Abstract
Metabolic syndrome (MetS) remains a controversial entity. Specific clusters of MetS components - rather than MetS per se’- were associated with accelerated arterial ageing and with CV events. To investigate whether the distribution of the “risky” clusters of MetS components differed cross-culturally, we studied 34,821 subjects from 12 cohorts from 10 European countries and 1 from US participants in the MARE (Metabolic syndrome and Arteries REsearch) Consortium. In accordance with the ATP III criteria, MetS was defined as an alteration ≥3 of the following 5 components: elevated glucose (G): fasting glucose ≥110 mg/dl; low HDL cholesterol (H): <40 mg/dl for M or < 50 mg/dl for W; high triglycerides (T) ≥150 mg/dl; elevated BP (B): ≥130/≥85 mmHg; abdominal obesity (W): waist circumference > 102 cm for M or >88 cm for W.
MetS had a 24.3% prevalence (8468 subjects) (23.9% in men vs 24.6% in women, p<0.001) with an age-associated increase in its prevalence in all the cohorts.
The age-adjusted prevalence of the clusters of MetS components previously associated with greater arterial and CV burden differed across countries (p< 0.0001) and in men and women (gender effect p<0.0001). In details, the cluster T-B-W was observed in 12% of the subjects with MetS, but was far more common in the cohorts from UK (32.3%), Sardinia in Italy (19.6%), and Germany (18.5%) and less prevalent in the cohorts from Sweden (1.2%), Spain (2.6%), and USA (2.5%). The cluster G-B-W accounted for 12.7% of subjects with MetS with higher occurrence in Southern Europe (Italy, Spain, and Portugal - with 31.4%, 18.4%, and 17.1% respectively) and in Belgium (20.4%), than in Northern Europe (Germany, Sweden, and Lithuania - with 7.6%, 9.4%, and 9.6% respectively).
The analysis of the distribution of MetS suggested that what follows under the common definition of MetS is not a unique entity rather a constellation of cluster of MetS components, likely selectively risky for CV disease, whose occurrence differs across countries.
Keywords: blood pressure, epidemiology, Europe, glucose, metabolic syndrome, triglycerides, HDL cholesterol, waist circumference
INTRODUCTION
Even though the metabolic syndrome (MetS) remains a controversial entity (1–2), it has previously been shown to be associated with higher odds of CV events even in older subjects (3–6) – likely through increasing arterial stiffness and thickness, i.e. accelerating arterial ageing (7–8).
Yet, whether MetS is a single syndrome or a constellation of different syndromes carrying different CV risk remains an unanswered question and a scarcely investigated topic (9). Of note, it was recently reported that specific clusters of altered MetS components - rather than MetS per se’-were associated with accelerated arterial aging in the SardiNIA Study (7). Similarly, only specific clusters of altered MetS components conferred a higher risk of CV events in the Framingham Study (10).
The aim of the present study was to investigate whether the distribution of clusters of MetS components differed across countries, and whether these differences were gender specific. To investigate this question, we studied 34821 subjects from 12 cohorts representing 10 different European countries and the US participating in the MARE (Metabolic syndrome and Arteries REsearch) Consortium
SUBJECTS AND METHODS
The MARE Consortium
The original MARE (Metabolic syndrome and Artery REsearch) Consortium was established as a collaboration among eleven European and one American centre studying population-based cohorts to identify any cross-cultural differences in clustering of MetS altered components and associations with arterial aging; to disentangle the specific role of genes and lifestyle factors (and their interactions) on the clinical presentation of MetS and on the CV risk attributable to MetS; and to develop new strategies to prevent CV events through identification of potential lifestyle changes.
The MARE Consortium is open to additional participating cohorts, provided that data on the MetS components and on arterial properties were available for the recruited subjects.
Ethic statement
The cohorts participating to the present analysis are briefly described in Supplemental Material and Supplemental Table 1; the age distribution of the enrolled subjects are presented in Figure 1.
Figure 1.
Age distribution (mean and SD) of subject enrolled in participating cohorts of the MARE Consortium
Each Cohort had its protocol approved by local Ethic Committee. Each participant signed written informed consent form, previously approved by site-specific Ethic Committee.
Definition of the Metabolic Syndrome
The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) (11) defined the MetS as an alteration in three or more of the following five components: abdominal obesity (W), high triglycerides (T), low HDL cholesterol (H), elevated blood pressure (systolic or diastolic) (B), and elevated fasting glucose (G). The following cut-off values are used to define each altered component: waist circumference > 102 cm for men or >88 cm for women, triglycerides ≥150 mg/dl, HDL cholesterol <40 mg/dl for men or < 50 mg/dl for women, blood pressure ≥130/≥85 mmHg, and fasting glucose ≥110 mg/dl.
Because MetS is defined by the presence of three or more altered components, subjects with MetS may have different combinations of the individual components of MetS.
Statistical analysis
All analyses were performed using the SAS package for Windows (9.1 Version Cary, NC, USA). Data are presented as mean ± SD for continuous variables or as percentages for categorical variables. Differences in the prevalence for each of the measured variables among groups were compared by ANOVA. ANCOVA analysis was used to assess interactions between sex and cohort on the distribution of MetS components or clusters of components, independently of the possible effects of age on the same variables. A two-sided p value <0.05 indicated statistical significance.
RESULTS
Complete data were available for 34821 subjects. MetS had in general a 24.3% prevalence (8468 subjects) that increased with advancing age (from 3.7 in the group aged 20–29 years to more than 30% in the subjects 70 years and older) (Figure 2 top panel). The significant difference in the MetS prevalence across countries illustrated in Figure 2 middle panel (p< 0.0001) likely reflects primarily different population characteristic (age distribution, general population versus higher CV risk subjects), but it did not seem to parallel the prevalence of diabetes mellitus in the same populations. Overall, there was a small but significant gender difference in the prevalence of Mets (23.9% in men and 24.6% in women, p<0.001) (Figure 2 bottom panel).
Figure 2.
Overall prevalence of MetS in different age groups (top panel),and diabetes mellitus in participating cohorts (middle panel); gender-specific prevalence of MetS in participating cohorts (bottom panel) of the MARE Consortium
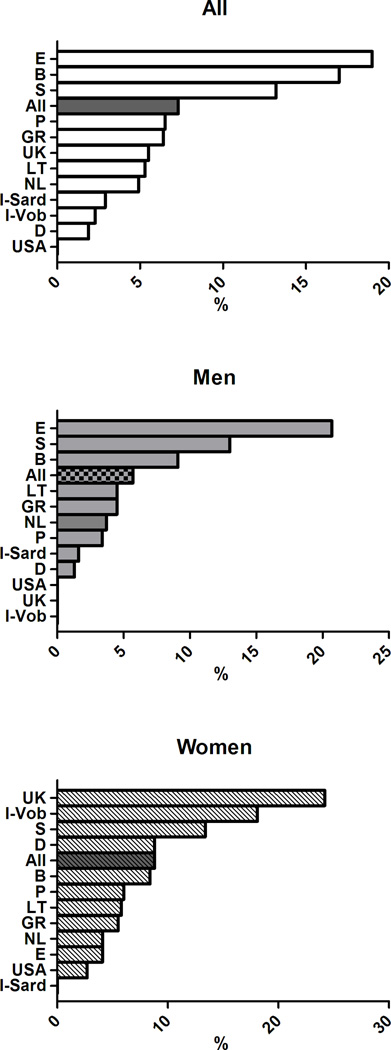
In subjects with MetS, the distribution of altered components differed between men and women and cross-country wise (see Figure 3). Specifically, elevated glucose (G) was highly prevalent in cohorts from Belgium, UK, Lithuania, Spain and Sardinia in Italy, whereas the lowest prevalence was observed in the Greek cohort. Low HDL cholesterol (H) showed the highest prevalence in the cohorts from Sweden, USA, the Netherlands and the lowest in Italian Sardinia and in the UK. Elevated triglycerides (T) were highly prevalent in the cohort from Germany, UK, and Lithuania and less prevalent in Spain and Sweden. Elevated blood pressure (B) had a prevalence >90% in the cohorts from Sweden, Germany, and Greece with the lowest prevalence in the cohorts from USA and Lithuania. The prevalence of abdominal obesity (W) showed a wide significant gender difference (75.6% in men and 90.6% % in women, p< 0.0001). Of note, waist circumference exceeded the cut-off values in more than 90% of women from USA, UK, and Mediterranean countries, whereas it was less frequently altered in the Swedish cohort.
Figure 3.
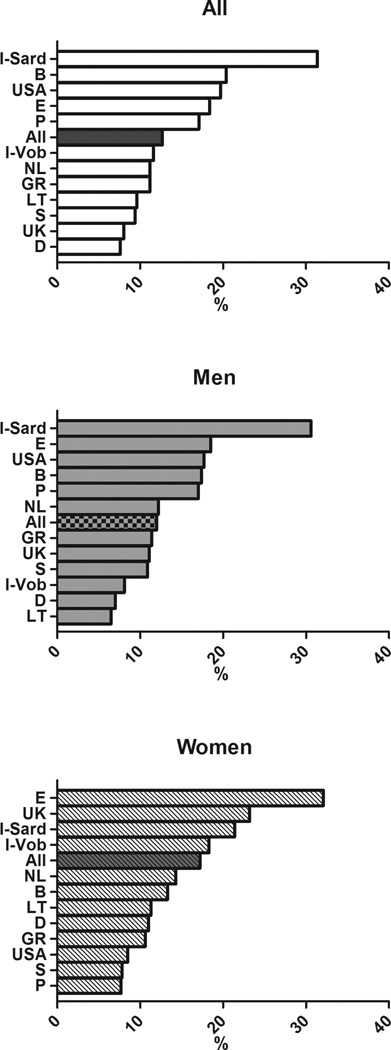
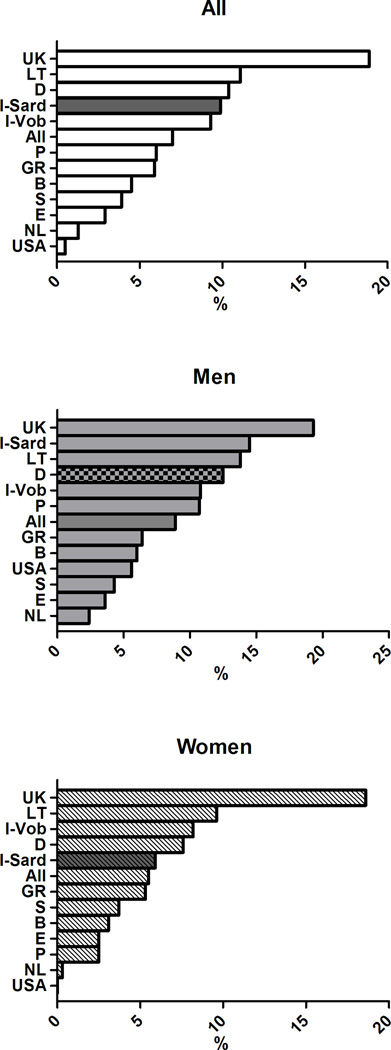
Distribution of MetS components in subjects with MetS in participating cohorts of the MARE Consortium
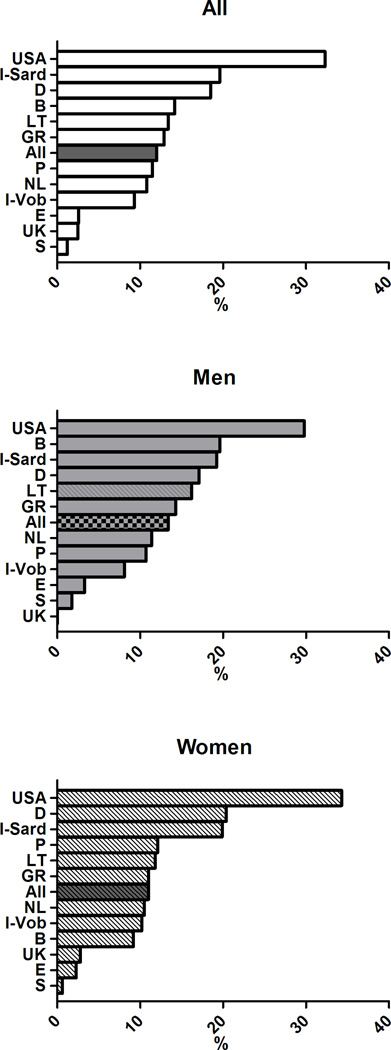
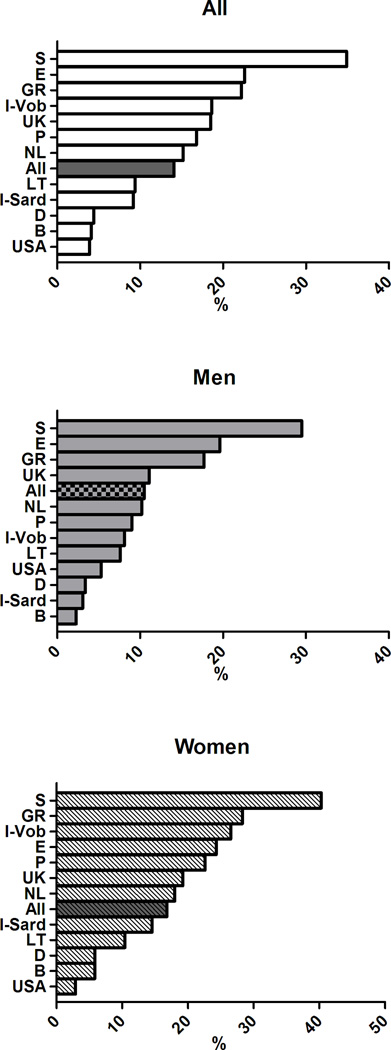
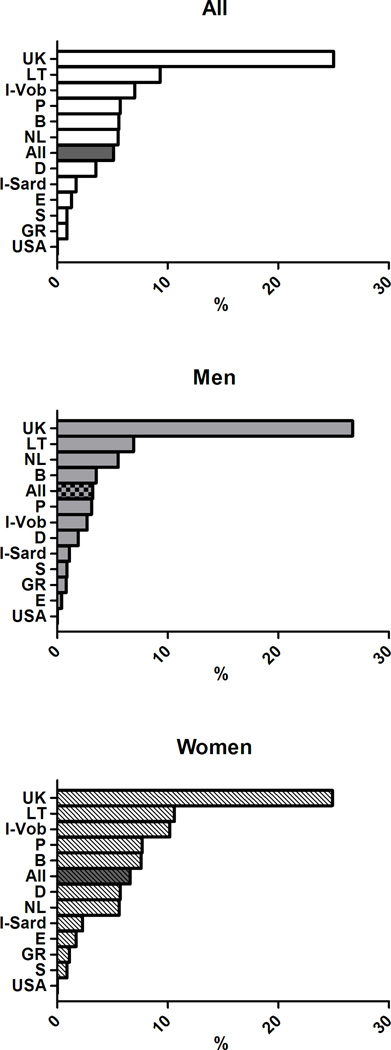
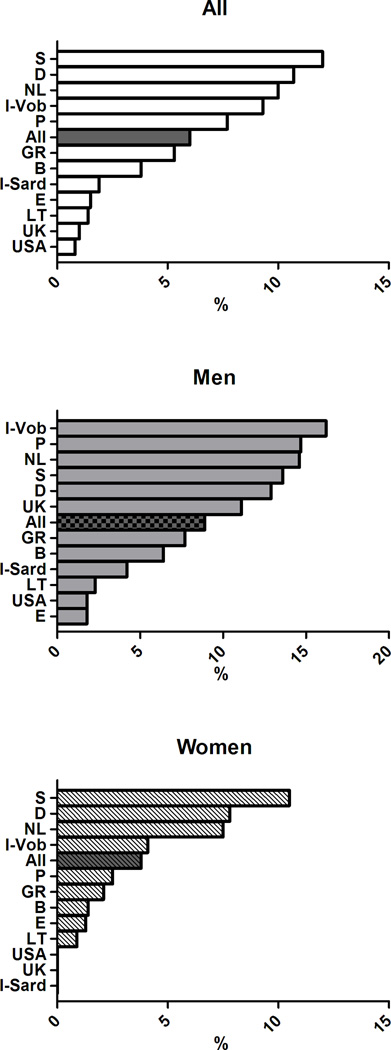
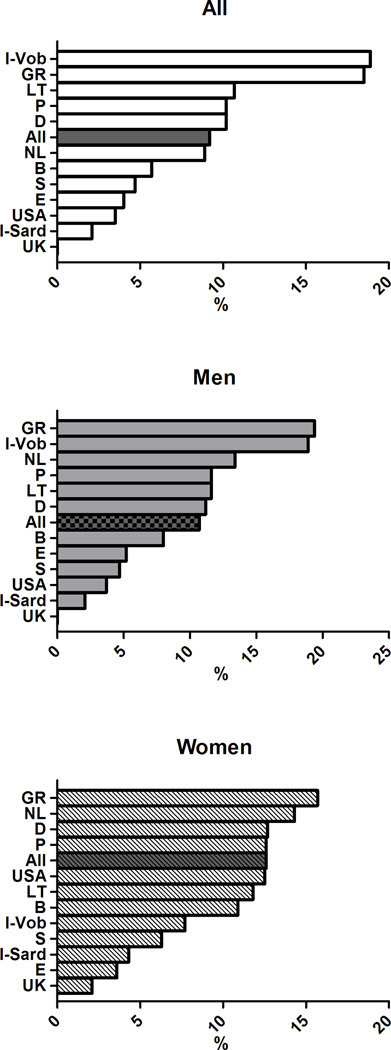
The age-adjusted prevalence of the clusters of MetS components previously associated with greater arterial and CV burden differed across European countries (p< 0.0001) (Figure 4) and in men and women (gender effect p<0.0001). The significant interaction term Gender*Cohort in the ANCOVA analyses also suggested that gender differences in the clusters of MetS components differed across countries.
Figure 4.
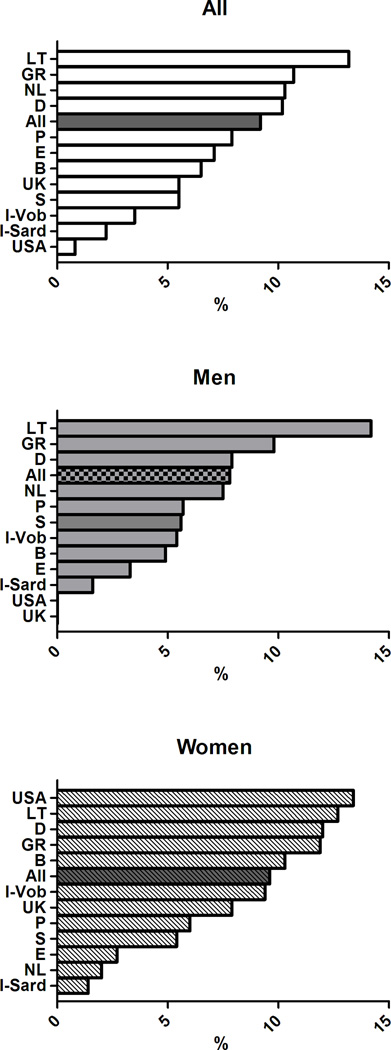
Age-adjusted prevalence of clusters of MetS components in subjects with MetS in participating cohorts of the MARE Consortium: overall prevalence (top panel), men (middle panel), women (bottom panel).
In details, the cluster T-B-W was observed in 12% of the subjects with MetS, but was far more common in the cohorts from UK (32.3%), Sardinia in Italy (19.6%), and Germany (18.5%) but less prevalent in the cohorts from Sweden (1.2%), Spain (2.6%), and USA (2.5%). The cluster G-B-W accounted for 12.7% of subjects with MetS with the highest occurrence in Southern Europe (Italy, Spain, and Portugal - with 31.4%, 18.4%, and 17.1% respectively) and in Belgium (20.4%), and with the lowest occurrence in Northern Europe (Germany, Sweden, and Lithuania - with 7.6%, 9.4%, and 9.6% respectively). The simultaneous alteration in all the five MetS components had a greater prevalence in Lithuania (13%,) and a considerably lower prevalence in the Italian cohorts (2–3%). More details are provided in Supplementary Figures 1–9, where the overall and the gender-specific occurrence of each cluster of MetS with an overall prevalence ≥5% are illustrated.
DISCUSSION
The main goal of the present study was not to investigate differences in MetS prevalence across studies, likely reflecting also differences in recruitment strategy across studies. Rather, we focused on subjects with MetS to identify whether within subjects who had the same diagnosis (MetS defined in accordance with ATPIII criteria) clusters of MetS components significantly differed across studies and countries.
The occurrence of low HDL cholesterol or elevated triglycerides in subjects with MetS was far more common in North European countries and in the USA, whereas elevated blood pressure was observable in more than 90% of subjects with MetS in Southern Europe. Similarly, it is remarkable that abdominal obesity was present in virtually all women with MetS from Southern Europe, UK, and the USA.
That the prevalence of specific clusters of MetS components may differ across countries can be expected based upon varying prevalence according to the various MetS definitions used – from the “glucocentric” WHO definition to the “obesity-centric” IDF one, for instance (12–14). Similarly, there has been inconsistency concerning the CV burden of MetS when different definitions of MetS were adopted (3–4, 15–20). Yet, to date no study has investigated cross-countries distribution of specific clusters of MetS components, which have been recognized previously as associated with greater arterial pathology or risk of CV events (7–8, 10).
The present study has some limitations. The included cohorts may be representative of the recruitment regions, but less representative of their countries. Further limitations arise from the fact that existing data were pooled; there was no harmonization of the studies prior to the data collection. Therefore, our findings cannot immediately be generalized.
An additional limitation may be represented by the adoption of the first ATP III MetS definition rather than of the harmonized 2009 definition (12). This strategy will result in a lower MetS and MetS components prevalence estimates. A similar effect may be the consequence of a population recruitment that occurred in different years across cohorts. Yet, the goal of the MARE Consortium is not primarily to provide the most accurate estimate of MetS prevalence in western countries. Rather, we wanted to highlight cross-country differences in the distribution of separate phenotypes currently falling under the common definition of MetS.
The MARE Consortium cohorts have also major strengths. The large number of men and women of a broad age range, from 12 different countries allow us to conclude that the current definition of MetS comprises constellation of syndromes rather than a single condition. An additional strength of the present findings is represented by the adoption of a single definition of MetS – namely the original ATP III definition – that favours comparison across studies.
The design of the present study does not allow speculation about whether different prevalence rate of MetS components and cluster of MetS components previously recognized to confer higher risk of arterial aging and CV events reflect cross-cultural differences in genetic “risky” allele distribution, in lifestyle ( including food consumption, nutritional intake, and physical exercise) , or in public health policies. For instance, a larger waist circumference – a trat that has been reported to also predict new cases of MetS (21) - may be a consequence of lower socioeconomic status (22), greater caloric intake (6) or less physical exercise, that impacts skeletal muscle energy processing (23–25).
We will further investigate whether the impact of specific clusters of MetS components are constantly associated with accelerated arterial aging, i.e. arterial stiffness and intima.media thickness, across countries as well as in men and women (26) and we will try to identify possible lifestyle and/or genetic factors differing among countries and potentially underlying cross-cultural differences in the distribution of clusters of MetS components
In conclusion, what follows under the common definition of MetS is not a unique entity rather a constellation of cluster of MetS components, likely selectively risky for CV disease, whose occurrence differs across countries.
Supplementary Material
SUMMARY TABLE.
'What is known about topic'
Metabolic syndrome is risky for CV events in younger and older subjects.
Specific clusters of metabolic syndrome components rather than metabolic syndrome per se’ have been associated with accelerated arterial aging, a risk factor for CV events.
'What this study adds'
The distribution of altered components of the metabolic syndrome differs across European countries and US
The prevalence of clusters of metabolic syndrome components previously associated with CV risk change cross-culturally
Future studies will establish whether the above described difference in regional prevalence of cluster of metabolic syndrome components are constantly associated with greater CV burden in populations.
ACKNOWLEDGMENTS
We thank all participants in the cohorts for contributing data to the MARE consortium as well as the funding agencies supporting these cohorts.
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. The metabolic syndrome still lives. Clin Chem. 2005;51:1352–1354. doi: 10.1373/clinchem.2005.050989. [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A, Najjar SS, Morrell CH, Lakatta EG. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005;28:882–887. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 4.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Osugi S, Shimokata K, Honjo H, Morita Y, Yamashita K, Maeda K, Muramatsu T, Shintani S, Matsushita K, Murohara T. Metabolic syndrome and all-cause mortality, cardiac events, and cardiovascular events: a follow-up study in 25,471 young- and middle-aged Japanese men. Eur J Cardiovasc Prev Rehabil. 2011;18:574–580. doi: 10.1177/1741826710389529. (2011) Epub 2011 Feb 14. [DOI] [PubMed] [Google Scholar]
- 6.Broekhuizen LN, Boekholdt SM, Arsenault BJ, Despres JP, Stroes ESG, Kastelein JJP, Khaw KT, Wareham NJ. Physical activity, metabolic syndrome, and coronary risk: the EPIC–Norfolk prospective population study. Eur J Cardiovasc Prev Rehabil. 2011. 2011;18:209–217. doi: 10.1177/1741826710389397. Epub 2011 Jan 28. [DOI] [PubMed] [Google Scholar]
- 7.Scuteri A, Najjar SS, Orru' M, Usala G, Piras MG, Ferrucci L, Cao A, Schlessinger D, Uda M, Lakatta EG. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J. 2010;31:602–613. doi: 10.1093/eurheartj/ehp491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. JACC. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Kahn R, Buse J, Ferranini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 10.Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino R. Trajectoreis of entering the metabolic syndrome. The Framingham Heart Study. Circulation. 2009;120:1943–1950. doi: 10.1161/CIRCULATIONAHA.109.855817. [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III).Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 13.Athyros VG, Ganotakis ES, Tziomalos K, Papageorgiou AA, Anagnostis P, Griva T, Kargiotis K, Mitsiou EK, Karagiannis A, Mikhailidis DP. Comparison of four definitions of the metabolic syndrome in a Greek (Mediterranean) population. Curr Med Res Opin. 2010;26:713–719. doi: 10.1185/03007991003590597. [DOI] [PubMed] [Google Scholar]
- 14.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, Morris R, Zavaroni I, van Dam R, Feskins E, Gabriel R, Diet M, Nilsson P, Hedblad B. European Group For The Study Of Insulin Resistance (EGIR). Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–376. [PubMed] [Google Scholar]
- 15.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, Ford I, Forouhi NG, Freeman DJ, Jukema JW, Lennon L, Macfarlane PW, Murphy MB, Packard CJ, Stott DJ, Westendorp RG, Whincup PH, Shepherd J, Wannamethee SG. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Scuteri A, Orru' M, Morrell CH, Tarasov K, Schlessinger D, Uda M, Lakatta EG. Associations of large artery structure and function with adiposity: Effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis. 2012;221:189–197. doi: 10.1016/j.atherosclerosis.2011.11.045. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timoteo A, Santos R, Lima S, Mamede A, Fernandes R, Ferreira R. Does the new International Diabetes Federation definition of metabolic syndrome improve prediction of coronary artery disease and carotid intima-media thickening? Rev Port Cardiol. 2009;28:173–181. [PubMed] [Google Scholar]
- 19.Ma WY, Li HY, Hung CS, Lin MS, Chiu FC, Lin CH, Shih SR, Chuang LM, Wei JN. Metabolic syndrome defined by IDF and AHA/NHLBI correlates better to carotid intima-media thickness than that defined by NCEP ATP III and WHO. Diabetes Res Clin Pract. 2009;85:335–341. doi: 10.1016/j.diabres.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K DECODE Study Group. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- 21.Scuteri A, Morrell CH, Najjar SS, Muller D, Andres R, Ferrucci L, Lakatta EG. Longitudinal paths to the metabolic syndrome: can the incidence of the metabolic syndrome be predicted? The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2009;64:590–598. doi: 10.1093/gerona/glp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boissonnet C, Schargrodsky H, Pellegrini F, Macchia A, Champagne BM, Wilson E, Tognoni G. Educational inequalities in obesity, abdominal obesity, and metabolic syndrome in seven Latin American cities: the CARMELA Study. Eur J Cardiovasc Prev Rehabil. 2011;18:550–556. doi: 10.1177/1741826710389418. Epub 2011 Jan 31. [DOI] [PubMed] [Google Scholar]
- 23.Van Berendoncks AM, Stensvold D, Garnier A, Fortin D, Sente T, Vrints CJ, Arild SS, Ventura-Clapier R, Wisløff U, Conraads VM. Disturbed adiponectin – AMPK system in skeletal muscle of patients with metabolic syndrome. European Journal of Preventive Cardiology. 2013 doi: 10.1177/2047487313508034. 2047487313508034, first published on October 8, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Andersen K, Lind L, Ingelsson E, Ärnlöv J, Byberg L, Michaëlsson K, Sundström J. Skeletal muscle morphology and risk of cardiovascular disease in elderly men. Eur J Prev Cardiol. 2013 Oct 3; doi: 10.1177/2047487313506828. (2013) [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Landaeta-Díaz L, Fernández JM, Da Silva-Grigoletto M, Rosado-Alvarez D, Gómez-Garduño A, Gómez-Delgado F, López-Miranda J, Pérez-Jiménez F, Fuentes-Jiménez F. Mediterranean diet, moderate-to-high intensity training, and health-related quality of life in adults with metabolic syndrome. Eur J Prev Cardiol. 2013;20:555–564. doi: 10.1177/2047487312445000. Epub 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- 26.Scuteri A, Cunha PG, Cucca F, Cockcroft J, Mattace Raso FU, Muiesan ML, Ryliškytė L, Rietzschel E, Strait J, Vlachopoulos C, Laurent S, Nilsson PM, Lakatta EG for The Metabolic syndrome and Arteries REsearch (MARE) Consortium. Arterial stiffness and influences of the metabolic syndrome: a cross-country study. Atherosclerosis. 2014 (in press) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


