Abstract
We explored the use of qualitative interviews to discuss discrepancies between two sources of information on unprotected sex: biomarker results and self-reported survey data. The study context was a randomized trial in Kingston, Jamaica examining the effect of STI counseling messages on recent sexual behavior using prostate-specific antigen (PSA) as the primary study outcome. Twenty women were interviewed. Eleven participants were selected because they tested positive for PSA indicating recent semen exposure, yet reported no unprotected sex in a quantitative survey (“discordant”): 5 reported abstinence and 6 reported condom use. Nine participants who also tested positive for PSA but reported unprotected sex in the survey were interviewed for comparison (“concordant”). Qualitative interviews with 6 of the 11 discordant participants provided possible explanations for their PSA test results, and 5 of those were prompted by direct discussion of those results. Rapid PSA testing combined with qualitative interviews provides a novel tool for investigating and complementing self-reported sexual behavior.
Keywords: Qualitative study, Self-reported data, Semen biomarker, Prostate-specific antigen, Jamaica
Introduction
The limitations of self-reported data are well-known. Self-reported data on sensitive topics such as sexual behavior are prone to recall bias, misunderstandings about survey questions, and social-desirability bias [1, 2]. Several strategies have been implemented to reduce the effect of recall bias and misunderstandings, including improving question phrasing, adding definitions to ambiguous terms, and using shorter time frames or reference points for questions. Primary strategies used to minimize social-desirability bias include changing the mode of survey administration (e.g., shifting from interview-administered to self-administered surveys and using computer aids, or ballot box approaches) and changing the characteristics of the interviewer (e.g., matching by gender, age, and ethnicity). These and other strategies have been used to increase the reporting accuracy of sensitive behaviors, with mixed results [2–9]. Additional approaches are needed to help minimize and control for these issues [10, 11].
Biomarkers represent additional tools for addressing and understanding the limitations of self-reported data, particularly when studying sensitive topics like sexual behavior. Studies that involve testing for sexually transmitted infections (STIs) have used that biological information to identify inaccuracies in self-reported data [12]. Objective markers of semen exposure, such as prostate-specific antigen (PSA), also have been used for this purpose. Long used in the forensic sciences for sexual assault cases, PSA is found in high concentrations in semen and serves as a reliable, valid biomarker for recent (<48 h) semen exposure [13–16]. In controlled trials, the sensitivity of PSA tests to the presence of even moderate levels of semen in the vagina was 99 % [17]. However, studies also show that PSA degrades rapidly in the vagina, returning to baseline levels in 24–48 h after exposure [18]. Studies comparing PSA test results with self-reported sexual behaviors have identified high levels of discordance between the two, confirming concerns about the under-reporting of unprotected sexual activity and over-reporting of condom use [7, 19–23].
Few studies have been able to compare survey data with not only biomarker test results but also qualitative data, which can provide another perspective on important study topics. In a study of Tanzanian youth, Plummer et al. compared biological markers of sexual activity (i.e., STI test results), self-reported survey data, and qualitative interviewing and found that the qualitative interview data best matched the biological markers but still contained inconsistencies [24]. Notably, in Plummer’s study and most other studies that compare biomarker data to self-reported data, the analysis took place months or years after data collection. In a microbicide trial among women in Africa, Pool et al. [25] further demonstrated both the difficulty and utility of triangulating data this way, using an objective marker (i.e., returned, used vaginal microbicide gel applicators), survey data, coital diaries, and qualitative interviews. Interviewing study participants just days after they had returned gel applicators and completed questionnaires, Pool et al. determined that data from qualitative interviews were more accurate than the coital diary or survey data and matched most closely the information provided by the returned applicators. Further, their findings highlight that misreporting is often unintentional and can be remedied through brief discussions with study participants.
In this study, we examine the use of qualitative interviews to better understand discordance identified between PSA rapid test results and quantitative survey data on recent sexual behavior. The primary aim was to assess whether and how qualitative interviews conducted immediately after survey completion and laboratory testing could yield explanations for discordant data and semen exposure indicated by PSA test results. We also attempted to gain a better understanding of why some participants misreported and had discordant data, by comparing them to participants whose data were concordant.
Methods
This study was a part of the Assessing Counseling Messages Effectiveness (ACME) trial, the details of which are forthcoming elsewhere [26]. In brief, the ACME trial involved 300 women ages 18 or older attending a public STI clinic in Kingston, Jamaica from August 2010 to March 2011. At the initial visit, enrolled women were randomized to receive one of two counseling messages during the period of syndromic treatment for STI or reproductive tract infections: (1) a single message promoting short-term abstinence (“abstinence only”) or (2) a hierarchical message promoting abstinence backed up by the promotion and provision of condoms (“abstinence plus condoms”). Staff also administered a quantitative survey on sexual behavior and collected vaginal swabs at the time of enrollment for STI testing.
The present study was conducted during participants’ single follow-up visit during the ACME trial, which was scheduled 6 days after enrollment. At that time, staff again collected vaginal swabs for STI testing and subsequently administered a quantitative survey about recent sexual behavior, including condom use. Then staff asked participants for written consent to test the already collected vaginal swabs for the presence of PSA. We employed this sequence of procedures to minimize bias from participants modifying their sexual behavior, or their reporting of sexual behavior, as a result of having prior knowledge of the biomarker testing. Staff then immediately tested vaginal swabs from the follow-up visit on-site with ABAcard p30 (Abacus Diagnostics, West Hills, CA), a rapid, semi-quantitative test for PSA, following published procedures [27].1 Results were ready in approximately 15 min, prior to the end of each participant’s follow-up visit.
The qualitative study component of the ACME trial involved the conduct of qualitative interviews with participants who had positive PSA test results at the follow-up visit. This substudy began about 3 months after the trial was initiated. Recruitment involved the laboratory technician alerting the qualitative interviewer when a positive PSA test result was obtained during follow-up. After reviewing the participant’s quantitative survey responses, the interviewer approached the participant to assess her interest in participating in a separate qualitative sub-study. For those who agreed to participate, the interviewer then conducted a short, face-to-face, semi-structured interview based on an interview guide. The participant’s study arm assignment was unknown to laboratory and qualitative interview staff and was not factored into recruitment or the interview.
We purposively sought three categories of participants in the qualitative study: (1) those who reported no vaginal sex (i.e., abstinence) in the previous 3 days in the quantitative survey yet tested positive for PSA (Group A); (2) those who reported consistent and correct condom use in the previous 3 days in the quantitative survey yet tested positive for PSA (Group B); and (3) those who reported recent unprotected sex in the quantitative survey and tested positive for PSA (Group C). Groups A and B were classified as “discordant” between their self-reported quantitative survey and biomarker data, while Group C was classified as “concordant” and served as a comparison group. Our initial target was to have 15 participants in each of Groups A and B, and 10 in Group C, for a total of 40 interviews, which we felt should have been sufficient to make comparisons and reach saturation of themes.
During the interview, participants first were asked to recount their recent sexual behaviors. For participants with discordant results, the aim was to determine whether the more flexible format of the qualitative interview could yield explanations for having detectable semen exposure despite reporting no unprotected sex and whether the information on their PSA test results prompted explanations. Some interview questions repeated those asked in the survey (e.g., number of sex acts, condom use), while other questions probed different areas (e.g., level of comfort talking about sex). Women also were given the opportunity to identify other potential sources of semen exposure apart from vaginal intercourse (e.g., anal sex). Like the quantitative survey, the qualitative interview focused on events in the previous 3 days, to capture the 48 h window of the PSA test period.
By design, the interviewer did not mention the positive PSA test result to participants until the end of the qualitative interview, both to avoid being confrontational and to assess whether the qualitative format alone could yield explanations for semen exposure. The two interviewers [AB and MT], both Jamaican women with relevant interview experience, were attuned to the potential discomfort that this situation might evoke in some participants (e.g., feeling that they were being accused of lying) and strove to set a professional, yet empathetic, tone for each interview. Interviewers were trained not to press women too hard for an explanation following presentation of their PSA test results. Though false positives are rare with this particular test [18], the interviewers were trained to tell women that there might have been a laboratory error in their testing in the case that a participant showed signs of discomfort with the test result.
Interviews took place in a private office in the building where the follow-up visits were conducted. Interviews lasted approximately 15–20 min and were audio-recorded with participants’ consent. Participants received compensation for their time in the form of cell phone credits. Immediately after each interview, interviewers completed a short form to describe the participant’s body language, demeanor, and other features of the interview unlikely to be captured by the audio recording. One interviewer [MT] transcribed each interview verbatim, translating portions of the Jamaican dialect into standard English during transcription, to ensure clarity of meaning to all analysts.
We performed content analysis on the interview data. We began by summarizing the interviews immediately after completion, with modifications made to the summaries after the transcripts were completed. Three staff [MC, AB, and MCS] developed and applied a set of codes to the transcripts using a qualitative data software package (ATLAS.ti®) to index information on issues identified a priori as important to our study questions, e.g., participants’ study experiences, sexual behaviors, condom use, the qualities of their sexual relationships, and when data discordance was discussed and/or resolved. Four transcripts then were coded independently by two analysts. Coding was then compared, and coders discussed and reconciled all differences in coding application. The subsequent 16 transcripts were single-coded, and then reviewed by one analyst [MC]. Using the coded data, we created spreadsheets that summarized key information by participant and group (A, B, and C) and used these summaries to answer the key study questions. Among the discordant participants, we categorized any explanations for PSA exposure, when provided, as either implausible, possible, or probable, indicating higher degrees of certainty that the explanation was the true cause of PSA exposure. Socio-demographic data from the questionnaire used at the enrollment visit for the ACME study were also used to further describe participants. The ACME study protocol, including the qualitative study, was reviewed and approved by the Institutional Review Boards of the US Centers for Disease Control and Prevention and the Jamaica Ministry of Health.
Results
Description of Participants
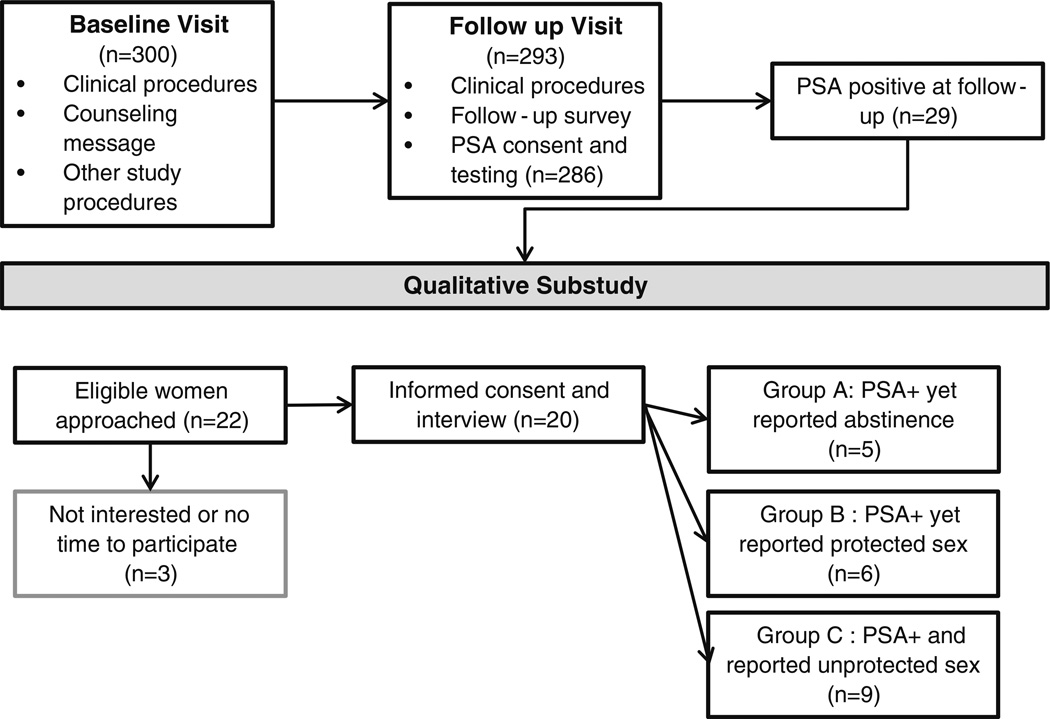
Twenty-two women who had positive PSA test results were approached to participate in the sub-study between November 2010 and March 2011, and twenty (91 %) agreed to participate (Fig. 1). We did not reach our targets for recruitment in the three groups largely because of low prevalence of PSA. These 20 participants were nearly three-quarters of all of the women who had positive PSA test results at follow-up in the larger study. As shown in Table 1, they ranged in age from 18 to 53, with a mean of 30 years. Women described their current sexual relationships as visiting partner [28]2 (n = 9), married or cohabiting (n = 8), and single (n = 3). Most of these were long-term, live-in relationships that women described as generally loving, although there were two relationships with a history of intimate partner violence. Participants demonstrated a range of communication styles during the interviews. Most women described themselves as open to talking about sex and seemed relaxed during the interviews, though some women were fairly reticent. All participants reported that they liked being a part of the larger ACME study and had no problems with any of the procedures or forms. Each Group (A, B, and C) included participants from both of the larger study’s counseling message arms (the abstinence message and the abstinence + condoms back-up message).
Fig. 1.
ACME qualitative study procedures
Table 1.
Description of participants (n = 20)
| Participant | Age | Marital status |
Number of children |
Education, attainment (years) |
Self-described assessment of main partner |
Notes on demeanor during interview |
|---|---|---|---|---|---|---|
| Group A (PSA + yet reported abstinence) | ||||||
| A | 21 | Visiting partner | 3 | 10 | Good, but they were stressed financially | Shy at first but warmed up |
| B | 32 | Visiting partner | 2 | 7 | Good, he was understanding | Relaxed |
| C | 23 | Visiting partner | 1 | 10 | Good, he was quiet and easy-going | Relaxed but bashful at times |
| D | 19 | Living with partner | 0 | 11 | Good, he was kind and caring | Shy but comfortable |
| E | 29 | Single | 4 | 8 | Bad, he cheated on her | Relaxed |
| Group B (PSA + yet reported protected sex) | ||||||
| F | 27 | Living with partner | 2 | 11 | Good, he was easy-going | Relaxed |
| G | 18 | Visiting partner | 0 | 11 | Good, he was kind and caring | Relaxed |
| H | 18 | Visiting partner | 1 | 10 | Bad, he had history of violence | Shy but comfortable |
| I | 26 | Single | 2 | 12 | Good, he was kind and caring | Relaxed |
| J | 43 | Visiting partner | 3 | 11 | Good, he was easy-going | Shy at first but warmed up |
| K | 53 | Living with partner | 6 | 9 | Bad, he was easily upset and moody | Uneasy and fidgety |
| Group C (PSA + and reported unprotected sex) | ||||||
| L | 34 | Married | 2 | 9 | Good, he was easy-going | Relaxed |
| M | 27 | Single | 3 | 10 | Bad, he was moody and untrustworthy | Relaxed |
| N | 36 | Living with partner | 1 | 11 | Bad, he was jealous and violent at times | Embarrassed but pleasant |
| O | 38 | Living with partner | 2 | 9 | Good, he was easy-going | Relaxed |
| P | 26 | Visiting partner | 3 | 11 | Bad, he was uncommunicative and uncooperative | Relaxed |
| Q | 25 | Visiting partner | 7 | 10 | Bad, they quarrelled often, she had side partner | Relaxed |
| R | 36 | Living with partner | 2 | 14 | Good, he was easy-going but had commitment issues | Relaxed |
| S | 32 | Visiting partner | 2 | 10 | Good, he treated her well but she wondered if he was cheating on her | Relaxed |
| T | 34 | Living with partner | 1 | 9 | Good, he treated her well but she had side partner | Relaxed |
Information on age, marital status, parity, and education are from the baseline quantitative survey. Information on relationship quality and demeanor is from the qualitative interviews
Did The Qualitative Interviews Yield Explanations for The Data Discordance?
Group A: PSA Positive yet Reported Abstinence
Among the 20 participants, eleven provided information in the survey that was discordant with their positive PSA lab result. Five reported abstinence (Group A), and of those, three provided explanations for their positive PSA test result, two of which were deemed possible (Table 2). The most possible case was Participant D, who, after being presented with her test results, readily explained that her partner’s semen had gotten near her vagina. She noted, “[the semen was there] because before he discharged… it kind of (pointing to vagina).” Participant B had reported earlier in the interview that her partner had ejaculated in her hand, which may have been one route of PSA exposure; however, she became uncomfortable upon hearing about her test result and did not believe she had gotten his semen in her vagina. She insisted, “I know we didn’t have any sex, ” and proceeded to ask about the motives of the study overall. The interviewer explained the study objectives, and the participant appeared at ease at the end of the interview.
Table 2.
Explanations provided for semen exposure, by Group
| Participant | Explanation of semen exposure provided? |
Reason or explanation for semen exposure |
Explanation possible or probable? |
Reaction to test results, according to interviewer notes |
|---|---|---|---|---|
| Group A (PSA + yet reported abstinence) | ||||
| A | Yes | Possible assault while sleeping | No, implausible | Surprised, insisted on abstinence, then suggested possible assault |
| B | Yes | Touched partner’s discharge | Yes, possible | Visibly upset, then in probing, mentioned discharge |
| C | No | No sexual contact | N/A | Not upset and insisted on non exposure |
| D | Yes | Partner discharged near vagina | Yes, possible | Not embarassed or upset |
| E | No | No sexual contact | N/A | Visibly upset and insisted on non exposure |
| Group B (PSA + yet reported protected sex) | ||||
| F | Yes | Possible burst condom | Yes, possible | Calmly offered explanation when told results |
| G | No | Condom used without incident | N/A | Lost eye contact but insisted on non-exposure |
| H | Yes | Condom slipped | Yes, probable | Calmly offered explanation prior to being told results |
| I | No | Attempted sex with condom but partner did not ejaculate | N/A | Not upset and insisted on non exposure |
| J | Yes | Condoms not used in the “second round” of sex | Yes, probable | Embarassed and offered explanation when told results |
| K | Yes | Possible slippage or bursting | Yes, possible | Embarassed, lost eye contact when told results |
| Group C (PSA + and reported unprotected sex): reasons for unprotected sex | ||||
| L | Condom slipped | |||
| M | Sex without a condom; no condoms on hand; not used to condoms; partner argument | |||
| N | Sex without a condom; did not ask partner to use a condom | |||
| O | Sex without a condom; misunderstood message | |||
| P | Sex without a condom; no condoms on hand; not used to condoms; fearful of partner | |||
| Q | Sex without a condom; felt partner would not abstain if asked; condom did not fit partner well | |||
| R | Sex without a condom; partner wants child with her | |||
| S | Partner removed condom during sex without her consent | |||
| T | She removed condom from her partner during sex; misunderstood message | |||
Participant A insisted that she had been abstinent even after being presented with her PSA test result. When asked to provide an explanation for semen exposure, she suggested that she may have been assaulted in her sleep by a man who lived in her household. Given her positive demeanor prior to this, some inconsistencies in her interview, and other information she provided, this explanation seemed an unlikely scenario to the interviewer and other study staff who discussed this case. By protocol, the interviewer reminded her of the possibility that the laboratory results were in error. The remaining two women in Group A (Participants C and E) did not provide any explanation for their PSA test results, and one became visibly uncomfortable with the news of her result.
Group B: PSA Positive yet Reported Protected Sex
Among the six women in Group B, four (Participants F, H, J, and K) provided explanations for semen exposure, two of which were deemed possible, and two deemed probable. One of the probable explanations came from Participant H, who, after the interviewer had queried her —prior to the presentation of the PSA test results— about her reported condom use for two recent sex acts, said that in fact the second condom had slipped off. She said, “the condom like it musi come off, and then mi start cry… and mi seh no mi don’t want get sick again (the condom must have come off and I started to cry… and I said I don’t want to get sick again.)” She did not say why she had not reported this incident when administered the survey questionnaire.
This was not the case for Participant J, who also provided a probable explanation, admitting that she did not in fact use a condom both of the times she had sex, as she had reported in the survey:
Interviewer: When they took the swab from your vagina, it shows that there is semen in your vagina. That’s the man’s sex discharge… In your vagina. So amm, and yuh said you used a condom both times
Participant: (laughs)
Interviewer: Right? Suh how yuh think that semen got there?
Participant: Mi use a condom first, but mi neva use a condom the last time [I used a condom the first (time) but I did not use a condom the last (time)]
The interviewer then asked if she had felt embarrassed to report the unprotected sex act, to which the participant responded, “Yeah, because true the doctor did want it [did not want me to do that].”
In the course of similar conversations with two other women from this group, involving direct discussion of their PSA test results, women began to reflect upon whether a condom might have slipped off or burst. Participant F reflected, “mi nuh know if [the condom] burst. Or wah (I don’t know if the condom burst or what)” and then shared that she had noticed some discharge on her foot after sex, suggesting a possible condom break. The other (Participant K) was upset upon hearing her results and wondered if the condom burst, given her vagina felt wet after sex. She then added that her partner had to slide the condom back up his penis during sex. Two other participants from Group B (Participants G and I) provided no explanation for their positive PSA test results.
In sum, among the 11 cases with discordant data, seven provided an explanation during the qualitative interview for semen exposure, six of which were deemed possible or probable causes (two in Group A, four in Group B). Five cases remained entirely unexplained. There were no discernible differences between the women who provided an explanation for their discordance and those who did not, by Group (A or B), communication/interview style, relationship contexts, or demographic data (Tables 1, 2). All but one explanation from participants in both groups came after the presentation of the test results.
What Leads Some Participants to have Discordant Data, and Others Not to?
Group C consisted of participants who had positive PSA test results and reported having unprotected sex in the survey; their data were concordant. We compared participants from Group A and B with participants from Group C to try to identify factors that might lead certain women to report unprotected sex openly in the survey and others not to. The only difference we could detect was the prominence of partner influences in the explanations provided by Group C participants, compared to those from Groups A and B (Table 2). Five women in Group C reported that their partners had strongly influenced whether they had sex recently and/or used a condom, while no women in Groups A and B did so (aside from the relatively implausible suggestion of assault by Participant A). For example, Participant N noted that, when she had asked her partner previously about using a condom, “Him seh him not using any. (He said he’s not using it.),” and she didn’t ask him to use one this time either, despite the counseling message she had received. She described this partner as quick to anger as well. Participant S was furious that her partner removed the condom without her consent, explaining, “Mi put on the condom and him did a dweet. But true mi guh tun back way now, when him guh in mi start feel suppen different. Suh mi look… him tek off di condom. (I put on the condom and he started having sex. But because I changed position and turned my back to him I started feeling something different so I looked… he took off the condom).” We were unable to identify differences by socio-demographic or other characteristics.
Discussion
This study assessed the utility of conducting semi-structured qualitative interviews with women immediately after completing a quantitative survey on sexual behavior and being tested for vaginal exposure to semen via a rapid test. This methodology enabled us to probe women who provided information on recent sexual activity that was not in agreement with the test results (i.e., women who had presence of PSA despite reporting no unprotected sex in the past 3 days). This approach yielded possible explanations for about half the cases of discordance between survey data and PSA biomarker results, including sexual contact other than intercourse, condom use problems, and condom non-use. Although most explanations were possible, we were limited in our ability to assess their veracity; self-reported data are inherently subjective, regardless of the format. As others have shown, qualitative data, even when collected in order to clarify issues, can still contain inaccuracies [24, 25].
While both Group A and B participants rendered possible explanations, the only two probable explanations came from Group B participants who had already reported some sexual activity (albeit condom-protected sex) in the quantitative survey. It is possible that the qualitative interviews were more effective at yielding explanations for women in Group B, who might have found it easier to explain semen exposure than participants in Group A. Group A participants were on record as having no sex and thus may have faced a potentially more embarrassing reversal to their account. With such small numbers in each group, however, this is speculative.
Four participants provided no explanation for their semen exposure during the qualitative interview. While there is a possibility of laboratory error, we believe this was unlikely, given the high specificity of PSA rapid tests. A more likely explanation is that the reasons these participants had for not initially explaining their semen exposure did not change as a result of the qualitative interview. For such women, the alternative format, approach, and information provided (i.e., laboratory evidence of PSA) employed in this study apparently had no effect. Alternatively, such women may truly not have known that semen exposure had occurred (e.g., that a condom in fact had broken or slipped off). Such cases demonstrate the value of having a biomarker to assess the study’s primary outcomes, rather than only self-reported data on sexual behavior.
The study also raised questions about who might be more likely to openly disclose behavior that, in this study context, was counter to their clinician’s counseling messages. While the reasons for misreporting in surveys are well-known (e.g., social desirability bias, recall bias), efforts to identify who is more likely to have discrepancies between self-reported survey data and biomarkers, including PSA, have had little success [12, 19–21]. In this study, we found that for several women in Group C (concordant), partner influences played a dominant role in their account of why they had unprotected sex, unlike women in Groups A and B (discordant). We speculate that participants in Group C may have found it easier to report having had unprotected sex if the reason was perceived as further outside of women’s own control. When the issue involved a joint decision not to use a condom or a problem in using a condom, however, it may have been harder for some women to report openly in the survey (or in the qualitative format), because these reasons implicated their own decisions or skills and were more personally embarrassing. In a separate analysis, we plan to further explore how participants from all Groups (A, B, and C) understood their clinician’s counseling messages and describe the barriers they faced to acting on those.
In addition to informing our understanding of the randomized trial’s outcomes (i.e., positive PSA test results), these findings provide ideas for future study methodology. For example, given some women’s explanations for their positive PSA test results, researchers may consider including in their survey instruments not only questions about whether a condom broke or slipped off but also questions about whether participants had any suspicion that it might have broken or slipped off, and whether they had any sexual contact with their partner’s ejaculate. Moreover, this study points to additional ways to effectively and efficiently utilize PSA as a semen biomarker and qualitative research methods. The commercial availability of a low cost (~$5) and rapid PSA assay for detection of recent semen exposure made our study design feasible. The qualitative interviews were easily integrated within the context of participants’ second study visit, without requiring extensive additional time or resources for women or study staff or interfering with the conduct of the larger ACME trial. This design also minimized recall bias about participants’ recent sexual experiences.
Obtaining explanations for positive PSA test results relied on first building rapport with participants and then presenting them with their PSA test results; only one of six participants who provided an explanation for PSA exposure did so prior to learning her test results. Being informed of their PSA test results may have effectively pressured some participants to admit to having unprotected sex. However, in our estimation, it served primarily to encourage women to think back with more focus on what occurred [See also, 29]. Prior knowledge that testing would be done is unlikely to have reduced discordance, as shown in an earlier study [30]. Future studies could opt to build rapport in ways other than the specific approach used in this study, and could, for example, avoid the repetitive recounting of events we asked participants to do. Regardless, participants’ reactions to learning their positive PSA test result will likely vary as they did in this study. However, with skillful interviewers and an empathetic approach, we believe discomfort was, and can be, minimized.
Our study was small and exploratory in nature and subject to additional limitations. Specifically, the small number of participants in each group limited our ability to delve much into differences between them. It would be helpful to replicate this kind of study in different populations, to determine how much the utility of these interviews may vary across social and study contexts, and to assess the feasibility of incorporating this study design into larger studies. Also, while our qualitative interviews were relatively short and thus easier to conduct and analyze, more in-depth interviews may have been more successful in explaining more of the discordant data and in identifying reasons some participants had discordant data and others did not.
This study expands upon a history of using qualitative methods to better understand and complement data obtained from quantitative surveys, as well as to improve the validity of survey data [e.g., 24, 25, 29, 31]. The primary innovation represented by this study is the additional incorporation of commercially available PSA rapid test results. By virtue of its validity and immediacy, PSA rapid testing can be utilized in other studies of sexual behavior, in conjunction with other quantitative and qualitative methods, to gain additional information about study participants’ experiences in real-time. The utilization of PSA and other semen biomarkers help illuminate bias in self-reported data, and can also serve as primary study outcomes [5, 22, 32]. As this trend continues, it represents a breakthrough for sexual health research.
Acknowledgments
The authors thank Maria Gallo, Shashauna Eastman, and Melrose Ellis for their support of this project. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
This rapid test performed favorably compared to a more extensive, quantitative laboratory test for PSA, with 99 % sensitivity and 96 % specificity [27]. Quality control measures conducted during the ACME trial also found excellent agreement between the rapid test results and quantitative laboratory tests.
Visiting partner is a relationship term commonly used in Jamaica and the Caribbean, to denote fairly serious or stable relationships in which one partner, usually the man, lives separately and visits regularly (see, for example, [28]).
Contributor Information
Marion W. Carter, Email: acq0@cdc.gov, Division of Reproductive Health, Centers for Disease Control and Prevention, 4770 Buford Highway, MS-K-34, Atlanta, GA 30341, USA.
Althea Bailey, Department of Community Health and Psychiatry, University of the West Indies, Kingston, Jamaica.
Margaret C. Snead, Division of Reproductive Health, Centers for Disease Control and Prevention, 4770 Buford Highway, MS-K-34, Atlanta, GA 30341, USA
Elizabeth Costenbader, Behavioral and Social Science Department, FHI 360, Durham, NC, USA.
Malene Townsend, Comprehensive Health Centre (CHC)/Epidemiology Research and Training Unit (ERTU), Jamaica Ministry of Health, Kingston, Jamaica.
Maurizio Macaluso, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Denise J. Jamieson, Division of Reproductive Health, Centers for Disease Control and Prevention, 4770 Buford Highway, MS-K-34, Atlanta, GA 30341, USA
Tina Hylton-Kong, Comprehensive Health Centre (CHC)/Epidemiology Research and Training Unit (ERTU), Jamaica Ministry of Health, Kingston, Jamaica.
Lee Warner, Division of Reproductive Health, Centers for Disease Control and Prevention, 4770 Buford Highway, MS-K-34, Atlanta, GA 30341, USA.
Markus J. Steiner, Clinical Sciences Department, FHI 360, Durham, NC, USA
References
- 1.Tourangeau R, Groves RM, Redline CD. Sensitive topics and reluctant respondents: demonstrating a link between nonresponse bias and measurement error. Public Opin Q. 2010;74(3):413–432. [Google Scholar]
- 2.Tourangeau R, Yan T. Sensitive questions in surveys. Psychol Bull. 2007;133(5):859–883. doi: 10.1037/0033-2909.133.5.859. [DOI] [PubMed] [Google Scholar]
- 3.Davis RE, Couper MP, Janz NK, Caldwell CH, Resnicow K. Interviewer effects in public health surveys. Health Educ Res. 2010;25(1):14–26. doi: 10.1093/her/cyp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langhaug LF, Sherr L, Cowan FM. How to improve the validity of sexual behaviour reporting: systematic review of questionnaire delivery modes in developing countries. Trop Med Intl Health. 2010;15(3):362–381. doi: 10.1111/j.1365-3156.2009.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mensch BS, Hewett PC, Abbott S, Rankin J, Littlefield S, Ahmed K, et al. Assessing the reporting of adherence and sexual activity in a simulated microbicide trial in South Africa: an interview mode experiment using a placebo gel. AIDS Behav. 2011;15(2):407–421. doi: 10.1007/s10461-010-9791-z. [DOI] [PubMed] [Google Scholar]
- 6.Mensch BS, Hewett PC, Jones HE, Luppi CG, Lippman SA, Pinho AA, et al. Consistency in women’s reports of sensitive behavior in an interview mode experiment, Sao Paulo, Brazil. Int Fam Plan Perspect. 2008;34(4):169–176. doi: 10.1363/ifpp.34.169.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minnis AM, Steiner MJ, Gallo MF, Warner L, Hobbs MM, van der Straten A, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009;170(7):918–924. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips AE, Gomez GB, Boily MC, Garnett GP. A systematic review and meta-analysis of quantitative interviewing tools to investigate self-reported HIV and STI associated behaviours in low- and middle-income countries. Int J Epidemiol. 2010;39(6):1541–1555. doi: 10.1093/ije/dyq114. [DOI] [PubMed] [Google Scholar]
- 9.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: II Accuracy of self-reports. Ann Behav Med. 2003;26(2):104–123. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart GS, Grimes DA. Social desirability bias in family planning studies: a neglected problem. Contraception. 2009;80(2):108–112. doi: 10.1016/j.contraception.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Turner AN, De Kock AE, Meehan-Ritter A, Blanchard K, Sebola MH, Hoosen AA, et al. Many vaginal microbicide trial participants acknowledged they had misreported sensitive sexual behavior in face-to-face interviews. J Clin Epidemiol. 2009;62(7):759–765. doi: 10.1016/j.jclinepi.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Diclemente RJ, Sales JM, Danner F, Crosby RA. Association between sexually transmitted diseases and young adults’ self-reported abstinence. Pediatrics. 2011;127(2):208–213. doi: 10.1542/peds.2009-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochmeister MN, Budowle B, Rudin O, Gehrig C, Borer U, Thali M, et al. Evaluation of prostate-specific antigen (PSA) membrane test assays for the forensic identification of seminal fluid. J Forensic Sci. 1999;44(5):1057–1060. [PubMed] [Google Scholar]
- 14.Kearsey J, Louie H, Poon H. Validation study of the “OneStep ABAcard PSA Test” kit for RCMP casework. Can Soc Forensic Sci J. 2001;34(2):63–72. [Google Scholar]
- 15.Graves HC, Sensabaugh GF, Blake ET. Postcoital detection of a male-specific semen protein. Application to the investigation of rape. N Eng J Med. 1985;312(6):338–343. doi: 10.1056/NEJM198502073120603. [DOI] [PubMed] [Google Scholar]
- 16.Kamenev L, Leclercq M, Francois-Gerard C. An enzyme immunoassay for prostate-specific p30 antigen detection in the postcoital vaginal tract. J Forensic Sci Soc. 1989;29(4):233–241. doi: 10.1016/s0015-7368(89)73257-6. [DOI] [PubMed] [Google Scholar]
- 17.Lawson ML, Maculuso M, Bloom A, Hortin G, Hammond KR, Blackwell R. Objective markers of condom failure. Sex Transm Dis. 1998;25(8):427–432. doi: 10.1097/00007435-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Macaluso M, Lawson L, Akers R, Valappil T, Hammond K, Blackwell R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59(3):195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 19.Aho J, Koushik A, Diakite SL, Loua KM, Nguyen VK, Rashed S. Biological validation of self-reported condom use among sex workers in Guinea. AIDS Behav. 2009;170(7):918–924. doi: 10.1007/s10461-009-9602-6. [DOI] [PubMed] [Google Scholar]
- 20.Gallo MF, Behets FM, Steiner MJ, Hobbs MM, Hoke TH, Van Damme K, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis. 2006;33(8):476–479. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 21.Gallo MF, Behets FM, Steiner MJ, Thomsen SC, Ombidi W, Luchters S, et al. Validity of self reported ‘safe sex’ among female sex workers in Mombasa, Kenya–PSA analysis. Int J STD AIDS. 2007;18(1):33–38. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]
- 22.Gallo MF, Steiner MJ, Hobbs MM, Weaver MA, Hoke TH, Van Damme K, et al. Predictors of unprotected sex among female sex workers in Madagascar: comparing semen biomarkers and self-reported data. AIDS Behav. 2010;14(6):1279–1286. doi: 10.1007/s10461-010-9742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen MP, Macaluso M, Blackwell R, Galvao L, Kulczycki A, Diaz J, et al. Self-reported mechanical problems during condom use and semen exposure. Comparison of two randomized trials in the United States of America and Brazil. Sex Transm Dis. 2007;34(8):557–562. doi: 10.1097/01.olq.0000258487.38309.b9. [DOI] [PubMed] [Google Scholar]
- 24.Plummer ML, Ross DA, Wight D, Changalucha J, Mshana G, Wamoyi J, et al. “A bit more truthful”: the validity of adolescent sexual behaviour data collected in rural northern Tanzania using five methods. Sex Transm Infect. 2004;80(Suppl 2:ii):49–56. doi: 10.1136/sti.2004.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pool R, Montgomery CM, Morar NS, Mweemba O, Ssali A, Gafos M, et al. Assessing the accuracy of adherence and sexual behaviour data in the MDP301 vaginal microbicides trial using a mixed methods and triangulation model. PLoS One. 2010;5(7):e11632. doi: 10.1371/journal.pone.0011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson C, Gallo M, Hylton-Kong T, Steiner M, Hobbs M, Macaluso M, et al. Randomized controlled trial on the effectiveness of counseling messages for avoiding unprotected sexual intercourse during STI and RTI treatment among female STI clinic patients. Sex Transm Dis. doi: 10.1097/OLQ.0b013e31827938a1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs MM, Steiner MJ, Rich KD, Gallo MF, Alam A, Rahman M, et al. Good performance of rapid prostate-specific antigen test for detection of semen exposure in women: implications for qualitative research. Sex Transm Dis. 2009;36(8):501–506. doi: 10.1097/OLQ.0b013e3181a2b4bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreher M, Hudgins R. Maternal conjugal multiplicity and child development in rural Jamaica. Fam Relations. 2010;59(5):495–505. [Google Scholar]
- 29.Mitchell K, Wellings K, Elam G, Erens B, Fenton K, Johnson A. How can we facilitate reliable reporting in surveys of sexual behaviour? Evidence from qualitative research. Cult Health Sex. 2007;9(5):519–531. doi: 10.1080/13691050701432561. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen SC, Gallo MF, Ombidi W, Omungo Z, Janowitz B, Hawken M, et al. Randomised controlled trial on whether advance knowledge of prostate-specific antigen testing improves participant reporting of unprotected sex. Sex Transm Infect. 2007;83(5):419–420. doi: 10.1136/sti.2006.022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chillag K, Guest G, Bunce A, Johnson L, Kilmarx P, Smith DK. Talking about sex in Botswana: social desirability bias and possible implications for HIV-prevention research. Afr J AIDS Res. 2006;5(2):123–131. doi: 10.2989/16085900609490372. [DOI] [PubMed] [Google Scholar]
- 32.Mauck CK, Doncel GF. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75(6):407–419. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]