographic information. Medicare is a federally funded program that provides health insurance for >95% of elderly individuals (aged 65 and over). Medicare data are collected by the Health Care Financing Administration (HCFA) and comprise of all claims and dates of claims for inpatient hospitalization, outpatient hospital services, physician services, and hospice care for persons with fee-for-service coverage. These claim files use The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes, Current Procedural Terminology (CPT)-4 codes (www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/cpt.page), and/or Health Care Procedure Codes (HCPCS) (www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/index.html). The SEER-Medicare data contain de-identified patient data, hence this study was given exempt status by the Institutional Review Board review by the Office of Human Subject Research at the National Institutes of Health.
Study Population
Individuals eligible for analysis were defined as persons diagnosed with esophageal cancer defined using The International Classification of Diseases, Ninth Revision (ICD-9) codes 150.0–159.0 with no restriction based on histology. Eligible cases were diagnosed during the period 1995–2008, and aged 66 years or greater at diagnosis. Medicare billing information for EUS is available from 1994 to 2008 and specific PET codes were introduced to bill for CT-PET procedures in 2001. The one year gap between code introduction and use for this analysis is used to reduce misclassification. Cases were required to have a minimum of 12 months of Medicare enrollment prior to date of cancer diagnosis to classify individuals according to a modified (inpatient and outpatient) Charlson comorbidity score.12 In the SEER staging system, esophageal cancer is staged as carcinoma in situ, local stage (localized cancer, including involvement of muscularis propria), regional stage (esophageal cancer with regional involvement by lymph node or direct extension), and distant stage (involvement of distal site[s]/lymph node[s]).13 Exclusions included: enrollment in a Medicare Health Maintenance Organization between age 65 years and date of esophageal cancer diagnosis; lack of enrollment in Medicare Part B; inconsistency between the SEER and Medicare databases in terms of common data captured by the two databases; and eligibility for Medicare on the basis of end-stage renal failure or disability. All patients with incomplete data and individuals with esophageal cancer without staging information were excluded.
Study groups
Cases were split into the following four analytic groups based on whether or not they received the specified staging modalities within the period 1 month prior- to 3 months post-date of cancer diagnosis: 1. EUS – esophageal cancer cases who received endoscopic ultrasound only (CPT-4: 43231, 43232, 43237, 43238, 43242, 43259, 76975); 2. CT-PET – cases who received computed tomography-positron emission tomography only (CPT-4: 78814, 78815, 78816, 78810; HCPCS: G0226, G0227, G0228, G0235); 3. EUS and CT-PET – cases who received both; 4. “No EUS or CT-PET” – esophageal cancer cases who received neither EUS nor CT-PET. This last group (“no EUS or CT-PET”) was used as the comparison group for all other staging modality groups.
All analyses were conducted for esophageal cancer, and then repeated for esophageal adenocarcinoma (ICD-9: 150.0–150.9; International Classification of Disease for Oncology (ICD-O): 8140–8575) and then for esophageal squamous cell carcinoma (ICD-9: 150.0–150.9; ICD-O: 8050–8084).
Statistical Analysis
Descriptive characteristics were calculated for each group with comparisons to the “no EUS or CT-PET” group using chi-square tests for categorical variables and unpaired t-tests for continuous variables. Median survival times (with interquartile range) were estimated by the Kaplan-Meier method and compared by using the log-rank test for significant differences between the specified case-groups. Multivariable Cox proportional hazards regression models were used to compare 1, 3 and 5-year survival of each of the groups to the “no EUS or CT-PET” comparison group in order to estimate hazard ratios (HR) and 95 percent confidence intervals (CI). Covariates included age (categorical), race (categorical), gender (dichotomous), tumor histology (categorical, EA, ESCC, as well as NOS for main esophageal cancer analysis), tumor stage (categorical, SEER Historic Stage A), registry (categorical), year of diagnosis (continuous), Medicare/Medicaid dual enrollment (dichotomous), census tract median household income (categorical), and modified Charlson comorbidity index (categorical). Individuals were right-censored at last date of follow-up (12/31/2008), death, or 1, 3, or 5 years post-diagnosis (dependent on the analysis), whichever occurred first. The proportional hazards assumption was assessed by using the Wald test to test for significance of non-proportionality of hazards over time.
Sensitivity analyses included: 1) excluding individuals who died within three months of diagnosis, as this forms part of the case group classification period; 2) excluding cases aged 80 years or greater at diagnosis; 3) using age as the underlying time metric. All analyses were conducted using SAS v9.2. All tests were two sided and p<0.05 were considered statistically significant.
RESULTS
A total of 6,436 patients with a diagnosis of esophageal cancer between 1995 and 2008 met the inclusion criteria. The distribution of esophageal cancer based on histology was as follows: EAC (n=3,526, 55%), ESCC (n=2,411, 37%), and other/unknown (n=499, 8%). Table 1 summarizes the baseline characteristics and cancer-related variables in the overall group and patients with EAC and ESCC. The vast majority of patients were white men with a mean age of 75.9 (SD 6.6) years. Localized, regional and distant disease each accounted for approximately 32% of the total, while stage 0 (carcinoma in situ) represented just 2.6%. The survival and therapies received for the entire cohort and based on histology are summarized in Table 1. Radiation was the predominant treatment modality (n=2,914, 45.3%) followed by chemotherapy (n=2,296, 35.7%). Only 3.7% and 2.1% of the overall cohort underwent esophageal resection and endoscopic eradication therapies, respectively.
Table 1.
Demographics and cancer-related variables of esophageal cancer patients who met the inclusion criteria
| Variable | Overall EC n=6,436 |
EAC n=3,526 |
ESCC n=2,411 |
|---|---|---|---|
| Age (mean, SD) | 75.9 (6.6) | 75.8 (6.6) | 75.7 (6.5) |
| Males (n, %) | 4,546 (70.6) | 2,862 (81.2) | 1,367 (56.7) |
| Race | |||
| White (n, %) | 5,613 (87.2) | 3,385 (96) | 1,786 (74.1) |
| Black (n, %) | 486 (7.6) | 52 (1.5) | 399 (16.6) |
| Other (n, %) | 337 (5.2) | 89 (2.5) | 226 (9.4) |
| Medicaid Enrollment: No (n, %) | 6,122 (95.1) | 3,406 (96.6) | 2,235 (92.7) |
| Charlson Co-morbidity Index (n, %) | |||
| 0 | 3,910 (60.8) | 2,075 (58.9) | 1,571 (65.2) |
| 1 | 1,477 (23) | 866 (24.6) | 463 (19.2) |
| 2 | 602 (9.4) | 339 (9.6) | 216 (9.0) |
| ≥3 | 447 (7) | 246 (7.0) | 161 (6.7) |
| Stage (n, %) | |||
| 0 (in situ) | 165 (2.6) | 76 (2.2) | 44 (1.8) |
| 1 (localized) | 2,087 (32.4) | 1,133 (32.13) | 822 (34.1) |
| 2 (regional) | 2,261 (35.1) | 1,224 (34.7) | 920 (38.2) |
| 4 (distant) | 1,923 (29.9) | 1,093 (31) | 625 (25.9) |
| Mean survival in days (n, SD) | |||
| 0 (in situ) | 1,329.3 (1119.3) | 1,450.12 (1063.3) | 1,310.6 (1206.8) |
| 1 (localized) | 801.0 (892.9) | 898.1 (950.9) | 671.3 (782.2) |
| 2 (regional) | 552.70 (674.2) | 569.9 (658.1) | 549.5 (691.3) |
| 4 (distant) | 258.3 (381.7) | 255.2 (341.1) | 289.6 (457.6) |
| Therapy (n, %) | |||
| EMR/Ablation | 135 (2.1) | 90 (2.6) | 27 (1.1) |
| Chemotherapy | 2,296 (35.7) | 1,240 (35.2) | 928 (38.5) |
| Radiotherapy | 2,914 (45.3) | 1,437 (40.8) | 1,339 (55.5) |
| Esophageal Resection | 239 (3.7) | 168 (4.8) | 62 (2.6) |
| Chemotherapy and radiation | 1,689 (26.2) | 855 (24.3) | 763 (31.7) |
| Chemotherapy, radiation and surgery | 12 (0.2) | 5 (0.14) | 6 (0.25) |
Abbreviations: EAC, esophageal adenocarcinoma; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; SD, standard deviation.
Univariate comparisons of patients with esophageal cancer with and without EUS evaluation
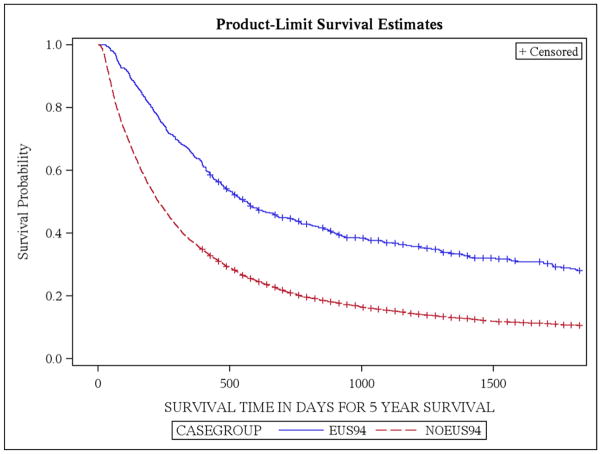
A total of 524 patients underwent EUS examinations; approximately 8% of patients with esophageal cancer (Table 2). In univariate comparisons, patients undergoing EUS were younger (p<0.0001), more likely to be white (p=0.0002), and were more likely to demonstrate EAC on histology (p<0.0001) compared with the “no EUS or CT-PET” group. Significant regional variation was noted in the receipt of EUS (p<0.0001) and patients with locoregional disease were more likely to undergo EUS evaluations (p<0.0001). There was no difference between the two groups with regards to the Charlson comorbidity indices. Patients undergoing EUS had improved survival for all stages (p<0.0001 for all univariate comparisons) with the exception for patients with Stage 0 disease. Receipt of EUS increased the likelihood of esophageal cancer patients receiving endoscopic eradication therapies (p<0.0001), esophageal resection (p=0.002) and chemotherapy and radiation (p=0.01) (Table 2). Overall survival by the Kaplan-Meier’s estimate was higher in the EUS group (log-rank test, p<0.05) (Figure 1). Similar results were noted in a subgroup analysis that included patients with EAC and ESCC (data not shown).
Table 2.
Univariate analysis comparing patients with esophageal cancer with and without EUS evaluation
| Variable | No EUS or CT-PET n=4,723 |
EUS n=524 |
p value |
|---|---|---|---|
| Age (mean, SD) | 76.3 (6.7) | 74.5 (5.9) | <0.0001 |
| Males (n, %) | 3,296 (69.8) | 384 (73.3) | 0.09 |
| Race | |||
| White (n, %) | 4,038 (85.5) | 480 (91.6) | 0.0002 |
| Black (n, %) | 420 (8.89) | 20 (3.8) | |
| Other (n, %) | 265 (5.61) | 24 (4.6) | |
| Registry Site | <0.0001 | ||
| Medicaid enrollment: No (n, %) | 4,503 (95.3) | 496 (94.7) | 0.48 |
| Histology | |||
| EAC | 2,488 (52.7) | 348 (66.4) | <0.0001 |
| ESCC | 1,835 (38.85) | 145 (27.7) | |
| Other | 400 (8.47) | 31 (5.9) | |
| Charlson Co-morbidity Index (n, %) | |||
| 0 | 2,883 (61.04) | 334 (63.7) | 0.61 |
| 1 | 1058 (22.4) | 113 (21.6) | |
| 2 | 452 (9.57) | 46 (8.8) | |
| ≥ 3 | 330 (6.99) | 31 (5.9) | |
| Stage (n, %) | |||
| 0 (in situ) | 133 (2.8) | 20 (3.8) | <0.0001 |
| 1(localized) | 1,568 (33.2) | 183 (34.9) | |
| 2 (regional) | 1,466 (31.04) | 264 (50.4) | |
| 4 (distant) | 1,556 (33) | 57 (10.9) | |
| Mean survival in days (n, SD) | |||
| 0 (in situ) | 1,303 (1178.7) | 1,566.2 (901.5) | 0.34 |
| 1(localized) | 741.5 (900.6) | 1,330.7 (1071.1) | <0.0001 |
| 2 (regional) | 508.5 (690.2) | 710.2 (774.4) | <0.0001 |
| 4 (distant) | 230.4 (372.3) | 431.3 (534.1) | <0.0001 |
| Therapy (n, %) | |||
| EMR/Ablation | 79 (1.67) | 39 (7.4) | <0.0001 |
| Chemotherapy | 1,500 (31.8) | 197 (37.6) | 0.007 |
| Radiotherapy | 1,962 (41.5) | 237 (45.2) | 0.10 |
| Esophageal Resection | 163 (3.4) | 32 (6.1) | 0.002 |
| Chemotherapy and radiation | 1,072 (22.7) | 145 (27.7) | 0.01 |
| Chemotherapy, radiation and surgery | 10 (0.21) | 1 (0.2) | 0.92 |
Abbreviations: EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; SD, standard deviation.
Figure 1.
Overall survival by the Kaplan-Meier’s estimate in EUS and no EUS groups
Univariate comparisons of patients with esophageal cancer with and without CT-PET evaluation
For the comparison of CT-PET, a sub-cohort of individuals diagnosed with esophageal cancer between 2002 and 2008 was used given that these codes were only introduced in 2001 (as described in methods). A total of 853 (~22% of 3,799 patients in the 2002–2008 sub-cohort) cases underwent CT-PET examination (Supplementary Table 1). In univariate comparisons, improved survival was noted among patients receiving CT-PET for all stages (p<0.01) except patients with Stage 0 disease (p=0.59). Receipt of CT-PET increased the likelihood of chemotherapy and radiation (p<0.0001) but had no impact on endoscopic eradication therapies and esophageal resection. Among patients with EAC (n=1,839), undergoing CT-PET did not result in improvement in survival in patients with in situ and locoregional disease but improved survival in patients with distant disease (p<0.0001). Patients with ESCC (n=1,180) undergoing CT-PET had improved survival for all stages (p<0.01 for all univariate comparisons) with exception on those with Stage 0 disease (data not shown).
For a fair comparison, receipt of EUS alone was also included in this sub-cohort. A total of 318 patients (~8%) of 3,799 underwent EUS. As shown in Supplementary Table 1, univariate analyses were similar in this sub-cohort compared with entire EUS cohort shown in Table 2.
Univariate comparisons of patients with esophageal cancer with and without EUS+CT-PET evaluation
Only 189 patients (~5%) of those with esophageal cancer underwent both EUS and CT-PET examinations between 2002 and 2008. Compared with patients not undergoing any staging modality, patients receiving EUS+CT-PET were more likely to be younger (p<0.0001), white (p=0.004), demonstrate EAC histology (p<0.001), and have locoregional disease (p<0.001) (Supplementary Table 1). There was no difference between these two groups with regards to Charlson comorbidity index. Receipt of EUS+CT-PET resulted in improved survival for all stages (p<0.01 for all univariate comparisons) with the exception of patients with Stage 0 disease. EUS+CT-PET staging resulted in increased frequency of chemotherapy and radiation (p<0.0001) but not with frequency of endoscopic eradication therapies or esophageal resections.
Predictors of survival
Multivariable Cox proportional hazards regression models showed that receipt of EUS was a significant predictor for improved 1-year (HR 0.54, 95% CI 0.46–0.62, p<0.0001), 3-year (HR 0.60, 95% CI 0.54–0.68, p<0.0001) and 5-year (HR 0.61, 95% CI 0.55–0.68, p<.0001) survival in the 1995 to 2008 cohort of esophageal cancer cases compared with no EUS or CT-PET (Table 3). Similar results were noted in the sub-cohort of esophageal cancer cases from 2002 to 2008. In addition, receipt of EUS was associated with improved survival in patients with EAC and ESCC when analyses were stratified based on histology (Supplementary Tables 2 and 3). In this multivariable model, increasing age, black race, ESCC, increasing Charlson comorbidity index and stage, registry site and year of diagnosis were significant predictors of survival (Supplementary Table 4).
Table 3.
Multivariable Cox proportional hazards regression models based on 1, 3 and 5 years of follow-up along with time-dependent analysis
| Analysis/Yrs of FU included | Overall HR (95% CI) | Time-dependent HRs | p value for proportional hazards assumption | ||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||
| EUS vs. no EUS or CT-PET (1995–2008) | |||||
| 1 | 0.54 (0.46–0.62) | 0.39 | 0.65 | 0.68 | 0.0029 |
| 3 | 0.60 (0.53–0.67) | 0.45 | 0.73 | 0.72 | 0.0006 |
| 5 | 0.61 (0.54–0.68) | 0.46 | 0.78 | 0.69 | 0.0003 |
| EUS vs. no EUS or CT-PET (2002–2008) | |||||
| 1 | 0.49 (0.39–0.59) | 0.34 | 0.61 | 0.63 | 0.0199 |
| 3 | 0.57 (0.48–0.66) | 0.41 | 0.66 | 0.77 | 0.0025 |
| 5 | 0.59 (0.5–0.68) | 0.43 | 0.68 | 0.79 | 0.003 |
| CTPET vs. no EUS or CT-PET (2002–2008) | |||||
| 1 | 0.57 (0.51–0.64) | 0.36 | 0.84 | 0.82 | <.0001 |
| 3 | 0.66 (0.60–0.72) | 0.45 | 0.78 | 1.00 | <.0001 |
| 5 | 0.67 (0.61–0.73) | 0.46 | 0.79 | 1.02 | <.0001 |
| EUS+CTPET vs. no EUS or CT-PET (2002–2008) | |||||
| 1 | 0.49 (0.38–0.62) | 0.31 | 0.50 | 1.10 | 0.0002 |
| 3 | 0.58 (0.47–0.69) | 0.35 | 0.94 | 0.78 | <.0001 |
| 5 | 0.60 (0.49–0.71) | 0.37 | 0.95 | 0.78 | <.0001 |
Similarly, receipt of CT-PET was a significant predictor of improved 1-year (HR 0.57, 95% CI 0.51–0.64, p<0.0001), 3-year (HR 0.66, 95% CI 0.60–0.73, p<0.0001) and 5-year (HR 0.67, 95% CI 0.62–0.74, p<0.0001) year survival in the 2002 to 2008 cohort of patients with esophageal cancer (Table 3). Similar improved survival was noted for each histology (EAC and ESCC) when analyzed separately (Supplementary Tables 2 and 3). In this multivariable model, increasing age, ESCC, increasing Charlson comorbidity index and stage, and registry site were significant predictors of survival (Supplementary Table 4). Combination of EUS and CT-PET similarly resulted in improved 1-year (HR 0.49, 95% CI 0.39–0.63, p<0.0001), 3-year (HR 0.58, 95% CI 0.48–0.70, p<0.0001) and 5-year (HR 0.60, 95% CI 0.50–0.71, p<0.0001) survivals (Table 3 and Supplementary Table 5).
There was evidence that the decreased hazard of death associated with each staging modality was not proportional over time (Table 3). As such we also conducted each analysis stratified into three periods of time, with cut-points for these periods defined by each period containing an equal number of outcomes (deaths). As can be seen in Table 3, the reduction in risk associated with each staging modality attenuates as time progresses. The overall hazard ratio for each analysis therefore represents the average hazard over the specified amount of follow-up (1, 3 or 5-years).
Sensitivity analysis
Sensitivity analyses that involved excluding individuals who died within three months of diagnosis, aged 80 years or greater at diagnosis and using age as the underlying time metric did not materially affect the results of our analyses (data not shown).
DISCUSSION
EUS is an accurate staging modality recommended by evidence-based guidelines in TNM staging of esophageal cancer.14 It has been shown to impact treatment strategies in a significant proportion of patients with esophageal cancer.15 An international multicenter study that compared EUS staging of esophageal cancer to CT alone showed that the additional information obtained by EUS changed patient management in one-third of cases with the majority (85%) of these changes being the advocation of nonsurgical and palliative measures due to identification of advanced disease.16
Although EUS and CT-PET are accurate staging modalities shown to have an impact on treatment regimens in individuals diagnosed with esophageal cancer, there is limited data on whether receipt of these staging modalities translates into improved overall survival in routine clinical practice; an important meaningful endpoint. In a previous analysis by Das et al, using the SEER-Medicare linked database from January 1994 and December 1999 identified 2830 patients with esophageal cancer. Similar to the current study, only 10.7% of patients underwent EUS examination. Patients who underwent EUS examination were more likely to undergo esophageal resection (21.1% vs. 14.7%, p=0.01) and more likely to receive adjuvant therapy (11.2% vs. 6.7%, p=0.008). When adjusted for age at diagnosis, race, gender, comorbidity, histology, and tumor stage, receipt of EUS was associated with a reduced risk of death (relative hazard, 0.59; 95% CI 0.52–0.68, p=0.001).13 However, this study was limited by the small sample size of patients who underwent EUS in a relatively early era of EUS application. In addition this study did not assess the impact of CT-PET on survival in patients with esophageal cancer. Results of our large population-based study, that used the linked SEER-Medicare database, identified 6,436 patients aged 66 years and older at diagnosis of esophageal cancer. This showed that only 8% of these patients underwent EUS staging. Undergoing EUS was associated with improved survival for all stages with the exception for patients with Stage 0 disease. Receipt of EUS increased the likelihood of esophageal cancer patients receiving endoscopic eradication therapies, esophageal resection, chemotherapy and radiation. Multivariable Cox proportional hazards regression models showed that receipt of EUS was a significant predictor for improved 1, 3 and 5-year survival. The improvement in survival is most likely related to accurate staging of patients with esophageal cancer resulting in appropriate stage-specific therapies. Lack of improvement in survival in patients with Stage 0 disease is not surprising as the role of EUS has been questioned in the setting of Barrett’s-related neoplasia (high-grade dysplasia and intramucosal cancer).17 EUS has moderate accuracy rates in differentiating mucosal (T1a) versus submucosal (T1b) esophageal cancer and is largely being supplanted by endoscopic mucosal resection and direct pathological staging. It should also be noted that EUS has no role in staging of esophageal cancer patients with clear evidence of metastasis on standard CT imaging.
CT-PET is a staging modality frequently used to rule out metastatic disease. A pooled analysis showed that the sensitivity and specificity for detection of distant metastases were 67% (95% CI 58–76) and 97% (90–100), respectively.18 Studies have shown that CT-PET can identify 5–28% with metastatic disease especially at sites not detected on CT alone.5, 19, 20 This study demonstrates an improved survival in a cohort of patients undergoing CT-PET staging, compared to the no EUS or CT-PET group, and that CT-PET was associated with a higher rate of treatment with chemoradiation. The exact reason for lack of improvement in survival in the subgroup of patients with locoregional esophageal adenocarcinoma is unclear. This may be related to the limitations of CT-PET in accurate locoregional staging (differentiation between tumor and surrounding peritumoral lymphadenopathy). The association of the assessed staging modalities with survival, following a diagnosis of esophageal cancer, may advocate for regular inclusion of EUS in clinical management of such cancers cases in individuals age 66 years and greater at diagnosis. Despite the favorable outcomes demonstrated, results of this large population based study showed that only a fraction of eligible patients underwent CT-PET and an even smaller percentage of patients with esophageal cancer underwent an EUS examination. While cost and accessibility are obvious likely barriers, further efforts should focus on determining why a small fraction of patients with esophageal cancer undergo appropriate staging. The authors acknowledge that limited availability of EUS from 1994 to early 2000s and use of EUS being limited to academic/tertiary care centers for a significant time period of the study where a multidisciplinary approach to treatment was likely utilized probably explains the low rate of EUS utilization in this study. Interestingly, results from our study demonstrate that the advantages of both EUS and CT-PET in terms of survival appears to be most pronounced during the time close to the cancer diagnosis (within the first 2-years).
There are several limitations of this study that merit discussion. The possibility of selection bias, where only patients who were healthier, with accessibility to tertiary referral centers and more likely to undergo surgery or neoadjuvant chemoradiation followed by surgery are referred for EUS/CT-PET, cannot be excluded. However, there was no difference in the Charlson comorbidity index between patients undergoing EUS/CT-PET compared to those not undergoing any staging investigation. The incremental yield of EUS-FNA in staging of esophageal cancer by interrogating peri-esophageal and, in particular, celiac nodes were not evaluated in this study because of relatively small number of patients undergoing EUS-FNA precluding an accurate evaluation of its true benefit in terms of survival. However, EUS-FNA for lymph node staging in esophageal cancer has been shown to enhance overall staging accuracy, compared to EUS alone and thus, our conclusion about the positive impact of EUS on outcome should be still valid.15 This study did not evaluate the impact of neoadjuvant chemoradiation on patient survival and its temporal association with esophagectomy. This study was not designed to compare differences in survival between EUS and CT-PET in patients with esophageal cancer. However, the addition of CT-PET does not appear to add to the survival benefit observed in patients undergoing EUS alone (Table 3). Confounding bias due to measured (age, race, histology, stage) and unmeasured (treatment at tertiary care centers) factors cannot be excluded. Generalizability of results is limited by the fact that this study only includes individuals ages 66 years and older with esophageal cancer, although the vast majority of esophageal cancer patients are diagnosed during these ages. While randomized controlled trials remain the gold standard for assessment of efficacy and outcomes, such a study would not be ethical as staging with EUS and CT-PET in patients with esophageal cancer has been associated with improved survival. The impact of staging modalities in cancer on stage migration, or the “Will Rogers phenomenon”, is well described.21 This refers to the scenario in which a more sensitive diagnostic test causes an upward shift in assigned cancer stages due to an increased ability to detect small metastases. This results in an apparent improvement in the prognosis of each individual stage through removal (reassignment) of those previously misclassified at an inaccurately low stage, even though overall prognosis is not improved. This study not only reported an improvement in survival but also reported a high frequency of therapies in patients undergoing staging with EUS and CT-PET. However, the investigators acknowledge that the effect of the “Will Rogers phenomenon” on the overall results cannot be excluded.
Strengths of this study include the use of a large population-based database with data that captures most of patient experiences in a prospective fashion from multiple institutions across the country (academic and community). The SEER-Medicare dataset has proven to be a valuable source of population-based studies with a high level of validity. Most importantly, this study addresses a meaningful endpoint in the field of oncology—patient survival.
In conclusion, receipt of either EUS or CT-PET alone in patients with esophageal cancer are associated with improved 1, 3 and 5-year survival. Future prospective studies should be designed to identify barriers to dissemination of these staging modalities for higher utilization, determine proper order and cost-effectiveness, and how the use of multimodality staging will influence other clinically relevant outcomes besides survival, such as, quality of life and performance status.
Supplementary Material
TAKE-HOME MESSAGE.
Patients undergoing EUS, CT-PET and EUS+CT-PET had improved survival for all stages, all compared with “no EUS or CT-PET”, with the exception of stage 0 disease.
Receipt of EUS was a significant predictor for improved 1, 3 and 5-year survival.
Similar results were noted when results were stratified based on histology, as well as for CT-PET and EUS+CT-PET groups.
Acknowledgments
No funding was obtained or provided for this study.
Footnotes
No writing assistance was provided for this manuscript.
Results of this study were presented in part as an Oral Presentation, Digestive Disease Week 2013, Orlando.
Disclosures: Sachin Wani, MD is supported by the AGA Takeda Research Scholar Award in GERD and Barrett’s esophagus. Jennifer Drahos, PhD, MPH and Michael B. Cook, PhD are supported by the Intramural Program of the National Institutes of Health. None of the other authors have any disclosures relevant to the manuscript.
References
- 1.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Wallace MB, Hoffman BJ, et al. Predictors of survival for esophageal cancer patients with and without celiac axis lymphadenopathy: impact of staging endosonography. Ann Thorac Surg. 2001;72:212–9. doi: 10.1016/s0003-4975(01)02616-9. discussion 219–20. [DOI] [PubMed] [Google Scholar]
- 4.Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg. 2001;72:306–13. doi: 10.1016/s0003-4975(00)02570-4. [DOI] [PubMed] [Google Scholar]
- 5.Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377–84. doi: 10.1016/j.gie.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–30. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483–9. doi: 10.1200/JCO.2005.20.644. [DOI] [PubMed] [Google Scholar]
- 8.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534–9. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Westreenen HL, Heeren PA, van Dullemen HM, et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54–61. doi: 10.1016/j.gassur.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 10.Schreurs LM, Janssens AC, Groen H, et al. Value of EUS in Determining Curative Resectability in Reference to CT and FDG-PET: The Optimal Sequence in Preoperative Staging of Esophageal Cancer? Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace MB, Nietert PJ, Earle C, et al. An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg. 2002;74:1026–32. doi: 10.1016/s0003-4975(02)03875-4. [DOI] [PubMed] [Google Scholar]
- 12.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das A, Chak A, Sivak MV, Jr, et al. Endoscopic ultrasonography and prognosis of esophageal cancer. Clin Gastroenterol Hepatol. 2006;4:695–700. doi: 10.1016/j.cgh.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Maluf-Filho F, Dotti CM, Halwan B, et al. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy. 2009;41:979–87. doi: 10.1055/s-0029-1215192. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626–35. doi: 10.1053/j.gastro.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Mortensen MB, Edwin B, Hunerbein M, et al. Impact of endoscopic ultrasonography (EUS) on surgical decision-making in upper gastrointestinal tract cancer: an international multicenter study. Surg Endosc. 2007;21:431–8. doi: 10.1007/s00464-006-9029-3. [DOI] [PubMed] [Google Scholar]
- 17.Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 8:1037–41. doi: 10.1016/j.cgh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Ott K, Weber W, Siewert JR. The importance of PET in the diagnosis and response evaluation of esophageal cancer. Dis Esophagus. 2006;19:433–42. doi: 10.1111/j.1442-2050.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 19.Kato H, Miyazaki T, Nakajima M, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–56. doi: 10.1002/cncr.20724. [DOI] [PubMed] [Google Scholar]
- 20.Meyers BF, Downey RJ, Decker PA, et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg. 2007;133:738–45. doi: 10.1016/j.jtcvs.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 21.Christensen D. The Will Rogers phenomenon: Roping the effects of a new cancer staging system. J Natl Cancer Inst. 2003;95:1105–6. doi: 10.1093/jnci/95.15.1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.