Abstract
The kidney is essential for fluid homeostasis, blood pressure regulation and filtration of waste from the body. The fundamental unit of kidney function is the nephron. Mammals are able to repair existing nephrons after injury, but lose the ability to form new nephrons soon after birth. In contrast to mammals, adult fish produce new nephrons (neonephrogenesis) throughout their lives in response to growth requirements or injury. Recently, lhx1a has been shown to mark nephron progenitor cells in the adult zebrafish kidney, however mechanisms controlling the formation of new nephrons after injury remain unknown. Here we show our method for robust and reproducible injury in the adult zebrafish kidney by intraperitoneal (i.p.) injection of gentamicin, which uses a noninvasive visual screening process to select for fish with strong but nonlethal injury. Using this method, we can determine optimal gentamicin dosages for injury and go on to demonstrate the effect of higher temperatures on kidney regeneration in zebrafish.
Keywords: Developmental Biology, Issue 102, kidney, mesonephros, gentamicin, injection, zebrafish, injury, regeneration, Lhx1a, heat shock, adult
Introduction
The kidney is essential for fluid homeostasis, blood pressure regulation and filtration of waste from the body. Although mammals are able to repair existing nephrons after injury using differentiated epithelial cells1-4, they seem to lack a pool of reserved stem cells5 and are unable to form new nephrons de novo. In contrast to mammals, adult fish are able to form new nephrons throughout adult life to support the growth of the fish and in response to injury6,7. The zebrafish, Danio rerio, is an invaluable model organism for the study of organ regeneration8-10 and has the potential to provide powerful insights into applications for engineering the repair of human kidneys. The Tg(Lhx1a:EGFP) transgene11 has been shown to label a pool of nephron progenitor cells in the adult zebrafish kidney12, however mechanisms controlling the response of lhx1a+ cells to injury remain unclear.
The aminoglycoside gentamicin is a widely used antibiotic with known nephrotoxic and ototoxic effects in humans13. Intraperitoneal injection of gentamicin is an established method of inducing acute kidney injury in fish6. This injury in fish mimics the loss of tubular epithelium and scarring of glomeruli that occurs in humans after gentamicin overdose14. Inducing injury in zebrafish by gentamicin injection is a convenient way of inducing a strong, synchronous regeneration response, with many new nephrons produced and simultaneously proceeding through stages of formation, proliferation and differentiation.
This protocol details our method for robust and reproducible injury in the adult zebrafish kidney by utilizing a noninvasive visual screening process to minimize outliers. We take advantage of the fact that injury with gentamicin leads to death of epithelial kidney tissue and formation of renal tubular casts, which then accumulate into masses in the mesonephric ducts and cloaca. These are passed by the fish and can be observed visually in the water. This allows us to screen for fish with strong nonlethal injury, which can then be pooled for further experimentation. Minimizing the numbers of uninjured fish or fish that die before they reach the endpoint of the experiment leads to more uniform and efficient data collection and analysis. In addition, no special devices or reagents are required, making this method cost-effective and appropriate for use in an academic or teaching setting. Using our method, here we show the increasing effects of gentamicin dose on kidney regeneration as well as the effect of increased temperature.
Protocol
NOTE: Ethics Statement: All experiments were conducted in accordance with Massachusetts General Hospital guidelines for animal use in research.
1. Advance Preparation
Determine how many adult zebrafish 6-12 months old to injure. Plan to injure 10-20% more fish than will be needed. In order to minimize variability, use age matched sibling fish reared together in the same tank so that they are roughly the same size and have less genetic variability. Female fish are much easier to inject compared to the male fish, due to the larger capacity of the abdomen. However, males and females give similar results.
When doing the experiment for the first time, plot a dose curve to determine the appropriate gentamicin dosage for a specific strain of fish. Test each new batch of gentamicin before use in experiments since the purity of gentamicin varies from batch to batch. Proper dosage should be determined by assessing expression of injury markers such as lhx1a. (See Figure 1A-D).
Prepare gentamicin solution. Gentamicin stock solution may be stored in -20 °C and thawed for later use. For example, for an 80 mg/kg dose (80 mg gentamicin/kg of bodyweight) in a fish weighing 0.5 g, prepare 2 mg/ml of gentamicin in phosphate buffered saline as a convenient working solution. This provides a 20 µl of gentamicin for intraperitoneal injection into the fish. NOTE: In general, 80-120 mg/kg achieve good results, but doses as low as 40 mg/kg may be appropriate. Gentamicin may also be purchased in solution to minimize hazardous exposure to lab personnel. NOTE: Gentamicin in high doses can be toxic. Wear gloves and mask when weighing powder.
Prepare 100 ml of 0.016% tricaine water for anesthetizing fish. Prepare a 25x stock of tricaine (4 g/1 L) dissolved in sterile milliQ water adjust to pH 7 and store at 4 °C until use. Dilute 4 ml in 100 ml of fish water for a working concentration of 0.016%. NOTE: Tricaine is an anesthetic and skin irritant, wear gloves when handling.
Make a fish scoop out of a plastic transfer pipet by cutting the bulb into a scoop shape and cutting 2 slots in the bottom to drain water. Load 1ml syringes (with 10 µl gradations) with the prepared gentamicin solution and attach a 30½ G needle. Remove any air bubbles. Twist the needle on the syringe to make sure that the angled tip on the needle is facing away and the syringe markings are facing forward and readable. For control injections, prepare a syringe and needle with sterile PBS.
Prepare a scale with a clean surface or weigh boat as well as paper towels for drying fish and for holding fish for injection.
Prepare individual small half-liter containers with lids for holding fish for observation overnight post injection. Small transparent plastic mating cages are useful for this purpose. These containers should NOT be white - ideally they should be clear so that the white epithelial casts shed by the fish can be observed on a black bench top. Fill each container with enough fish water for the fish to swim comfortably.
2. Intraperitoneal Injection of Gentamicin
Anesthetize the fish in 0.016% tricaine solution in fish water. Wait for the gill ventilation rate to slow and for the fish to no longer respond to touch.
Scoop up the fish using the fish scoop, moving from head toward tail in order to avoid injuring the gills or fins. Place the fish on paper towels to absorb the excess water by turning the fish out gently on its side and shake off excess water from the scoop.
Scoop up the fish again and place it in the weigh boat on the zeroed scale and weigh the fish. Round to the nearest 0.25 g, and calculate the appropriate amount of gentamicin to inject. NOTE: For a 0.5 g fish use a 20 µl injection at 80 mg/kg dose.
Scoop up the fish again and place it on a dry folded paper towel. If injecting using the right hand, hold the paper towel in the left hand and place the fish’s head pointing left, with the belly easily accessible.
Hold the syringe with the needle at a 45° angle to the skin of the belly, anterior to the cloaca. Push the needle just under the skin, then decrease the angle and slide the needle forward under the skin, avoiding the internal organs. Depress the plunger the appropriate amount, pause to make sure no liquid is coming out around the needle, then withdraw the needle. If holding the fish steady is a problem, brace elbows against torso to stabilize them. NOTE: Wear gloves when handling gentamicin solution.
Drop each fish into an individual container. Observe the fish and ensure its recovery from anesthesia. Keep fish at 28.5 °C overnight. NOTE: If the fish doesn’t revive immediately, use a plastic transfer pipet to irrigate water across its gills to revive it.
Use the same syringe and needle when injecting multiple fish with the same dose of gentamicin. Dispose of the used syringe and needle into the appropriate biohazard sharps container.
3. Post Injection Observation of Injury
The next day (1 day post injury), place the injected fish in its container on a dark surface. White casts of dead epithelial tissue excreted by the injured fish should be visible. (See Figure 1E-H). If there are no casts, either the fish was not injured by the gentamicin injection (either the dose was too low, or some of the gentamicin leaked during the injection process) or the fish was severely injured resulting in complete blockage of the ureters and cloaca with sloughed tissue. NOTE: If the fish is unable to clear the casts from its body it will usually die within 2-3 days and is therefore unusable for longer assays. If no casts are visible, euthanize the fish in tricaine water by immersing for at least 10 min after gill movement stops.
If white tissue casts are visible, set the fish aside and continue checking the other fish. An appropriate dose of gentamicin will result in 80-90% of the fish being usable. Pool the injured fish and then split them into different treatment groups if desired.
4. Care of Recovering Fish
Keep fish in clean water and uncrowded environment. Dirty water and crowding will lead to infections and unintended death. Try to keep no more than 6 fish/500 ml of fish water and change the water daily if possible. NOTE: This is a minimum volume for conservation of space or if the investigator wishes to perform experiments using expensive drug treatments.
Do not feed the fish until 3 days post injury. Recovering fish will not eat at first, and any food in the tank will decompose and promote bacteria growth. Starting at 3 days post injury, feed a small amount once a day.
5. Analysis of Injured Kidneys
For in situ hybridization for lhx1a mRNA expression, kidneys can be harvested from fish at desired timepoints. Euthanize fish in tricaine water on ice at least 10 min until gill movement stops, then remove the head with a razor blade and open the body cavity and remove the internal organs with forceps.
- Leave the kidney in place (pigmented organ attached to the dorsal body wall) and fix the fish in 4% paraformaldehyde in PBS overnight. Dissect out the kidney15 and continue with standard in situ hybridization.
- Briefly, wash the kidneys in PBST (phosphate buffered saline 0.5%Tween 20), permeabilized with proteinase K in PBST (10 µg/ml) for 1 hr at room temperature. Post-fix with 4% paraformaldehyde, and washed again with PBST.
- Then pre-hybridize the kidneys overnight at 68 °C in hybridization buffer (50% formamide, 5x SSC, 50 µg/ml heparin, 500 µg/ml tRNA, 0.1% Tween20, pH 6.0).
- Incubate the samples with digoxigenin labeled probe in hybridization buffer, then wash for 5-10 min each with 100%, 75%, 50%, 25% hybridization buffer at 68 °C, then moved to room temperature and wash twice for 30 min with 2x SSC with 0.1% Tween20, and wash twice for 30 min with 0.2x SSC with 0.1% Tween20.
- Equilibrate the sample 3x 5 min in MAB (0.1 M maleic acid, 0.15 M NaCl, pH 7.5) and blocked overnight at 4 °C in MAB with 10% goat serum. Then incubate samples overnight in anti-digoxigenin-AP Fab 1:5,000 in MAB.
- Wash the sample 5x for 1 hr in MAB, and equilibrate 3x for 15 min in NTMT (0.1 M Tris pH9.5, 0.05 M MgCl2, 0.01 M NaCl, 50 µl Tween20).
- Then treat the kidneys with NBT/BCIP to detect signal. Fix the kidneys with 4% paraformaldehyde and incubated in dimethylformamide to remove excess NBT/BCIP, bleached overnight in deionized water. Wash the kidneys and then photograph in PBST with 50% glycerol under a coverslip.
Representative Results
Gentamicin injury can be confirmed visually by the observation of renal epithelial casts in the water (Figure 1). Different dosages of gentamicin were used to injure adult wildtype TuAB zebrafish, resulting in increasing numbers of lhx1a+ cellular aggregates in the regenerating kidney (Figure 1A-D). White casts can easily be seen in the water 1 day after injury (Figure 1E-H). A low dose of gentamicin resulted in most fish having no casts, while higher doses resulted in over 75% of fish having casts (Figure 1I). In all groups, selecting for fish with casts resulted in a higher average injury level (Figure 1J).
After visual confirmation of injury, fish can be pooled and then split into control and treatment groups for experiments (Figure 2). Wildtype TuAB adult zebrafish were injured with an 80 mg/kg dose of gentamicin. One day after injury, fish with casts were selected, pooled and then split into heat shock and no heat shock groups. Heat shock fish were exposed to 37 °C for 16 hr overnight and an 8 hr rest period at 28.5 °C each day. At 4 days and 7 days post injury fish were fixed for whole mount in situ hybridization and injury level was assessed by density of lhx1a+ aggregates. Heat shock alone does not produce an injury response (Figure 2A, D), but heat shock accelerates the response in injured fish compared to no heat shock controls (Figure 2B, C, G). By 7 days post injury there was no longer a significant difference between injured groups (Figure E, F, H).
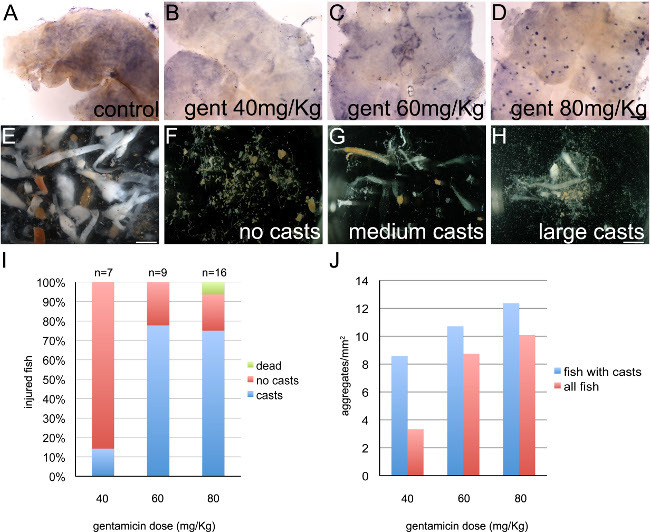 Figure 1: Regeneration in response to acute kidney injury by gentamicin is dose dependent. (A-D) lhx1a mRNA expression marks cellular aggregates, which are new nephron progenitors. (A) 7 days after control i.p. injection, wildtype TuAB adult zebrafish normally have 0-2 lhx1a+ cellular aggregates in the whole kidney. (B-D) 7 days after gentamicin injection fish display many lhx1a+ cellular aggregates, indicating robust neonephrogenesis.This response is dose dependent, with higher concentrations of gentamicin resulting in more lhx1a+ clusters. (E-H) 1 day after injection of gentamicin, fish excrete white casts consisting of dead epithelial tissue from the kidneys. (E) A collection of casts from a total of 12 fish showing the variety of shapes and sizes. Note the difference between white casts indicating injury compared to reddish-orange digested brine shrimp. (F-H) Examples of casts collected from individual fish 1 day after injury by allowing the water in the tank to settle, pipetting the sediment out and observing under a microscope. (F) Excrement with no casts, indicating no injury from a fish treated with the 40 mg/kg dose. (G) Medium sized casts indicating injury from a fish treated with the 60 mg/kg dose. (H) Larger casts with additional sloughed tissue indicating injury from a fish treated with the 80 mg/kg dose. (I) The percentage of injured fish with casts is increased at 60 mg/kg and 80 mg/kg. (J) After 7 days, injected fish were processed for whole mount in situ hybridization to assess injury. lhx1a+ aggregates were counted in a single 5x picture taken at the widest point of each kidney and kidney area was measured using ImageJ. Average number of aggregates/mm2 is shown for each dose. In each case, fish with casts had a higher average than the total group at each dose. p <0.5 for 40 mg/kg compared to 80 mg/kg. Scale bar in A-D = 0.2 mm, E = 1 mm, F-H = 2mm.
Figure 1: Regeneration in response to acute kidney injury by gentamicin is dose dependent. (A-D) lhx1a mRNA expression marks cellular aggregates, which are new nephron progenitors. (A) 7 days after control i.p. injection, wildtype TuAB adult zebrafish normally have 0-2 lhx1a+ cellular aggregates in the whole kidney. (B-D) 7 days after gentamicin injection fish display many lhx1a+ cellular aggregates, indicating robust neonephrogenesis.This response is dose dependent, with higher concentrations of gentamicin resulting in more lhx1a+ clusters. (E-H) 1 day after injection of gentamicin, fish excrete white casts consisting of dead epithelial tissue from the kidneys. (E) A collection of casts from a total of 12 fish showing the variety of shapes and sizes. Note the difference between white casts indicating injury compared to reddish-orange digested brine shrimp. (F-H) Examples of casts collected from individual fish 1 day after injury by allowing the water in the tank to settle, pipetting the sediment out and observing under a microscope. (F) Excrement with no casts, indicating no injury from a fish treated with the 40 mg/kg dose. (G) Medium sized casts indicating injury from a fish treated with the 60 mg/kg dose. (H) Larger casts with additional sloughed tissue indicating injury from a fish treated with the 80 mg/kg dose. (I) The percentage of injured fish with casts is increased at 60 mg/kg and 80 mg/kg. (J) After 7 days, injected fish were processed for whole mount in situ hybridization to assess injury. lhx1a+ aggregates were counted in a single 5x picture taken at the widest point of each kidney and kidney area was measured using ImageJ. Average number of aggregates/mm2 is shown for each dose. In each case, fish with casts had a higher average than the total group at each dose. p <0.5 for 40 mg/kg compared to 80 mg/kg. Scale bar in A-D = 0.2 mm, E = 1 mm, F-H = 2mm.
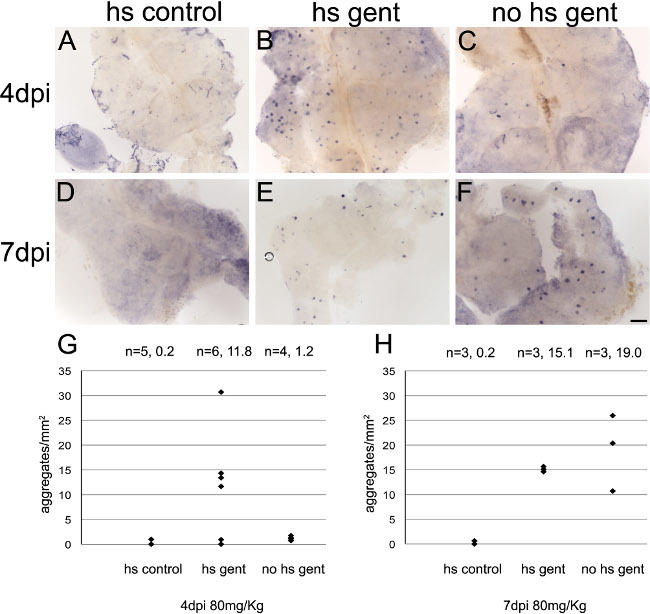 Figure 2: Temperature dependent regeneration. 24 hr after gentamicin injection, injured wildtype TuAB adult zebrafish with casts were pooled and then split into a heat shock group and a no heat shock group. Fish were maintained at 37 °C for 16 hr overnight each night afterward with an 8 hr resting period at 28.5 °C during each day. (A, D) Control uninjured fish did not express lhx1a after heat shock. (B, C) Heat shock accelerates the regeneration response, with more clusters already appearing at 4 days after injury compared to injured fish without heat shock (p <0.5, Student’s t-test). (E, F) After 7 days, there is no significant difference between injured fish treated with heat shock and no heat shock. (G, H) Quantification of injury by counting lhx1a+ aggregates per mm2. Each point represents an individual fish. Number of fish and average response are indicated above each group. Scale bar = 0.2 mm.
Figure 2: Temperature dependent regeneration. 24 hr after gentamicin injection, injured wildtype TuAB adult zebrafish with casts were pooled and then split into a heat shock group and a no heat shock group. Fish were maintained at 37 °C for 16 hr overnight each night afterward with an 8 hr resting period at 28.5 °C during each day. (A, D) Control uninjured fish did not express lhx1a after heat shock. (B, C) Heat shock accelerates the regeneration response, with more clusters already appearing at 4 days after injury compared to injured fish without heat shock (p <0.5, Student’s t-test). (E, F) After 7 days, there is no significant difference between injured fish treated with heat shock and no heat shock. (G, H) Quantification of injury by counting lhx1a+ aggregates per mm2. Each point represents an individual fish. Number of fish and average response are indicated above each group. Scale bar = 0.2 mm.
Discussion
The zebrafish is ideal for studying regeneration of adult organs including the adult mesonephric kidney6. Recent studies have taken advantage of molecular markers and new transgenic reporter lines to better characterize the steps that occur during nephron regeneration and what cells might be responsible7,12. Observation of urinary casts has been used for over a century to diagnose kidney disease in humans16. Here we use the presence of casts as an easy, noninvasive and early indicator of the extent of acute kidney injury after gentamicin injection. We observe that fish that do not produce casts are either only mildly injured (and do not form new nephrons) or too injured to recover and usually die within the first 3 days. Therefore, a critical step within the protocol is the individual observation of fish after injury, and the presence of urinary casts as an indicator of proper injury levels. We also see lhx1a expression in aggregates starting at day 4 after injury. This indicates that the first 3 days after injury are a crucial period for recovery.
If a large number of fish are uninjured, or too many fish are dying during the course of the experiment, check to make sure that the intraperitoneal injections are being done properly. It may help to add a nontoxic dye such as phenol red to the gentamicin solution to visualize any leakage during injection. Marking the needle with a permanent marker or a piece of tape 2 mm from the tip can serve as a guide to prevent pushing the needle too far into the fish. Additionally, the dosage of gentamicin may need to be adjusted. Inject fish with a range of gentamicin doses and test the kidneys for expression of lhx1a mRNA either by in situ hybridization or quantitative RT-PCR.
A limitation of this technique is that it requires housing individual fish overnight before selecting injured fish for experimentation. To investigate very early events in regeneration (within the first 24 hr after injury) fish may need to undergo continuous experimental treatment, even during the individual observation period. The cost of treating individual fish with drugs, for example, may be very high. Our technique is an improvement on the existing method of acute kidney injury using gentamicin, because it allows the researcher to eliminate outliers by utilizing a noninvasive visual screening process. This robust, uniform and reproducible injury model can now be used to effectively test hypotheses about kidney regeneration in the adult zebrafish.
We observed that although regeneration occurs in both male and female zebrafish (all data shown here are from females), regeneration in response to gentamicin injection in males is much more variable. This may be due to technical reasons, because their bodies are long, thin and more muscular, while females have a much larger belly with looser, thinner skin, allowing easier injection and a larger peritoneal cavity to accommodate injection volumes without leakage. An alternative possibility is that androgens produced by males may have some effect on the regeneration process17. Nachtrab et al. found that the effect of androgens was strain dependent, which indicates that genetic background might modulate androgen levels. Slight variations in genetic background of the TuAB fish used in our study might explain the variability seen in males, but presumably androgens are not the only source of variation and other genetic loci may contribute to heterogeneity in the response to injury. We have also found that different transgenic lines of zebrafish may respond differently to gentamicin injury. For example, the Tg(Lhx1a:GFP)12 line in our hands requires 120 mg/kg for efficient induction of injury (data not shown). These observations highlight the importance of using same-sex sibling controls in injury studies to minimize experimental variability.
Many zebrafish transgenic lines use a heat shock sensitive promoter as a means to induce expression of a transgene. Since zebrafish are poikilothermic and do not regulate their body temperature, they are forced to perform physiological functions at the temperature of their environment. In the context of experiments using the hsp70 promoter to express dominant transgenes to modulate the regeneration response, we unexpectedly observed that the regeneration response was accelerated by higher temperature. Therefore, determining the effect of temperature on the regeneration response in a given system becomes crucial to planning the timing of experiments (capturing the peak of the regeneration response for example) and makes comparison to regeneration at normal temperatures problematic. Other inducible systems using for instance CreERT and tamoxifen for transgene induction may be better suited to studies of adult kidney regeneration. Similarly, newer genetic methods of injury induction such as CreERT activation of toxic transgenes or inducible injury using bacterial nitroreductase/metronidazole18,19 will broaden the range of possible experiments to better understand neonephrogenesis and kidney regeneration.
Disclosures
The authors have no competing financial interests to disclose.
Acknowledgments
This work was supported by NIH grant F32DK091998 to CNK; NIH grant RO1DK041071 and Harvard Stem Cell Institute grant D001229 to IAD. The authors thank Neil Hukriede for the lhx1ain situ probe.
References
- Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JK, Cantley LG. Cellular maintenance and repair of the kidney. Annu Rev Physiol. 2010;72:357–376. doi: 10.1146/annurev.physiol.010908.163245. [DOI] [PubMed] [Google Scholar]
- Cirio MC, de Groh ED, de Caestecker MP, Davidson AJ, Hukriede NA. Kidney regeneration: common themes from the embryo to the adult. Pediatr Nephrol. 2013. [DOI] [PMC free article] [PubMed]
- Little MH, Bertram JF. Is there such a thing as a renal stem cell. J Am Soc Nephrol. 2009;20:2112–2117. doi: 10.1681/ASN.2009010066. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42:285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Goessling W, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanhart LM, et al. Characterization of an lhx1a transgenic reporter in zebrafish. Int J Dev Biol. 2010;54:731–736. doi: 10.1387/ijdb.092969ls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- Hentschel DM, et al. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–F929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- Gerlach GF, Schrader LN, Wingert RA. Dissection of the adult zebrafish kidney. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int. 1996;50:1058–1068. doi: 10.1038/ki.1996.409. [DOI] [PubMed] [Google Scholar]
- Nachtrab G, Czerwinski M, Poss KD. Sexually dimorphic fin regeneration in zebrafish controlled by androgen/GSK3 signaling. Curr Biol. 2011;21:1912–1917. doi: 10.1016/j.cub.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, et al. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013;83:1193–1200. doi: 10.1038/ki.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


