Abstract
Severe thrombosis and its ischemic consequences such as myocardial infarction, pulmonary embolism and stroke are major worldwide health issues. The ferric chloride injury is now a well-established technique to rapidly and accurately induce the formation of thrombi in exposed veins or artery of small and large diameter. This model has played a key role in the study of the pathophysiology of thrombosis, in the discovery and validation of novel antithrombotic drugs and in the understanding of the mechanism of action of these new agents. Here, the implementation of this technique on a mesenteric vessel and carotid artery in mice is presented. The method describes how to label circulating leukocytes and platelets with a fluorescent dye and to observe, by intravital microscopy on the exposed mesentery, their accumulation at the injured vessel wall which leads to the formation of a thrombus. On the carotid artery, the occlusion caused by the clot formation is measured by monitoring the blood flow with a Doppler probe.
Keywords: Medicine, Issue 100, thrombosis, ferric chloride, carotid artery, mesentery, vascular injury, intravital microscopy, doppler flow meter
Introduction
The study of the mechanisms involved in the development of thrombosis and the evaluation of the effectiveness of anti-thrombotic drugs requires well established experimental animal models. Large animal models were the first to be used as they provide large vessels more similar to humans than rodents1. However, high cost, the larger facilities required and the difficulty in manipulating them genetically are major drawbacks to their use and large animals are now limited to late preclinical studies once preliminary tests on rodents have given conclusive results2. With wide availability of transgenic and knockout strains and their small size that minimizes the quantity of antithrombotic drugs required for in vivo testing, mice are mainly used for thrombosis research. Therefore, several models of thrombotic disorders have been developed in mice3.
Many established thrombosis models disrupt the intima layer of the vessel wall, followed by the exposure of the sub endothelial extracellular matrix to the blood flow inducing the formation of blood clots4. The thrombi may result from the exposure of collagen which triggers platelets activation or/and from the exposure of tissue factor which activates the coagulation cascade5. Several techniques are then employed to achieve the initial vessel injury. Pierangeli et al. developed a mechanical disruption model with a microsurgery tool on the femoral vein6. Kikushi et al. described a model which consists in the administration of a photo reactive compound (Rose Bengal) which accumulates in the lipid bilayer of endothelial cells followed by the specific excitation of the vessel wall of interest with green light (540 nm)7. The injury can also be induced by a short high-intensity pulse laser illumination8. Another technique firstly established on the carotid artery of rats consists in the topical application of ferric chloride (FeCl3)9. In this case, the vessel denudation results from free radicals generated by FeCl3 which causes lipid peroxidation and destruction of endothelial cells10. The injury induces the expression of several adhesion molecules triggering platelet adhesion and aggregation as well as leukocytes recruitment. It has been demonstrated that leukocytes, particularly neutrophils, play a crucial role in the activation of the blood coagulation cascade leading to thrombosis11. This method is well suited to reproduce the coagulation cascade; investigators must keep in mind that, in this mouse model, thrombosis is typically induced in healthy vessels whereas thrombosis in humans is mainly occurring in diseased e.g. atherosclerotic vessels.
As this model is very simple to implement and is also effective in mice, it is now the mostly used thrombosis model for small animal in vivo studies. In addition, this technique offers the possibility to induce the formation of thrombi in a variety of vessels. Target vessels can be arteries or veins of large diameter (carotid, femoral, vena cava) or small diameter (mesentery, cremaster)12–14. More recently, it was also used on the proximal middle cerebral artery to develop a model of stroke15. The thrombosis formation may be directly observed by intravital microscopy after fluorescent labeling of platelet and leukocytes or monitored by measuring the blood flow decrease with a temperature probe or a Doppler probe12,16,17. Several parameters such as occlusion time, thrombus formation time or thrombus size may then be investigated. The physiological differences between the vessels investigated result in significant variations in the thrombi obtained. Therefore, investigators usually select the target vessel according to the parameters they want to measure and/or the disease setting they want to investigate. Typically, the model on the carotid artery is more relevant for research on atherothrombosis related to myocardial infarction or stroke whereas studies on the vena cava are more relevant for research on deep venous thrombosis. The accessibility of the different vessels also determines the method used to measure thrombus growth. For instance, the mesenteric vessels are easy to access making this model well suited for intravital microscopic observation and the study of the dynamics of thrombus formation. The carotid artery is less accessible but bigger enabling blood flow measurements and provide an excellent model to study occlusive thrombosis.
The ferric chloride induced thrombosis model has provided tremendous progress in the understanding of this pathology. It has been used in many studies focusing on the role of von Willebrand factor in thrombosis formation18,19. Combined with genetic modification techniques, it has allowed the identification of many specific gene involved in thrombotic disorders. Lamrani et al. for example have shown that a knock-in of the JAK2V617F gene is associated with an accelerating formation of unstable clot20. Zhang et al. have investigated the physiological implication of the P2Y12 platelet receptor and demonstrated that transgenic mice overexpressing specifically this receptor in platelets only, displayed a more rapid and stable thrombus formation in mesenteric artery injured with FeCl321. The crucial role of Tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) in the fibrin degradation process has also been investigated in this method22. Furthermore this model also provides a simple and accurate way of testing the fibrinolytic capacities of many novel drugs in vivo. For instance, Wang et al. have used this model for the preclinical validation of a novel recombinant plasminogen activator targeted against activated platelets23. This method also enabled the validation of therapeutic proteins isolated from the salivary of ticks, vampire bats, and mosquitos or from the venom of snakes with specific identification of the target24-27. These examples demonstrate the versatility of the ferric chloride model. In this article, we focus on two methods and study ferric chloride induced thrombosis on two different vessel type; mesenteric vessel and carotid artery.
Protocol
All experiments involving animals were approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee (E/1534/2015/B). All surgical manipulations were performed under full anesthesia and the animals did not experience pain at any stage. All experiments described are non-recovery.
1. Preparation
Cut thin bands of filter paper (1 mm x 2 mm).
Freshly prepare 2 solutions of ferric chloride of 4% (w/v) and 6% (w/v) diluted into deionized water. Prepare rhodamine 6G solution 0.3% in PBS, filtered through 0.22 µm.
Cut a small piece (5 mm x 1 cm) from the white plastic of syringe wrapper.
2. Mesentery Arteriole Thrombus Formation Observed by Intravital Microscopy
Weigh a 10-12 weeks old male C57Bl/6 wild type mouse and anesthetize accordingly with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) though intraperitoneal injection. Monitor the depth of anesthesia by response to toe, tail and/or skin pinch, reactivity from corneal and palpebral pinch, and absence of whiskers movement. If necessary, inject a second dose of ketamine (50 mg/kg) to maintain anesthesia of the animal. Apply vet ointment on the eyes to prevent dryness while under anesthesia. Place the mouse in a small petri dish placed on a heating pad adjusted to 37 °C. NOTE: Although IP injection may result in pain for the animals, the duration of this discomfort will be minimal (less than 3 sec). Pain and discomfort associated with injections will be minimized through the use of experienced and competent personnel and appropriate needle size (25 G). Following all the procedures, euthanize all animals using an overdose of ketamine and xylazine followed by cervical dislocation.
Perform an abdominal midline incision of about 3 cm in the skin and carefully cut the peritoneum.
Position the mouse on the side of the petri dish, gently exteriorize its intestines and carefully spread the mesentery with 2 cotton buds to bring a suitable vessel to the surface of the Petri dish. Dry properly with a delicate wiper. NOTE: To limit the movement of the mesenteric vessels, Papaverine may be used to inhibit gut peristalsis.
Soak the tail of the mouse in warm water to dilate the vessels and inject 30 µl of Rhodamine 6G (0.3%) into the tail vein of the mouse with a 29 G syringe to label leukocytes and platelets.
Place the Petri dish under an inverted microscope and focus on the chosen arteriole using the bright field channel.
Soak a band of filter paper with 6% (w/v) iron (III) chloride and apply the filter paper on the arteriole with two forceps; the first one to hold the filter paper, the second one to gently press it onto the area of interest. Observe thrombus formation in the first 10 sec following the deposition of the filter paper. NOTE: It is quite common to injure the surrounded microvasculature and the precision of the deposition of the filter paper and the gently pressure is therefore important to limit this issue.
Observe thrombus formation by fluorescence microscopy (TRITC channel: peak excitation 557 nm, peak emission 576 nm), through the filter paper. Observe the circulating leukocytes and platelets that have taken up the Rhodamine 6G and their aggregation into the thrombus is therefore easy to identify.
Take the filter paper off after 1 min of exposure to iron (III) chloride and continue to monitor the formation of the thrombus. Wash the vessel with PBS.
Observe and record the dynamic formation of the thrombus highlighted with the labelling of platelets and leukocytes with the Rhodamine 6G. Capture the images and measure the size of the thrombus. The herein presented images were obtained with an inverted intravital microscope, though an objective 4X, in the TRITC fluorescence channel.
Following all the procedures, euthanize the animal using an overdose of ketamine and xylazine followed by cervical dislocation.
3. Carotid Artery Thrombus Formation Assessed by Blood Flow Velocity Measurement
Weigh a 10-12 weeks old male C57Bl/6 mouse and anesthetize accordingly with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) though intraperitoneal injection. Apply vet ointment on the eyes to prevent dryness while under anesthesia.
Fix the mouse under an operating microscope using sticky tape on legs, on a heating pad adjusted to 37 °C.
Make a small pillow out of one wiper and tape it under the head of the mouse to elevate the head slightly. Use a thread loop with tongs to pull the snout down (use upper teeth). This will expose the region of the carotid artery for easy access.
Perform a small 5 mm deep incision of the skin directly below the jaw, down to the sternum.
Dissect the fascia and isolate a fragment of either the left or right common carotid artery above the bifurcation.
Carefully introduce tweezers in-between the artery and the nerve to separate them. Do not disturb the nerve running close to the artery and avoid touching too much of the carotid artery as it may causes damage to the vessel. Isolate a section of a least 5 mm of the artery.
Dry the area of the artery properly with wipers to avoid that any liquid interferes with the FeCl3.
Put the small white plastic piece under the isolated part of the common carotid so the FeCl3 does not soak in the surrounding tissues. For this purpose, use a second forceps to bring the piece to the first one then slowly slide the plastic paper under the artery.
Soak a piece of filter paper with 4% (w/v) or 6% (w/v) ferric chloride and place it all around the artery.
After 3 min exposure, take off the filter paper, rinse with PBS and dry the area with wipers.
Place the Doppler flow probe around the vessel at the injured area and start recording the changes in flow. In the healthy common carotid of adult mice, the flow is usually around 1 ml/min. Caution! contact of the probe with ferric chloride will damage the probe so any contact should be avoided. The herein presented data were obtained with a Transonic System Inc. Flow meter, TS420 perivascular module equipped with a Nano Doppler flow probe 0.5 PBS. NOTE:The concentration of ferric chloride can modify the kinetics of thrombus formation resulting in different occlusion times. Thus, an exposure to 6% (w/v) ferric chloride gives a faster occlusion than exposure to 4% (w/v) ferric chloride.
Following all the procedures, euthanize all animals using an overdose of ketamine and xylazine followed by cervical dislocation and carefully clean the Doppler probe.
Representative Results
The fluorescent intravital microscopy observation of the mesentery will reveal the accumulation of Rhodamine 6G labeled platelets and leukocytes along the vessel wall injured by FeCl3. The progressive formation of a partial thrombus is monitored in a 200 µm mesentery vessel (Figure 1). A thrombus slowly appears and is clearly identifiable after the first minute of exposure to FeCl3 (Figure 1, t = 60 sec). 40 sec after the removal of the filter paper soaked with FeCl3, the thrombosis rapidly progresses and is finally present on the wall of the whole vessel section observed (Figure 1, t = 100 sec).
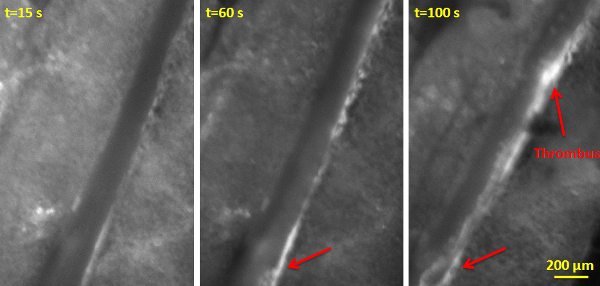 Figure 1: Thrombus Growth Observed by Fluorescent Intravital Microscopy on a Mesentery Vessel. Images were taken at 15 sec, 60 sec and 100 sec after the deposition of the filter paper soaked with 6% (w/v) FeCl3 solution. The filter paper was removed after 60 sec of exposure. Leukocytes and platelets were labeled through pre-injection of Rhodamine 6G (0.5% w/v). Red arrows indicate platelets/leukocytes aggregates. Scale bar 200 µm. Please click here to view a larger version of this figure.
Figure 1: Thrombus Growth Observed by Fluorescent Intravital Microscopy on a Mesentery Vessel. Images were taken at 15 sec, 60 sec and 100 sec after the deposition of the filter paper soaked with 6% (w/v) FeCl3 solution. The filter paper was removed after 60 sec of exposure. Leukocytes and platelets were labeled through pre-injection of Rhodamine 6G (0.5% w/v). Red arrows indicate platelets/leukocytes aggregates. Scale bar 200 µm. Please click here to view a larger version of this figure.
An intra-carotid thrombus is induced by the application of a filter paper soaked in FeCl3 solution around an isolated carotid artery and the changes in blood flow downstream of the injury is recorded with a Doppler flow probe (Figure 2). An overall constant blood flow around 1.1 ml/min is measured in the non-injured carotid artery. After a 3 min exposure of the vessel with a filter paper soaked in 4% (w/v) FeCl3 solution, an occlusive thrombus is obtained with an occlusion time of 13 min and 30 sec after the beginning of the exposure. After a 3 min exposure with a filter paper soaked in 6% (w/v) FeCl3, an occlusive thrombus is obtained with an occlusion time of 9 min and 30 sec after the beginning of the exposure.
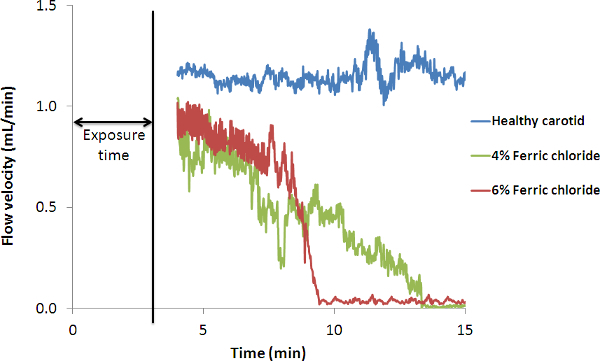 Figure 2. Representative Recordings of Blood Flow through the Carotid Artery after FeCl3 Injury. Blood flow was measured with a Doppler flow probe placed on the carotid artery just downstream of the filter paper soaked with 4% (w/v) or 6% (w/v) FeCl3. The filter paper was removed after 3 min of exposure. As a control blood flow was obtained by measuring the healthy carotid artery.
Figure 2. Representative Recordings of Blood Flow through the Carotid Artery after FeCl3 Injury. Blood flow was measured with a Doppler flow probe placed on the carotid artery just downstream of the filter paper soaked with 4% (w/v) or 6% (w/v) FeCl3. The filter paper was removed after 3 min of exposure. As a control blood flow was obtained by measuring the healthy carotid artery.
Discussion
The ferric chloride induced thrombosis model is an excellent research tool. As shown in this study, it is extremely easy to implement and when used in combination with intravital microscopy or Doppler flowmeter, it provides a good real-time monitoring of thrombus formation. Adjusting the time exposure and the concentration of FeCl3, it also offers the possibility to produce either non-occlusive or occlusive thrombi.
However, this method also has some limitations. In the carotid artery, the major drawback is that although the occlusion time can effectively be modified, the reproducibility of the model remains too weak to precisely control thrombus size and growth rate10. Several groups have worked on a standardization of the model28,29. Owens et al. suggested that reliable and reproducible occlusion time may be obtained with practice and by reducing all the variation factors such as the age of the mice, the genetic background of the mice, the anesthesia utilized, the technique for the ferric chloride exposition and the concentration of the ferric chloride solution28. The Doppler probe itself also has some limitations with a certain degree of background signal present which can affect the determination of the occlusion. The blood flow may also be altered by the formation of unstable thrombi.
On the mesenteric vessel, the reproducibility may be affected by the size of the vessel that varies more than the carotid arteries and the presence of fat that may decreases the extent of the injury. It has been reported that the thrombi obtained differ according to the size of the vessel wall lesion which may restrain to endothelium shedding or also affect the smooth muscle cells of the media layer30. The laser irradiation model constitutes a good alternative of the ferric chloride model which provides a better reproducibility8. However, it is limited to small vessels that are transparent enough to enable the penetration of the laser. It should be also noticed that in this model, endothelial cells are destroyed after the ferric chloride application and it is therefore not suitable for studies on the role of endothelial cells. However, it is possible to replace the ferric chloride by calcium ionophore to obtain a weaker injury, restricted to the activation of the endothelium31.
Another limitation of this model is that it is not suitable to study long-term evolution of the disease. To fulfill this requirement, Boulaftali et al. have developed dorsal skinfold chambers which enable the monitoring of the same thrombus over several weeks32. This technique is especially well suited to examine the effects of thrombolytic drugs according to the thrombus maturity. In this study, the clot aging was found to impair the lytic action of a recombinant form of tissue plasminogen activator, which is currently the gold standard of thrombolytic drugs for human use.
Despite some drawbacks that must be taken in consideration, the FeCl3 model is relevant to the study of human thrombosis. The composition of the obtained thrombi has been analyzed on histological section and the presence of platelets, fibrin and red blood cells have been identified in the intra-carotid thrombi33. Besides, since atherothrombotic disorder is assumed to be initiated by the oxydation of lipoproteins, inducing the vessel injury though an oxido-reduction reaction the FeCl3 model is more likely to mimic the pathophysiology of the human disease than a mechanical, photo-chemical or laser induced injury34.
The thrombus formed though ferric chloride has also been described to be sensitive to both anticoagulant and anti-platelet drugs. Heparin and Clopidogrel for instance have been reported to extend the occlusion time of thrombi formed in the carotid artery29. The administration of a recombinant form of Hirudin has significantly prolonged the thrombus formation time on the mesentery microvasculature17. Therefore, the ferric chloride model provides excellent insights in thrombosis and is a highly relevant tool for the preclinical validation of new thrombolytic, anticoagulant and anti-platelet drugs.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to acknowledge technical support from Joy Yao and Dr. Karen Alt, as well as funding from the NHMRC and NHF.
References
- Leadley RJ, Chi L, Rebello SS, Gagnon A. Contribution of in vivo models of thrombosis to the discovery and development of novel antithrombotic agents. J Pharmacol Toxicol Methods. 2000;43(2):101–116. doi: 10.1016/s1056-8719(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Johnson GJ, Griggs TR, Badimon L. The utility of animal models in the preclinical study of interventions to prevent human coronary artery restenosis: analysis and recommendations. On behalf of the Subcommittee on Animal, Cellular and Molecular Models of Thrombosis and Haemostasis of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1999;81(5):835–843. [PubMed] [Google Scholar]
- Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92(3):486–494. [PubMed] [Google Scholar]
- Sachs UJH, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100(7):979–991. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- Pierangeli SS, Liu XW, Barker JH, Anderson G, Harris EN. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost. 1995;74(5):1361–1367. [PubMed] [Google Scholar]
- Kikuchi S, Umemura K, Kondo K, Saniabadi AR, Nakashima M. Photochemically induced endothelial injury in the mouse as a screening model for inhibitors of vascular intimal thickening. Arterioscler Thromb Vasc Biol. 1998;18(7):1069–1078. doi: 10.1161/01.atv.18.7.1069. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Raymond S, et al. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am J Pathol. 2001;158:1613–1622. doi: 10.1016/S0002-9440(10)64117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60(4):269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- Eckly A, Hechler B, et al. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9(4):779–789. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- Darbousset R, et al. Involvement of neutrophils in thrombus formation in living mice. Pathol Biol (Paris) 2014;62(1):1–9. doi: 10.1016/j.patbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Denis C, Methia N, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis) Proc Natl Acad Sci U S A. 1998;95(16):9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hagemeyer CE, et al. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012;125(25):3117–3126. doi: 10.1161/CIRCULATIONAHA.111.030312. [DOI] [PubMed] [Google Scholar]
- Wang X, Smith PL, Hsu M-Y, Ogletree ML, Schumacher WA. Murine model of ferric chloride-induced vena cava thrombosis: evidence for effect of potato carboxypeptidase inhibitor. J Thromb Haemost. 2006;4(2):403–410. doi: 10.1111/j.1538-7836.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- Karatas H, Erdener SE, et al. Thrombotic distal middle cerebral artery occlusion produced by topical FeCl(3) application: a novel model suitable for intravital microscopy and thrombolysis studies. J Cereb Blood Flow Metab. 2011;31(6):1452–1460. doi: 10.1038/jcbfm.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirousková M, Chereshnev I, Väänänen H, Degen JL, Coller BS. Antibody blockade or mutation of the fibrinogen gamma-chain C-terminus is more effective in inhibiting murine arterial thrombus formation than complete absence of fibrinogen. Blood. 2004;103(6):1995–2002. doi: 10.1182/blood-2003-10-3401. [DOI] [PubMed] [Google Scholar]
- Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107(10):3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete A-M, Casari C, et al. A murine model to characterize the antithrombotic effect of molecules targeting human von Willebrand factor. Blood. 2012;120(13):2723–2732. doi: 10.1182/blood-2012-03-420042. [DOI] [PubMed] [Google Scholar]
- Rayes J, Hollestelle MJ, et al. Mutation and ADAMTS13-dependent modulation of disease severity in a mouse model for von Willebrand disease type 2B. Blood. 2010;115(23):4870–4877. doi: 10.1182/blood-2009-11-254193. [DOI] [PubMed] [Google Scholar]
- Lamrani L, Lacout C, et al. Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood. 2014;124(7):1136–1145. doi: 10.1182/blood-2013-10-530832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ye J, et al. Increased platelet activation and thrombosis in transgenic mice expressing constitutively active P2Y12. J Thromb Haemost. 2012;10(10):2149–2157. doi: 10.1111/j.1538-7836.2012.04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer K, Konstantinides S, et al. Different mechanisms of increased luminal stenosis after arterial injury in mice deficient for urokinase- or tissue-type plasminogen activator. Circulation. 2002;106(14):1847–1852. doi: 10.1161/01.cir.0000031162.80988.2b. [DOI] [PubMed] [Google Scholar]
- Wang X, Palasubramaniam J, et al. Towards effective and safe thrombolysis and thromboprophylaxis: preclinical testing of a novel antibody-targeted recombinant plasminogen activator directed against activated platelets. Circ Res. 2014;114(7):1083–1093. doi: 10.1161/CIRCRESAHA.114.302514. [DOI] [PubMed] [Google Scholar]
- Decrem Y, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206(11):2381–2395. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, et al. Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood. 2013;122(25):4094–4106. doi: 10.1182/blood-2013-08-517474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb Haemost. 2014;111(2):279–289. doi: 10.1160/TH13-06-0490. [DOI] [PubMed] [Google Scholar]
- Waisberg M, et al. Plasmodium falciparum infection induces expression of a mosquito salivary protein (Agaphelin) that targets neutrophil function and inhibits thrombosis without impairing hemostasis. PLoS Pathog. 2014;10(9):e1004338. doi: 10.1371/journal.ppat.1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens AP, Lu Y, Whinna HC, Gachet C, Fay WP, Mackman N. Towards a standardization of the murine ferric chloride-induced carotid arterial thrombosis model. J Thromb Haemost. 2011;9(9):1862–1863. doi: 10.1111/j.1538-7836.2011.04287.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115(1-2):95–100. doi: 10.1016/j.thromres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Tseng MT, Dozier A, Haribabu B, Graham UM. Transendothelial migration of ferric ion in FeCl3 injured murine common carotid artery. Thromb Res. 2006;118(2):275–280. doi: 10.1016/j.thromres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Bonnard T, et al. Leukocyte mimetic polysaccharide microparticles tracked in vivo on activated endothelium and in abdominal aortic aneurysm. Acta Biomater. 2014;10(8):3535–3545. doi: 10.1016/j.actbio.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Boulaftali Y, Lamrani L, et al. The mouse dorsal skinfold chamber as a model for the study of thrombolysis by intravital microscopy. Thromb Haemost. 2012;107(5):962–971. doi: 10.1160/TH11-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinides S, Schäfer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001;103(4):576–583. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- Li W, McIntyre TM, Silverstein RL. Ferric chloride-induced murine carotid arterial injury: A model of redox pathology. Redox Biol. 2013;1(1):50–55. doi: 10.1016/j.redox.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


