Summary
Regions of the left inferotemporal cortex are involved in visual word recognition and semantics. We utilized functional magnetic resonance imaging to localize an inferotemporal language area and to demonstrate that this area is involved in the active maintenance of visually presented words in working memory. Maintenance activity in this inferotemporal area showed an effect of memory load for words, but not pseudowords. The selective modulation of this language-related inferotemporal area for the maintenance of words, in the absence of visual input, is accompanied by an increased functional connectivity with left prefrontal cortex. These results are the first demonstration of an involvement of inferotemporal cortex in verbal working memory. They provide neurophysiological support for the notion that nonphonological language representations can be recruited in the service of verbal working memory. More generally, they suggest that verbal working memory should be conceptualized as the frontally-guided, sustained activation of pre-existing cortical language representations.
Introduction
Verbal working memory, i.e. the temporary maintenance of behaviorally relevant verbal information in memory, relies to a large degree on brain regions involved in language processing. Using functional neuroimaging techniques, the left inferior parietal lobe has been proposed as a passive short-term store for phonological representations of verbal information (Awh et al., 1996; Jonides et al., 1998; Paulesu et al., 1993; Smith & Jonides, 1999). Broca's area in the left posterior inferior frontal gyrus, together with other premotor regions and the cerebellum, is thought to serve as a subvocal articulatory rehearsal mechanism that refreshes the decaying contents of the passive store (Awh et al., 1996; Paulesu et al., 1993; Smith & Jonides, 1999). These observations are consistent with the notion that verbal working memory relies on the human language system (Baddeley and Logie, 1999). However, it is at present an open question how other language regions of the brain contribute to verbal working memory.
One language-relevant region whose role in working memory has not been explored so far is the inferotemporal cortex (IT). IT is critically involved in the recognition of visual objects, scenes, faces, and written words (Malach et al., 2002). For the domain of language, more precisely visual word recognition, a functionally specialized processing stream is thought to exist within IT, representing visual words at increasingly higher levels of abstraction along a posterior-to-anterior axis. Intracranial electrophysiological recordings (Nobre et al., 1994) show that posterior IT differentiates letter strings from nonlinguistic complex visual objects. Brain activity in more anterior IT regions, in contrast, distinguishes words from nonwords and is affected by the semantic context of words, indicating that anterior IT holds more elaborate linguistic representations.
In domains other than language, human functional neuroimaging research has demonstrated that visual association areas in IT contribute to non-perceptual functions, in addition to their role in perception. For example, functionally defined IT areas such as the fusiform face area and the parahippocampal place area contribute to the temporary maintenance of faces or scenes, respectively, in visual working memory (Courtney et al., 1997; Druzgal and D'Esposito, 2001; Ranganath et al., 2004). The finding of a recruitment of visual association areas for working memory is consistent with neurophysiological data showing sustained firing of IT neurons while non-human primates maintain visual information in working memory (Fuster and Jervey, 1981; Miyashita and Chang, 1988). Given these results, working memory, from the neurophysiological perspective, is often understood as active memory, i.e., as a sustained activation of existing long-term representations stored in the posterior cortices that are involved in the initial processing of information to be memorized (Fuster, 1995). Active memory of posterior representations, according to this model, is under the `top-down' control of prefrontal cortex (PFC; Curtis & D'Esposito, 2003; Fuster et al., 1985).
The neurophysiological active memory model is consistent with cognitive models conceptualizing working memory as an activated state of long term memory representations (Cowan, 1999; Crowder, 1993). Under an active memory perspective of verbal working memory (Ruchkin et al., 2003), we accordingly hypothesized that active memory mechanisms analogous to the one observed for visual working memory should exist in IT language areas. We therefore expect that visually presented words are maintained not only using phonological codes (Baddeley and Logie, 1999), but also based on non-phonological, inferotemporal language representations. More specifically, we predict that the temporary maintenance of visually presented words in working memory should elicit sustained activation in left IT areas involved in visual word recognition.
We explored this hypothesis by investigating activation during the retention period of a verbal working memory task. During working memory retention, no visual information is present but recently presented words have to be actively kept available for later task performance. According to the active memory hypothesis for verbal working memory, inferotemporal language areas should be recruited more for the maintenance of words than pseudowords. Maintenance of pseudowords (i.e., orthographically legal and pronounceable nonwords) should not elicit strong sustained activation in such brain regions, as no stored representations pre-exist for these items. The present fMRI study tests these predictions using a delayed cued recall task. In addition to varying the nature of verbal stimuli to be maintained (i.e., words vs. pseudowords), we manipulate the number of items to be held in working memory (i.e., two vs. five). Assuming that cognitive processes related to working memory should be more strongly engaged under higher memory loads (Druzgal and D'Esposito, 2001), this load manipulation served to ensure that observed brain activation effects can indeed be attributed to working memory.
Results
Haemodynamic brain responses, known to be tightly coupled with regionally specific neural activity (Logothetis, 2003), were measured using fMRI while eleven participants performed (i) a simple word recognition experiment to localize an inferotemporal brain region responsive to visually presented words (IT) (Fig. 1A) and (ii), in a separate session, a verbal working memory task (Fig. 1B).
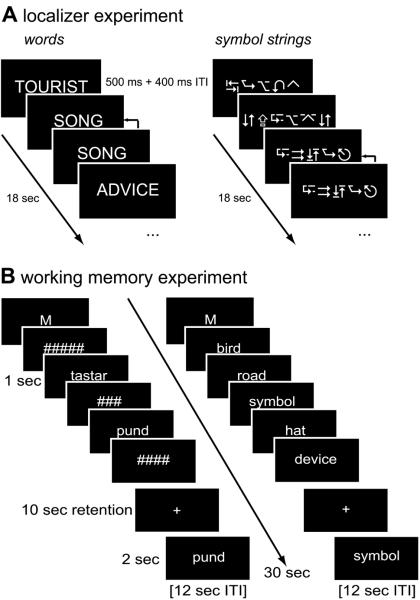
Figure 1. Experimental Paradigms.
(A) Localizer experiment for identifying the inferotemporal language-sensitive region of interest. Words, pseudowords (not shown), and symbol strings were presented in rapid sequences of 18 sec length. Participants detected immediate item repetitions, indicated by arrows in the figure (1-back task). (B) Delayed cued recall working memory experiment. Participants memorized two or five words or pseudowords, for a retention period of 10 sec. For display purposes only the two pseudowords (left) and five words conditions (right) are shown. The `M' cue (`memory') shown in the first frame indicates to the participants a working memory trial, as opposed to the case judgment control task which was cued by a `C'.
Localization of an Inferotemporal Language Area
From an independent localizer experiment, the IT region of interest (ROI) was identified individually for each participant as a brain area in left inferotemporal cortex that responds more to visually presented words than to length-matched, complex nonlinguistic strings of symbols (Fig. 1A). The IT ROI (Fig. 2A) was defined as the largest cluster of connected voxels more strongly activated for words than for length-matched strings of nonlinguistic symbols, restricted to left inferotemporal cortex. In seven individuals, the identified IT ROI was located in the fusiform gyrus or the medially bordering collateral sulcus. For four individuals, the IT ROI was localized more laterally, in the inferior temporal gyrus, with an extension into the inferior temporal sulcus in two individuals. Normalization of these individual IT ROIs into Talairach and Tournoux (1988) space results in a mean peak location in the anterior fusiform gyrus (x = −43; y = −37; z = −12). Statistical analysis of the mean parameter estimates from the general linear model (serving as an indicator of condition-specific activation) for the three stimulus types included in the localizer experiment, averaged across all voxels of the individually defined ROIs, demonstrates that not only words, but also length-matched pseudowords elicited greater activation than symbol strings [both t(10) > 3.6; p < .0025; one-tailed paired t-test]. Words and pseudowords, however, did not differ in their activation strengths [t(10) = 1.34; p > .2; two-tailed paired t-test] (Fig. 2B).
Figure 2. Inferotemporal Region of Interest.
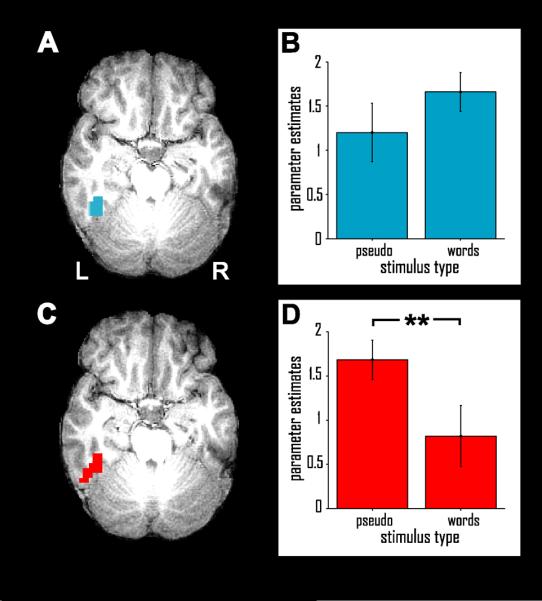
The inferotemporal language-sensitive region of interest (IT) shown in one representative participant (A) and group-level fMRI activation profile of IT during the processing of words and pseudowords, relative to length-matched symbol strings (B). (C) and (D) show the pseudoword-defined inferotemporal region of interest, in the same subject, and group-level activations for words and pseudowords relative to symbol strings. Displayed are mean parameter estimates (and st.err.) from the general linear model. **, p < .01.
In order to ensure that IT results reported below for verbal working memory are not a result of a bias towards words introduced by selecting the IT ROI using a contrast of words vs. symbols, we defined a second set of functional ROIs based on the contrast of pseudowords vs. symbols, also from the localizer experiment (Fig. 2C). Normalization of these new ROIs results in a mean peak location of x = −43; y = −41; z = −12, i.e., within less than half a centimeter distance in the anterior-posterior dimension from the word-based ROI. In this pseudoword-based ROI, again both words [t(10) = 2.4; p = .02; one-tailed t-test] and pseudowords [t(10) = 7.6; p < .001; one-tailed t-test] showed greater activity than nonlinguistic symbols. However, pseudowords elicited greater activity than words [t(10) > 3.2; p = .01; two-tailed t-test] (Fig. 2D). The pseudoword-based ROI will be used to verify the results obtained from the word-based IT ROI, in order to avoid a bias towards word-specific activity.
Behavioral Performance of Verbal Working Memory Task
The working memory task, conducted in a separate session, was a delayed cued recall task (Fig. 1B) that required participants to maintain either two or five words or pseudowords over a retention period of ten seconds. After the retention period, a recall probe appeared on the screen, cueing participants to recall and overtly pronounce one item from the memorized list.
Spoken responses were recorded in the scanner while participants performed the working memory task. Recall accuracy [2 words: 97% (SE 1.4); 5 words: 86% (5.6); 2 pseudowords: 99% (1.0); 5 pseudowords: 60% (4.5); cf. Fig. 3] was modulated by stimulus type [F(1,8) = 36.3; p < .001] and working memory load [F(1,8) = 24.2; p = .001]. These main effects were qualified by a stimulus type by load interaction [F(1,8) = 35.8; p < .001], due to significantly reduced accuracy at higher load for pseudowords [t(8) = −8.1; p < .001] but not words [t(8) = −1.8; p = .11].
Figure 3.

Recall accuracy (and st.err.) in the working memory task for pseudowords and words under low load (white bars) and high load (grey bars). ***, p < .0001.
Verbal Maintenance Activity in the Inferotemporal Cortex
Whole-brain results for average maintenance activation across all conditions, relative to the case judgment control task, are listed in Table S1 to provide context for the following results. During the retention period of the working memory task, the IT ROI showed significant activation for word maintenance relative to a visual case judgment control task that did not include memory demands (see Experimental Procedures) [t(10) = 2.96; p = .01] and a trend for such an effect for pseudoword maintenance [t(10) = 2.08; p = .06]. Furthermore, IT activity during the retention period showed a significant interaction between stimulus type (words vs. pseudowords) and working memory load (two vs. five items) [F(1,10) = 6.97; p = .025; Fig. 4B] but no main effects of stimulus type or load [both F < 1]. This interaction was due to a load effect for words (i.e., greater activation for maintenance of five vs. two words) [t(10) = 2.56; p = .01] but not pseudowords [t(10) = −.54; p = .6]. The selective activation increase in IT maintenance activity for five as compared to two words correlates with behavioral performance accuracy: Greater load effects (i.e., greater difficulties as a result of increased load) were accompanied by greater IT activation increases for words (r = .65; p = .08; Fig. 4C) but not pseudowords (p > .3).
Figure 4. Maintenance Activity in Inferotemporal Cortex.
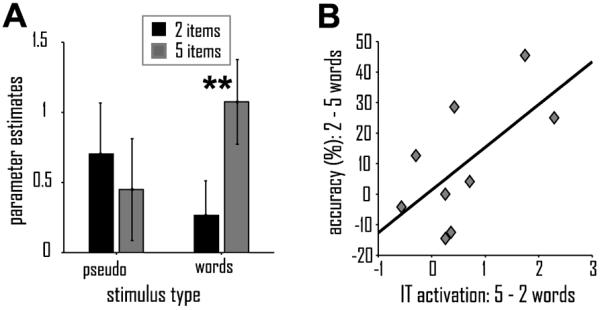
Group-level fMRI activation profile of IT during the maintenance of two (black) or five (grey) words or pseudowords in working memory (A). (B) Correlation between IT activation and performance accuracy (n = 9; see Experimental Procedures). Displayed values represent load effects, i.e., IT activation for 5 words – 2 words and performance accuracy for 2 words – 5 words. Displayed values (the mean parameter estimates from the general linear model and st.err.) reflect activation for the respective experimental condition relative to the corresponding task period of the visual case judgment task that served as control condition. **, p = .01.
In order to ensure that the observed stimulus type by load interaction is not a result of a bias introduced by functionally selecting the IT ROI as a word-selective area, we additionally probed the pseudoword-based IT ROI (see above and Experimental Procedures) for maintenance activity. This region shows again no main effects of stimulus type (F < 1) or working memory load [F(1,10) = 2; p = .19]. Analogous to the word-based IT ROI, a reliable stimulus type by load interaction [F(1,10) = 9.13; p = .013] is seen that results again from a selective load effect for words [t(10) = 2.89; p = .008] but not pseudowords [t(10) = −.04; p = .97]. As this analysis brought no indication of a bias introduced during functional ROI definition, the following results will be derived from the word-based IT region.
Functional Interaction between Inferotemporal and Prefrontal Cortex
Given that there was no visual input present during the retention period of the working memory task, IT maintenance activity cannot be attributed to the perceptual processing of new visual input. We hypothesized that the selectively increased IT engagement under high maintenance demands for words must be the result of top-down control exerted over this cortical region, with the goal of keeping language representations in IT at an elevated activation level throughout the retention period, to support successful working memory performance (Curtis and D'Esposito, 2003; Fuster, 1995). Although there are no direct anatomical projections between inferotemporal cortex and the dorsolateral PFC according to current knowledge derived from nonhuman primate neuroanatomy (Petrides and Pandya, 2002), dorsolateral PFC is proposed to be the primary source of higher cognitive control modulating processing in posterior brain regions (Miller and Cohen, 2001). Under the premise that functional interactions between brain regions are reflected in correlated activation patterns (Friston et al., 1993), the assumption of top-down modulation of IT during verbal maintenance can be tested by examining to what degree maintenance-related activity in IT and PFC covary over time. We thus expected to find a selectively increased correlation (i.e., functional connectivity) between IT and PFC activity for the maintenance of five as compared to two words and also relative to pseudoword maintenance.
To this end, we conducted a whole-brain functional connectivity analysis (Rissman et al., 2004), using each subject's IT ROI as a seed (see Experimental Procedures). Functional connectivity with the IT seed region was assessed for every voxel in the brain volume for the maintenance of five and two words, as well as for pseudoword maintenance. PFC activation in the left middle frontal gyrus correlates more strongly with IT activity during the maintenance of five words than during the maintenance of two words (Fig. 5A; peak voxel: x = −31, y = 39, z = 17; T(10) = 3.6). In addition, PFC activation correlates also more strongly with IT activity during the maintenance of five words than during pseudoword maintenance (Fig. 5B; peak voxel: x = −38, y = 40, z = 15; T(10) = 3.5).
Figure 5. Functional Connectivity between Inferotemporal and Prefrontal Cortex.
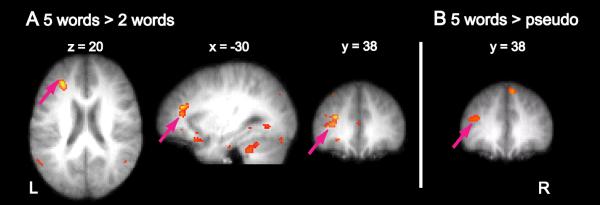
(A) Greater functional connectivity between the inferotermporal cortex (seed region) and left prefrontal cortex for five as compared to two word maintenance. Shown are axial, sagittal, and coronal sections through an average anatomical image generated from the individual images of 11 participants. (B) Increased functional connectivity between the inferotermporal cortex (seed region) and left prefrontal cortex for the maintenance of five words as compared to pseudoword maintenance. Pink arrows indicate significantly increased functional connectivity between PFC and IT (displayed here at p < .025 for illustrative purposes). (See Table S2 for more details.)
Complementary Mechanisms of Pseudoword Maintenance
The sustained activation of pre-existing IT language representations is not possible for pseudoword maintenance. The IT working memory mechanism for word maintenance that was demonstrated here may therefore be complementary to the phonological loop, adding an additional representational basis that makes word maintenance less difficult than pseudoword maintenance. Conversely, it follows that pseudoword maintenance may rely more on the phonological loop system than word maintenance, as lexical or semantic representations do not exist for unfamiliar pseudowords.
To test this assumption, we conducted a whole-brain analysis looking for brain regions more strongly activated during the maintenance of pseudowords than words. Such a main effect of lexicality was seen in the left inferior parietal lobule and in the posterior superior portion of the left inferior frontal gyrus (dorsal Broca's area) – i.e., in the phonological loop system (Figure 6A; cf. also Chein & Fiez, 2001). These brain regions are generally activated during verbal working memory, relative to the corresponding trial phase of the case judgment control task (Broca's area: words, t(10) = 3.8, p = .002; pseudowords, t(10) = 6.9, p < .001; inferior parietal lobule: words, t(10) = 1.9, p = .04; pseudowords, t(10) = 3.3, p = .004; one-tailed t-tests). In addition to the lexicality effect that identified these areas (F(1,10) for both areas > 20; p ≤ .001), both areas showed reliably greater activity for high than for low memory loads (both areas F(1,10) > 6; p < .05). In addition, the parietal area showed a stimulus type by load interaction (F(,10) = 5.46; p = .04) complementary to that seen for words in IT: Pseudoword maintenance (t(10) = 3.38; p = .007) elicited a greater memory load effect than word maintenance (t(10) = 2.04; p = .03).
Figure 6. Brain Activation and Functional Connectivity during Pseudoword Maintenance.
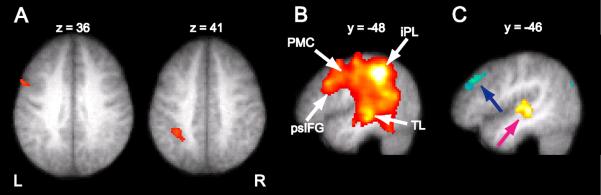
(A) Maintenance of pseudowords activated the left posterior inferior frontal gyrus (x = −57; y= 11; z = 31; t(10) = 4.98; left) and the left inferior parietal lobule (x = −34; y = −41; z = 39; t(10) = 5.24; right) more strongly than word maintenance. Displayed at p < .001. (B) Functional connectivity with the inferior parietal region during the maintenance of five pseudowords. iPL, inferior parietal lobe; psIFG, postero-superior inferior frontal gyrus; PMC, premotor cortex; TL, temporal lobe. (C) The left iPL shows stronger coupling with left TL (pink arrow) when maintaining five pseudowords as compared to five words, while it is more strongly coupled with left prefrontal cortex (blue arrow) when maintaining five words as compared to five pseudowords. Connectivity results displayed at p < .005. (See Table S3 for more details.)
Functional connectivity analyses show a broadly distributed correlation of the left inferior parietal area with bilateral parietal cortex, left superior temporal cortex, i.e., an area involved in the phonological processing of speech (Binder et al., 2000), and left premotor cortex, the dorsal portion of Broca's area, and the left posterior middle frontal gyrus (Fig. 6B), i.e., areas involved in phonological rehearsal. Statistical comparisons show that the inferior parietal region is coupled more strongly with left superior temporal cortex when subjects maintained five pseudowords than when they maintained five words in memory (Fig. 6C). Conversely, during maintenance of five words as compared to five pseudowords, inferior parietal cortex interacts more strongly with left PFC (Fig. 6C).
Discussion
The present study provides important new insights into the organization of verbal working memory in the brain. Behavioral results of the working memory task reported here support earlier claims that verbal working memory benefits from the preexistence of stored representations in long-term memory (Ruchkin et al., 2003). The brain activation results compellingly suggest that the recruitment of left inferotemporal cortex during word maintenance contributes to the better performance for words relative to pseudowords under high memory load. This is further supported by a task condition-specific correlation of IT activity with behavior, showing that this area is recruited more strongly the more difficult the maintenance task is for the individual. In addition, our data suggest that this inferotemporal working memory mechanism is controlled by a top-down signal from prefrontal cortex.
Inferotemporal Region of Interest
In recent years, a word-sensitive area in inferotemporal cortex, labeled as `visual word form area', has been studied in the context of visual word recognition. The IT language region explored in the present study is located somewhat anterior to the visual word form area (mean coordinate: x = −43; y = −54; z = −12; Cohen et al., 2002). We will therefore briefly discuss possible distinctions between the present IT ROI and the visual word form area. The visual word form area is typically defined as activation elicited by words relative to non-language stimuli, such as a fixation cross or a checkerboard grid (Cohen et al., 2000, 2002). IT activation for words relative to such low-level control stimuli extends along a significant length of IT cortex (e.g., from y = −75 to y = −35; Cohen et al., 2002; cf. also Dehaene et al., 2002), and the visual word form area is one among several peaks within this activation cluster. We chose a stricter functional definition of our language-sensitive IT region (i.e., relative to activation elicited by strings of complex symbols), in order to ensure that our IT ROI is specifically activated by strings of letters and not merely by complex combinations of visual features (Turkeltaub et al., 2003). We conclude that our definition of the IT language-sensitive area – controlling for complex but nono-rthographic shape processing – specifically identified an anterior component of the inferotemporal visual word response reported by others. However, it is important to note that the IT ROI defined here cannot correspond to more anterior inferior or medial temporal regions known to respond more to words than to pseudowords (Nobre et al., 1994), as comparable levels of activation were elicited during word and pseudoword processing in the localizer experiment.
The present result of an IT modulation during working memory maintenance of words but not pseudowords seems at odds with the observation of comparable activation levels for words and pseudowords during the localizer experiment. However, during the encoding and retrieval phases of the present working memory task, i.e., when words or pseudowords were actually presented to the participants, IT activation strength and IT load effects (5 vs. 2 items) did not distinguish between words and pseudowords (all effects p > .1 for both encoding and retrieval). The stimulus-specific top-down recruitment of IT was thus restricted to the non-perceptual maintenance phase of the working memory task.
Word Maintenance in Inferotemporal Cortex
The present result of a stimulus type × memory load interaction demonstrates that a language-sensitive left inferotemporal region contributes to the maintenance of visually presented words in working memory. Given that there was no visual input present during the retention period of the working memory task, IT maintenance activity cannot be attributed to the perceptual processing of new visual input. The observed IT effects for verbal maintenance are based on a covariate of the general linear model that reflects retention-period activation independent of encoding-related processes. Effects related to maintenance, therefore, cannot be attributed to residual encoding activity in the present experiment. One important conclusion of the present study is therefore that inferotemporal language functions are not exclusively driven by the perceptual processing of letter strings. Rather, IT language representations can be recruited for the performance of non-perceptual tasks.
Our observation of IT maintenance activity parallels data from monkey neurophysiology (Fuster & Jervey, 1981; Miyashita & Chang, 1988) and human functional neuroimaging (Courtney et al., 1997; Druzgal & D'Esposito, 2001; Ranganath et al., 2004) on the role of inferotemporal cortex in visual working memory. However, the present study is the first report of sustained activation during verbal working memory in a functionally defined region of IT that is involved in language processing. The present results, thus, extend the principles of active memory – i.e., the sustained activation of posterior cortical representations (Fuster, 1995) – that are established for visual working memory, to the domain of verbal working memory.
In addition to supporting an active memory model of working memory as discussed in the neurophysiological literature, the present data strongly support proceduralist cognitive models of working memory (Cowan, 1999; Pessoa and Ungerleider, 2004; Ruchkin et al., 2003). Proceduralist models of working memory claim that short term storage occurs in the same neural systems involved in processing the information to be maintained (Crowder, 1993). Attention-based models (e.g., Cowan, 1999) posit that working memory is the sustained activation of stored long-term memory representations. Critically, these models predict that non-phonological language representations contribute to the temporary maintenance of visually presented words in working memory. This is supported by behavioral (e.g., Penney, 1989; Bourassa & Besner, 1994; Logie et al., 2000) and electrophysiological (Ruchkin et al., 2003; Cameron et al., 2005) work, but has so far not been explored systematically using functional neuroimaging. The sensitivity of IT to word-related working memory demands observed in the present study clearly demonstrates a non-phonological mechanism for word maintenance. In addition, the present data suggest that this attention-based, or active, IT memory mechanism is specifically relevant under high working memory demands.
The Nature of IT Contributions to Verbal Working Memory
The present study demonstrates a contribution of left IT cortex to word maintenance under high memory load conditions. This inferotemporal memory mechanism is complementary to the phonological loop system; its additional recruitment is a likely source of the behavioral advantage for words relative to pseudowords in verbal working memory. However, the exact nature of the language representations that are activated in IT through the top-down influence of prefrontal cortex cannot be derived from haemodynamic response data. Behavioral work indicates that – besides phonological codes – visual, lexical-semantic as well as conceptual-semantic codes contribute independently to verbal working memory (e.g., Besner and Davelaar, 1982; Bourassa and Besner, 1994; Logie et al., 2000). The localization of the IT ROI in higher-level visual association cortex, as well as its functional definition that subtracts out complex visual processing, makes it unlikely that its contribution to verbal working memory is based on purely visual codes.
In studies of language processing, inferotemporal cortex has also been associated with prelexical processing of abstract word form (Cohen et al., 2000; Dehaene et al., 2005) and with conceptual-semantic processing (e.g., Bookheimer et al., 1995; Vandenberghe et al., 1996), independent of presentation modality (Cohen et al., 2004). The activation profile of the IT ROI in the localizer task (i.e., activity for words and pseudowords greater than that for complex symbol strings) is analogous to that reported for the more posteriorly located `visual word form area' (Dehaene et al., 2002; Polk & Farah, 2002). This may imply that the contribution of IT to verbal working memory is a pre-lexical, abstract visual-orthographic code. However, the fact that we observed a significant difference in maintenance activity between words and pseudowords, coupled with the more anterior location of our IT ROI, suggests that this region should not be anatomically or functionally equated with the visual word form area. Rather, our data seem to indicate that our IT ROI may represent higher-level linguistic codes.
Another functionally defined IT language region reported in the literature is the `basal temporal language area' (BTLA), identified by pre-operative electrical stimulation with subdural electrode implants (Lüders et al., 1991) and functional neuroimaging (Bookheimer et al., 1995). BTLA extends from 1 to around 9 cm posterior to the tip of the temporal lobe, including portions of the fusiform gyrus. BTLA stimulation leads to language deficits such as, most importantly, naming difficulties in about 30% (Lüders et al., 1991; Schäffler et al., 1996) to 80% (Krauss et al., 1996) of tested patients. Interpretation of the present IT area as BTLA could therefore potentially explain why words show a modulation during maintenance but not pseudowords. However, the specific relationship of BTLA to language has not been unequivocally established. In addition, BTLA resection is associated with naming decreases, but does not necessarily lead to major language deficits (Krauss et al., 1996). Finally, it was reported that language deficits occur only when BTLA stimulation causes remote discharges in the posterior superior temporal lobe (Ishitobi et al., 2000).
A final line of evidence links the present verbal working memory results for IT to more semantic aspects of word recognition. In a recent review of functional neuroimaging studies of concreteness and imageability effects in word recognition, we identified an area in the left anterior fusiform gyrus that is more strongly activated for highly imageable and concrete words than for more abstract words (Fiebach & Friederici, 2003). This activity was located slightly more medially than the present IT region, along the collateral sulcus, but at the same position of the anterior-to-posterior axis (i.e., with a peak at y = −41). An independent contribution of semantic codes to verbal working memory maintenance is also supported by behavioral studies (Bourassa & Besner, 1994) and electrophysiological research (Cameron et al., 2005). A semantic interpretation is further in line with findings of multimodal IT effects during word processing (Cohen et al., 2004). However, the question arises why the perception of pseudowords (in the localizer task and during working memory encoding and retrieval) activates semantic representations in IT to a comparable degree as words do. This may be the result of the automatic activation of semantic representations of orthographic neighbors of the pseudowords, i.e., of words that share orthographic features with the perceived pseudoword (Andrews, 1997). Such neighborhood effects are plausible only during word perception but not during top-down guided activation of word representations, as occurs during the WM retention interval.
Complementary Mechanisms for Word and Pseudoword Maintenance
The present results do not imply that the sustained activation of IT is the only cortical mechanism contributing to verbal working memory. It is well known that left inferior parietal cortex, Broca's area, the supplementary motor area and the cerebellum support the rehearsal of verbal information based on its phonological form (phonological loop; Awh et al., 1996; Paulesu et al., 1993; Smith and Jonides, 1999). The present study demonstrates that different language systems (such as parieto-frontal phonological vs. temporal visual-semantic) are flexibly recruited depending on the affordances of the to-be-maintained stimulus (e.g., pseudowords vs. words). In support of this assumption, behavioral studies suggest the involvement of visual, lexical and semantic codes during word maintenance. Such aspects of verbal short-term memory can compensate for difficulty at the level of phonological rehearsal introduced by phonological similarity (e.g., Hulme et al., 1991) or articulatory suppression (e.g., Besner and Davelaar, 1982; Bourassa and Besner, 1994; Logie et al., 2000). When recruited for non-perceptual task demands such as working memory, different language systems may thus have complementary roles.
Top-Down Recruitment of IT During Verbal Working Memory
We had hypothesized that the recruitment of IT during word maintenance is the result of a top-down signal from prefrontal cortex. The finding of a functional interaction between PFC and IT is consistent with this hypothesis, as well as with earlier reports of task-relevant functional interactions between IT and PFC during visual working memory in non-human primates and humans (e.g., Gazzaley et al., 2004). However, the present data cannot establish the directionality of IT-PFC coupling, i.e., whether PFC controls IT, or whether information primarily flows from IT to PFC during the retention period of the task. The rationale for interpreting the present result as a top-down influence of PFC on IT is that during the retention period of the present working memory paradigm, no visual input is processed. Observed functional connectivities therefore cannot reflect the bottom-up transmission of new words from temporal cortex to PFC, but rather must reflect the top-down activation of the inferotemporal brain region by the PFC.
In humans, an intact dorsolateral prefrontal cortex is critical for the successful maintenance of information held in temporal cortex (Chao & Knight, 1998). More direct evidence for the existence of top-down signals from PFC to posterior regions comes from neurophysiological studies in monkeys (Fuster et al., 1985; Tomita et al., 1999; Moore & Armstrong, 2003). Functional inactivation of PFC by reversible cooling (Fuster et al., 1985) results in reduced firing rates of IT neurons during working memory retention and in an increase in behavioral errors. By combining neurophysiological measures with permanent posterior callosal lesions, prospective memory signals have been recorded from inferotemporal cortex that cannot reflect bottom-up processing but have to be the result of prefrontal top-down signals (Tomita et al., 1999). Moore and Armstrong (2003) showed that electrical microstimulation of frontal eye field neurons enhanced neuronal responses in extrastriate visual cortex. These results provide direct evidence for the existence of top-down signaling from PFC to posterior cortices (see Miller & D'Esposito, 2005, for a more detailed review).
While previous work in human and non-human primates was carried out in the domain of visual working memory, the present result of a coupling between PFC and a langugae-sensitive IT region strongly suggests that fronto-posterior interactions are also critical for verbal working memory. The selectivity of the functional interaction between PFC and IT (i.e., only under high load and only for word maintenance) suggests that PFC copes with high task demands – under high verbal working memory load – by selectively integrating additional brain areas storing task-relevant language representations into verbal working memory networks. Crucially, this increased integration involves areas that are not necessarily critical for successful performance under lower task demands (such as maintaining only two words in memory). Interestingly, the coupling between inferior parietal cortex and the premotor cortex and Broca's area that is associated with phonologically mediated rehearsal of verbal material (Buchsbaum et al., 2005) is not affected by working memory load.
Taken together, the observed functional PFC-IT interaction, the unavailability of visual bottom-up signals during the retention period of the present task, and the established existence of fronto-temporal top-down signals strongly suggest a top-down modulation of IT during verbal working memory. The finding of a functional interaction between IT and PFC complements our observation of IT maintenance activity and demonstrates a possible neural mechanism for the recruitment of this brain region during verbal working memory. Sustaining task-relevant information over brief periods of time is one of the critical functions of PFC (Miller and Cohen, 2001). Inferotemporal areas, in contrast, typically are involved in the automatic, transient processing of visual-perceptual information. A sustained inferotemporal contribution to verbal working memory maintenance, thus, depends on its coupling with PFC. Maintenance, or storage, of verbal information in working memory, accordingly, involves the coordinated processing of posterior and frontal brain regions, with frontal regions controlling the sustained activation of posterior representations (Curtis and D'Esposito, 2003). In this respect, verbal working memory may be only a special case of frontal control over posterior language regions. Other situations, such as the inhibition of verbal responses or the selection of context-appropriate words may involve similar mechanisms of frontal control over the language system.
Conclusion
The present results demonstrate that a language-sensitive region of the left inferotemporal cortex is involved in the maintenance of words in verbal working memory. Specifically, this inferotemporal area shows a selective activation increase when memory load for words increases, but not for an increased load of pseudowords. The functional interaction between this inferotemporal area and prefrontal cortex is selectively enhanced for the maintenance of a high memory load for words. Taken together, these results provide neurophysiological evidence that the temporary maintenance of verbal information in working memory is not exclusively based on phonological codes, but relies also on other components of the language system. The inferotemporal working memory mechanism identified here is assumed to reflect a semantic contribution to word maintenance. More generally, these findings challenge the assumption of a special purpose neural system for verbal working memory and support attention-based, procedural models that describe working memory as a sustained activation of long term memory contents. This indicates that the neurophysiological principles of active memory, i.e. the sustained activation of representations in posterior cortices under dynamic prefrontal control (Fuster, 1995), that are established for visual working memory, are also operative in the domain of verbal working memory. Verbal and visual working memory, thus, rely on analogous neural mechanisms applied to different cortical representations. Finally, the present data specify a possible neural mechanisms for the control of activation-based working memory in the verbal domain, namely the task-dependent modulation of functional connectivities between prefrontal cortex and language areas in posterior cortex.
Experimental Procedures
Participants
Eleven undergraduate students (five females; mean age 21.4 years) participated as paid volunteers after giving informed consent according to a protocol approved by the University of California, Berkeley Committee for the Protection of Human Subjects. All participants were right handed native speakers of English, had normal vision, and were screened for presence of medical, neurological or psychiatric illnesses, or use of prescribed drugs.
Material & Experimental Procedures
Word recognition localizer experiment
160 words were selected from the MRC Psycholinguistic Database (Coltheart, 1981). Words had three to eight letters (mean 5.6), one or two syllables (mean 1.65), and a mean Kucera and Francis (1967) word frequency of 74.6 per million. Half of the words were abstract and half were concrete (means of 297.4 vs. 574.2; Welch two sample t-test: t(152.7) = 48.5, p < .001). Pseudoword stimuli were constructed in two steps. First, a pool of nine pseudoword items was generated for each word by repeatedly (i.e., nine times) replacing one, two, or three letters of the original word at randomly determined positions. Second, one pseudoword that was pronounceable and not similar to any existing word was manually selected from the pool for each word item. If no pseudoword from the pool of nine items fulfilled these two criteria, additional letters were manually replaced. Symbol string items were generated by replacing the letters of the word stimuli with symbols from a symbol font (see Fig. 1A for examples). Stimuli from all three conditions were thus identical in length. The localizer experiment was conducted with a blocked design, with eight 18 sec blocks from each condition, words, pseudowords, and symbol strings, as well as eight rest blocks during which only a fixation cross was presented. Blocks were presented in pseudo-random order, with transition frequencies between conditions equated, and acquired in one functional run of 10.9 minutes length. Each block consisted of 20 items centrally presented in white letters on black background for 500 ms (interstimulus interval 400 ms; Fig. 1A). Participants indicated by button press whenever they detected an immediate item repetition, of which there could be one, two, or three per block.
Verbal working memory task
The delayed cued recall task was conducted as a slow single-trial fMRI paradigm. During encoding, participants were presented with five visual frames, each for one second, which were used to present either two or five words or pseudowords (Fig. 1B). Word lengths were between three to seven letters (mean 5.1) and did not vary between two and five word trials [5.06 vs. 5.14; t(166) = .48; p = .63]. Words were comparable between conditions in their mean occurrence frequencies (Kucera and Francis, 1967) [71.8 vs. 67.9 per million; t(166) = .5; p = .62] and mean concreteness ratings [548.7 vs. 555.6; t(166) = .61; p = .54]. Pseudowords were generated as described above for the localizer experiment, ensuring that words and pseudowords did not differ in length. In two-item trials, the remaining three frames contained strings of pound signs (`#'), matched for length to the five-item conditions. After a 10 sec retention period, a probe item from the encoded list was presented for two seconds as a cue to recall the respective other item in the two-item trials, or, in five-item trials, the item that followed the probe. Participants' verbal responses were recorded using a microphone that was placed over the mouth in a rubber-mask to shield some of the scanner noise. After probe-offset, a 12 sec inter-trial interval followed.
In addition to the four working memory conditions, a control condition was included that consisted of the identical stimulus presentation and timing, but required participants to make a spoken upper/lower case judgment on the probe item. Before the beginning of the encoding period, the letters `M' or `C' were presented for one second to inform participants whether they performed the memory (M) or the case judgment (C) task. For the case judgment task, participants were explicitly instructed and pre-trained to not memorize the word list presented during the encoding phase. Trials from the five conditions (i.e, four working memory conditions plus control task) were presented in pseudorandom order with transition frequencies between conditions equated. Twenty-four items were presented per condition, distributed evenly across four 15 min runs of thirty trials each.
Separation of spoken responses from scanner noise was possible only in nine out of eleven individuals. For the remaining two subjects, larger head size prevented positioning the mask with the microphone close enough to the mouth to shield scanner noise effectively. Response times are not reported as they do not reflect true voice onset times. Rather, the obtained response time estimates indicate the time point at which the amplitude of the spoken response rises above the background scanner noise. This, however, may vary between individuals depending on the loudness of their spoken response and the degree to which the set up of the microphone could shield scanner noise.
No indications of systematic response related movements were noticed. Note that the interest of the present study is on activations during the retention phase of the working memory task, not during the retrieval phase during which the spoken response was given.
FMRI Acquisition
Functional MRI scanning was performed on a 4T Varian INOVA scanner using a T2*-weighted BOLD sensitive gradient echo echo-planar imaging sequence (TE = 28 ms, FOV = 22.4 × 22.4 cm, 64 × 64 matrix, resulting in-plane resolution 3.5 × 3.5 mm). Using a two-shot interleaved sequence, half of K-space was acquired in 1 second (i.e., total TR 2 sec). During reconstruction, each shot was interpolated with its neighbor, resulting in an effective TR of 1 sec. A phase map correction was applied to remove Nyquist ghosts. 18 oblique axial slices of 5 mm thickness (1 mm gap) were acquired that covered all of the frontal and temporal lobes and only excluded the dorsal-most aspect of the parietal lobe in some participants. Each run was preceded by 10 sec of dummy gradient RF pulses to achieve steady-state tissue magnetization. The experiment was conducted in two sessions. In the first session, the localizer experiment was conducted, as well as a further, independent experiment that is not reported here. In the second session, participants performed the working memory experiment. For purposes of coregistration of the functional scans between the two sessions, and to coregister functional data to high-resolution anatomical scans for purposes of localization and normalization, a T1-weighted anatomical scan with the identical slice prescription as the functional scans was acquired before the functional scans in both sessions. A T1-weighted, three-dimensional high resolution MP (magnetization-prepared)-Flash scan was obtained after the functional scans in the first session.
FMRI Analysis
Preprocessing
Acquired time series were corrected for slice acquisition time differences during reconstruction. Using the Statistical Parametric Mapping software package (SPM2, Wellcome Department of Cognitive Neurology, London, UK), data were corrected for motion using a 6-parameter, rigid-body, least-squares realignment to the first functional image acquired in each session. Using the session-specific anatomical scans, all scans from the second session were then coregistered to the T1-weighted anatomical scan acquired at the beginning of the first session, so that data from the two sessions could be analyzed together. As a final step of preprocessing, functional data were spatially smoothed with an 8 mm FWHM isotropic Gaussian kernel. All statistical analyses, with the exception of the group statistics in the functional connectivity analysis (see below) were performed in native space (i.e., without spatial normalization).
General Linear Model
Statistical analysis was performed using a general linear model (GLM) including a canonical hemodynamic response function and its temporal derivative (Friston et al., 1998). The four functional runs of the working memory task were modeled as separate sessions in subject-specific fixed-effect models. These models included separate covariates for instruction period (at 0 sec or TR 0 within the trial), encoding (at 1 sec) and retrieval phases (at 16 sec) and, for the retention period, a covariate reflecting early (from two to three seconds of the retention period; at 7 sec within the trial) and one modeling late (from five to eight seconds; at 10 sec in the trial) maintenance activity for each experimental condition. At these time points, the canonical hemodynamic response function provided by SPM2 was convolved with a box car function of the length of the respective trial period (i.e., instruction:1 sec; encoding: 5 sec; early delay: 2 sec; late delay: 4 sec; retrieval: 2 sec). This approach allowed us to estimate changes in hemodynamic signal for the four working memory conditions, relative to the control task, separately within each task phase (Postle et al., 2000; Zarahn et al., 1997). The early retention covariate was included in the GLM to reduce noise in the estimation of the baseline, but was not further analyzed as it is not assumed to be independent from the encoding phase (Postle et al., 2000; Zarahn et al., 1997). A temporal high pass filter with a cut-off of 128 sec was included. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm using a second-order autoregressive model (AR(2) model); resulting estimates were used for non-sphericity correction (whitening) during the model estimation.
Region of Interest Analyses
Given that strong a-priori hypotheses existed concerning the location of the IT region of interest, functional ROIs were defined by applying a statistical threshold of p < .01 (uncorrected) to an anatomically defined search space. The inferotemporal ROI was functionally defined for each participant as greater activation for words than for length-matched symbol strings in the localizer experiment. The IT ROI was selected by identifying a statistically significant activation cluster in left IT, as determined using an atlas of human brain anatomy (Duvernoy, 1999). Resulting ROIs had a mean size of 16.8 voxels (each of size 3.5 × 3.5 × 6 mm). IT ROIs were located either in the fusiform gyrus, collateral sulcus, or inferior temporal gyrus. To ensure that IT ROIs did not suffer from susceptibility-induced signal loss, average IT raw signals taken from each subject's mean epi image (as generated during SPM2 realignment) were compared to the average raw signal from an inferior parietal ROI. Raw signal strenghts did not differ between IT and inferior parietal lobe (t(10) = −.05; p = .96; Fig. S1). To determine the localization of functionally defined ROIs in stereotaxic space, the binary ROI masks were normalized to stereotaxic (MNI) space using the ICB 152 template and coordinates for the central voxel in each ROI were determined. All MNI coordinates were converted to Talairach and Tournoux (1988) space (as described by M. Brett; http://www.mrc-cbu.ac.uk/Imaging/Common/mnispace.shtml).
For the group-level analyses, condition-specific parameter estimates from the GLM – reflecting the strength of the involvement of each voxel during the late retention period of the working memory task – were averaged across voxels within each individual's ROI. These values reflect activation in the time period between five and eight seconds within the ten-second retention period. Condition-specific mean parameter estimates were extracted from the ROIs, corrected for parameter estimates from the respective task period of the control task (Courtney et al., 1997), and then submitted to a repeated-measures ANOVA with the factors stimulus type (words vs. pseudowords) and working memory load (2 vs. 5), treating subjects as random effects. Direct comparisons between conditions are conducted using paired two-sample t-tests. In case of pre-existing hypotheses (such as in the case of ROI definition), one-sample t-tests are used. This is stated explicitly in the text.
Functional Connectivity Analysis
Voxel-wise whole brain analyses of functional connectivity over time were calculated for the late retention period activity during maintenance of five and two words. These analyses were conducted in native space, with the IT ROI as the seed region. For each participant, a set of single trial activity estimates, or beta series, specific to the late retention period of each experimental condition were derived independently for every brain voxel, based on a separate GLM in which each task phase from each trial was modeled as a unique event (see Rissman et al., 2004, for a detailed description of this functional connectivity analysis procedure, and see Buchsbaum et al., 2005, and Gazzaley et al., 2004, for further examples of its application to the retention period of working memory tasks). For each condition, a seed beta series, averaged across all voxels of the seed ROI, was generated for each participant, and the correlation of each brain voxel's beta series with that of the seed was determined. The resulting condition-specific correlation maps were then normalized to MNI space using the ICB 152 template. Finally, correlation maps were compared between conditions at the group level using t-tests for paired samples. To test our a-priori hypothesis of increased functional coupling between IT and PFC, resulting t-maps were thresholded at t > 3.169 (p < .005; one-tailed; df = 10; uncorrected for multiple comparisons).
Whole-Brain Analysis
To complement the reported ROI-driven analyses with an analysis of brain regions activated more strongly for pseudowords as compared to words, contrast images resulting from individual GLMs were normalized to MNI space and then subjected to a random effects one-sample t-test. Brain regions more strongly activated for pseudowords were identified by thresholding the resulting group t-maps at t > 4.1437 (p < .001 one-tailed; df = 10; uncorrected).
Supplementary Material
Acknowledgements
The authors thank Joe Devlin, Adam Gazzaley, and three anonymous reviewers for their insightful comments on an earlier version of this manuscript. This research was supported by the German Academic Exchange Service (DAAD), the German Research Foundation (DFG, FI 848-2/1), and by NIH grant MH63901.
References
- Andrews S. The effect of orthographic similarity on lexical retrieval: Resolving neighborhood conflicts. Psych Bull Rev. 1997;4:439–461. [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychological Science. 1996;7:25–31. [Google Scholar]
- Baddeley AD, Logie RH. Working memory: The multiple-component model. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; Cambridge: 1999. pp. 28–61. [Google Scholar]
- Besner D, Davelaar E. Basic processes in reading: Two phonological codes. Canadian J Psychology. 1982;36:701–711. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cer Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp. 1995;3:93–106. [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Bourassa DC, Besner D. Beyond the articulatory loop: A semantic contribution to serial order recall of subspan lists. Psychonomic Bull Rev. 1994;1:122–125. doi: 10.3758/BF03200768. [DOI] [PubMed] [Google Scholar]
- Cameron KA, Haarmann HJ, Grafman J, Ruchkin DS. Long-term memory is the representational basis for semantic verbal short-term memory. Psychophysiol. 2005;42:643–653. doi: 10.1111/j.1469-8986.2005.00357.x. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosc. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex. 2001;11:1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. NeuroImage. 2004;23:1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In Models of Working Memory. In: Miyake AA, Shah P, editors. Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; Cambridge: 1999. pp. 62–101. [Google Scholar]
- Crowder RG. Short-term memory: where do we stand? Mem Cognit. 1993;21:142–145. doi: 10.3758/bf03202725. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D'Esposito M. Activity in fusiform face area modulated as a function of working memory load. Brain Res Cogn Brain Res. 2001;10:355–364. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The Human Brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply. Springer-Verlag; Wien: 1999. [Google Scholar]
- Fiebach CJ, Friederici AD. Processing concrete words: FMRI evidence against a specific right-hemisphere involvement. Neuropsychologia. 2003;42:62–70. doi: 10.1016/s0028-3932(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the cerebral cortex. The MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Jervey JP. Inferotemporal neurons distinguish and retain behaviorally relevant features of visual stimuli. Science. 1981;212:952–955. doi: 10.1126/science.7233192. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Hulme C, Maughan S, Brown GDA. Memory for familiar and unfamiliar words: Evidence for a long-term memory contribution to short-term memory span. J Mem Lang. 1991;30:658–701. [Google Scholar]
- Ishitobi M, Nakasato N, Suzuki K, Nagamatsu K, Shamoto H, Yoshimoto T. Remote discharges in the posterior language area during basal temporal stimulation. NeuroReport. 2000;11:2997–3000. doi: 10.1097/00001756-200009110-00034. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, Lesser RP. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-Day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Logie RH, Della Sala S, Wynn V, Baddeley AD. Visual similarity effects in immediate verbal serial recall. Q J Exp Psychol. 2000;53A:626–646. doi: 10.1080/713755916. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, Godoy J. Basal temporal language area. Brain. 1991;114:743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends Cogn Sci. 2002;6:176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Chang HS. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature. 1988;331:68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Penney CG. Modality effects and the structure of short-term verbal memory. Mem Cognit. 1989;17:398–422. doi: 10.3758/bf03202613. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Top-down mechanisms for working memory and attentional processes. In: Gazzaniga MS, editor. The Cognitive Neurosciences III. The MIT Press; Cambridge, MA: 2004. pp. 919–930. [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; Oxford: 2002. pp. 31–50. [Google Scholar]
- Polk TA, Farah MJ. Functional MRI evidence for an abstract, not perceptual, word-form area. J Exp Psychol Gen. 2002;131:65–72. doi: 10.1037//0096-3445.131.1.65. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Res Brain Res Protoc. 2000;5:57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Ranganath C, DeGutis J, D'Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Cogn Brain Res. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Grafman J, Cameron K, Berndt RS. Working memory retention systems: a state of activated long-term memory. Behav Brain Sci. 2003;26:709–728. doi: 10.1017/s0140525x03000165. discussion 728–777. [DOI] [PubMed] [Google Scholar]
- Schäffler L, Lüders HO, Beck GJ. Quantitative comparison of language deficits produced by extraoperative electrical stimulation of Broca's, Wernicke's, and Basal Temporal Language areas. Epilepsia. 1996;37:463–475. doi: 10.1111/j.1528-1157.1996.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in th frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Georg Thieme Verlag; Stuttgart: 1988. [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.