Abstract
We report a case of a 56-year-old man referred by his family physician with an asymptomatic cardiac murmur. Trans-thoracic echocardiography (TTE) suggested an unruptured right sinus of Valsalva aneurysm (SVA) causing extrinsic compression of the right ventricular outflow tract. This was confirmed with an ECG-gated cardiac CT showing a large right SVA measuring 35×37×42 mm in size. Coronary angiography demonstrated non-obstructive coronary artery disease. Ascending thoracic anterior in the right anterior oblique view delineated the right SVA. The patient underwent aortic valve sparing surgical repair of the aneurysm with an excellent result. Echocardiography confirmed obliteration of the aneurysm and normal aortic valve function postoperatively.
Background
Aneurysms of the sinus of Valsalva are rare condition. Unruptured sinus of Valsalva aneurysms are less frequently reported because in most instances they are clinically silent. As a result of this, most of the literature is biased in favour of ruptured sinus of Valsalva aneurysms (SVAs).
Case Summary
The aortic sinuses of Valsalva are three dilations of the aortic root that arise from the three closing cusps of the aortic valve. The right and left sinuses give rise to the right and left coronary arteries, while the non-coronary sinus has no coronary artery. They are thought to facilitate the smooth closure of the aortic valve, thereby avoiding the build-up of abnormal stress in the leaflets.1
Aneurysms of the sinus of Valsalva are rare and can be congenital or acquired. They originate usually from the right coronary sinus or the non-coronary sinus and, rarely, the left coronary sinus.2–4 The incidence is four times greater in males and more common in people of Asian origin.1 3 5 6
Pathophysiology
Congenital SVA is caused by dilatation, usually of a single sinus of Valsalva, from a separation between the aortic media and the annulus fibrosus. A deficiency of normal elastic tissue as seen in Marfan and Ehlers-Danlos syndromes along with an abnormal development of the bulbus cordis has been associated with the development of SVA. Other disease processes such as, atherosclerotic aneurysms, syphilis, endocarditis, cystic medial necrosis and chest trauma that involves the aortic root may also produce SVA. Associated congenital defects are ventricular septal defect (30–50%), aortic insufficiency (20–30%), bicuspid aortic valve (10%) and less commonly coarctation of the aorta, pulmonary stenosis and atrial septal defects.7
Clinical presentation
The true natural history of SVA is unclear. Approximately 25% of reported cases of SVA are clinically asymptomatic and detected on routine two-dimensional (2D) transthoracic echocardiography.
Clinical complications from SVA are often the initial presentation. Rupture of the aneurysmal sac may occur spontaneously or may be precipitated by exertion, trauma or cardiac catheterisation. The aneurysm generally ruptures into an adjoining cardiac chamber causing a fistula.6 Dyspnoea is the most common presenting symptom. Syncope may present secondary to obstruction of the left or right ventricular outflow tract. Atypically, SVA may present with infective endocarditis, which usually originates at the edges of the aneurysm. Myocardial ischaemia due to extrinsic compression and obstruction of epicardial coronary artery flow by an SVA has been infrequently reported as a mode of presentation (figures 1 and 2).8 Tamponade can occur if the aneurysm ruptures into the pericardium. When SVA ruptures, clinical signs of left-to-right shunting may become apparent and these are often indistinguishable from coronary arteriovenous fistulae. A loud, superficial, ‘machine-type’ continuous murmur is heard and accentuated in diastole. Occasionally, a palpable thrill along the right or left lower parasternal border is noticeable. Bounding pulses are sometimes present. Aortic regurgitation is a common association.
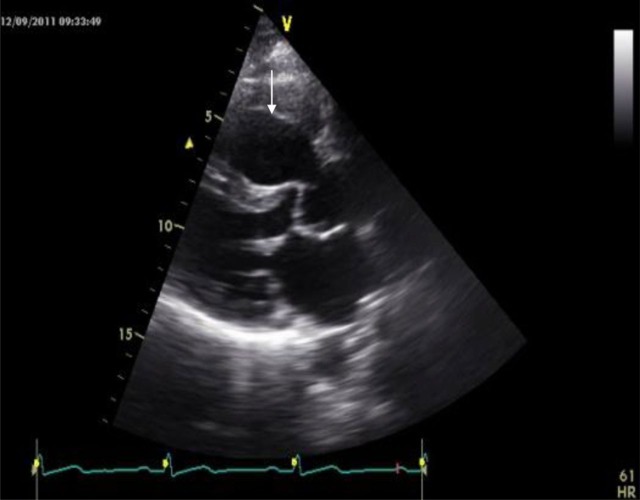
Figure 1.
Transthoracic echocardiography. Parasternal long axis view shows an aneurysm of the right coronary sinus (arrow).
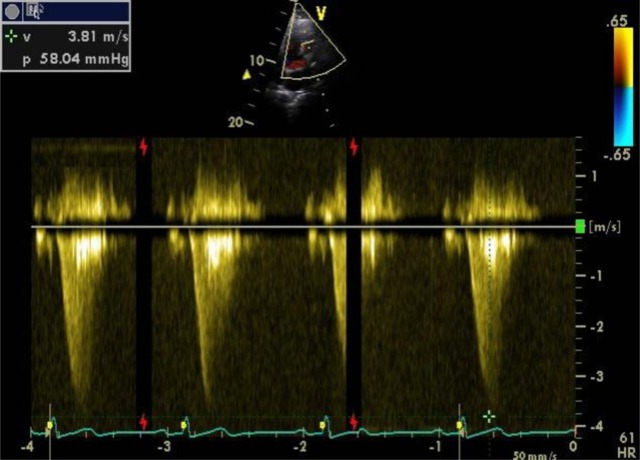
Figure 2.
Continuous wave Doppler demonstrating right ventricular outflow tract obstruction.
A ruptured SVA progresses in three stages as described by Blackshear et al:9
Acute chest or right upper quadrant pain.
Subacute dyspnoea on exertion or at rest (heart failure syndrome) with progressive or acute onset.
Progressive cough, dyspnoea, oedema and oliguria.
Diagnosis and management
Clinical suspicion followed by prompt echocardiographic confirmation is the key to the diagnosis. A 2D transthoracic echocardiography may detect as many as 75% of all patients with SVA. A 3D trans-thoracic echocardiography (TTE) is becoming a valuable tool. Usually CT or MRI is needed to confirm the diagnosis of SVA. This allows precise identification of coexistent structural anomalies and shunt locations for perioperative assessment. Although rarely necessary, the definitive diagnosis can be confirmed by performing retrograde thoracic aortography or cardiac catheterisation. Left-to-right shunting also can be demonstrated if SVA is ruptured (figure 3).
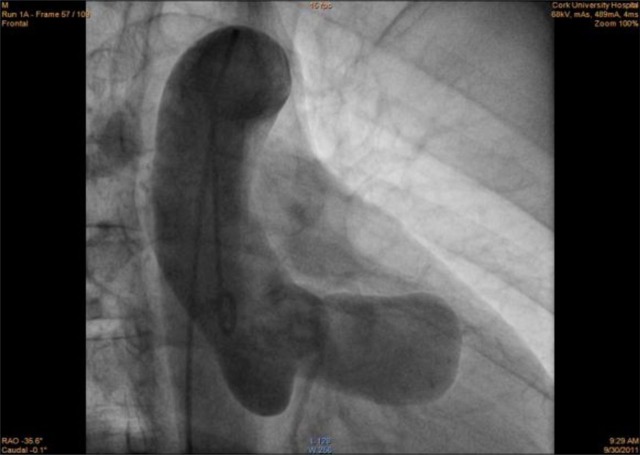
Figure 3.
Sinus of Valsalva aneurysm as seen on aortography.
Unruptured SVA can be managed medically. Treatment of those with aortic root diameter <5 cm includes tight blood pressure control through the use of drugs such as angiotensin receptor blockers and β-blockers; similar to the guidelines for dilated ascending thoracic aorta.10 Aggressive surgical correction of unruptured SVA, with aortic root diameter >5 cm is often recommended because of its association with increased morbidity and mortality. Urgent surgical repair is recommended in all patients with ruptured SVA, especially with intracardiac shunting as clinical deterioration can be rapid.
The surgical approach to repair depends on various factors, such as whether the aneurysm is ruptured, the need to repair or replace the aortic valve and the presence or absence of a ventricular septal defect. The goal is to repair the SVA without interfering with the native aortic valve if possible. Operative procedures commonly include simple placation of the aneurysm or patch repair.11 Aortic valve repair or replacement may occasionally be necessary. Prosthetic aortic root replacement and reimplantation of the coronary arteries are rarely performed in patients with a dilated annulus and multiple sinus involvement, where simple plication or patch repair is not possible, and where the aortic valve cannot be spared. Several investigators have suggested that direct closure with sutures may be associated with a higher incidence of aneurysm recurrence. This ultimately depends on the quality of the surrounding tissues and on the amount of tension on the sutures. Patch closure, therefore, is sometimes used. Although most fistulae repair can be accomplished solely through the chamber of termination, a combination of approaches through the chamber of termination and aortotomy is preferred as it allows observation of the situation of the aortic root and aortic valve simultaneously with the intention of preventing aortic valve tethering.1 6 12 In this patient the SVA was closed directly. Interrupted pledgeted sutures were used to attain a tension-free, secure closure. The sutures were placed close to the aortic valve leaflet but did not interfere with leaflet function as confirmed on postoperative Echo.
Learning points.
Sinus of Valsalva aneurysm aneurysms are rare and can be congenital or acquired.
It could be associated with other congenital defects.
The serious complication that could arise from its rupture combined with the low perioperative death rates have triggered some authors to recommend operating on asymptomatic patients.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Mayer ED, Ruffmann K, Saggau W, et al. Ruptured aneurysms of the sinus of Valsalva. Ann Thorac Surg 1986;42:81–5. [DOI] [PubMed] [Google Scholar]
- 2.Ring WS. Congenital Heart Surgery Nomenclature and Database Project: aortic aneurysm, sinus of Valsalva aneurysm, and aortic dissection. Ann Thorac Surg 2000;69:S147–63. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg N, Krasnow N. Sinus of Valsalva aneurysms. Clin Cardiol 1990;13:831–6. [DOI] [PubMed] [Google Scholar]
- 4.Ott DA. Aneurysm of the sinus of Valsalva. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2006:165–76. [DOI] [PubMed] [Google Scholar]
- 5.Bricker AO, Avutu B, Tan-Lucien H, et al. Valsalva sinus aneurysms: findings at CT and MR imaging 2010;30:99–110. [DOI] [PubMed] [Google Scholar]
- 6.Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg 1999;68:1573–7. [DOI] [PubMed] [Google Scholar]
- 7.Chu HS, Hung CR, How SS, et al. Ruptured aneurysms of the sinus of Valsalva in oriental patients. J Thorac Cardiovasc Surg 1990;99:288–98. [PubMed] [Google Scholar]
- 8.Brandt J, Jogi P, Luhrs C. Sinus of Valsalva aneurysm obstructing coronary arterial flow: case report and collective review of the literature. Eur Heart J 1985;6:1069–73. [DOI] [PubMed] [Google Scholar]
- 9.Blackshear JL, Safford RE, Lane GE, et al. Unruptured noncoronary sinus of Valsalva aneurysm: preoperative characterization by transesophageal echocardiography. J Am Soc Echocardiogr 1991;4(5):485–90. [DOI] [PubMed] [Google Scholar]
- 10.Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med 2008;358:2787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mourad F, Tang A. Sinus of valsalva aneurysm in Blau's syndrome. J Cardiothorac Surg 2010;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katayama S, Umetani N, Sugiura S, et al. The sinus of Valsalva relieves abnormal stress on aortic valve leaflets by facilitating smooth closure. J Thorac Cardiovasc Surg 2008;136:1528–35. [DOI] [PubMed] [Google Scholar]