Abstract
Environmental enrichment (EE) is a housing environment for mice that boosts mental and physical health compared to standard laboratory housing. Our recent studies demonstrate that environmental enrichment decreases adiposity, increases energy expenditure, resists diet induced obesity, and causes cancer remission and inhibition in mice. EE typically consists of larger living space, a variety of ‘toys’ to interact with, running wheels, and can include a number of other novel environmental changes. All of this fosters a more complex social engagement, cognitive and physical stimulations. Importantly, the toy location and type of toy is changed regularly, which encourages the mice to adapt to a frequently changing and novel environment. Many variables can be manipulated in EE to promote health effects in mice. Thus these approaches are difficult to control and must be properly managed to successfully replicate the associated phenotypes. Therefore, the goal of this video is to demonstrate how EE is properly set up and maintained to assure a complex, challenging, and controlled environment so that other researchers can easily reproduce the protective effects of EE against obesity and cancer.
Keywords: Behavior, Issue 100, Mouse, Environmental Enrichment, Animal Husbandry, Metabolism, Cancer, Hypothalamic-sympathoneural-adipocyte (HSA) axis.
Introduction
Environmental enrichment (EE) is an experimental paradigm used to explore the effects of a complex and challenging environment that does not stress the animals1. EE is an animal housing technique composed of increased space, physical activity, and social interactions, which in turn increases sensory, cognitive, motor, and social stimulation2. Igloos, running wheels, saucer wheels, tube mazes, and other objects in the housing environment foster this sensory cognitive, social, and motor stimulation by promoting exploration and interaction. The layout of the objects in the enclosure changes regularly to present a novel environment, to which the mice are forced to adapt. This strategy offers mild and brief challenges that induce a beneficial, benign, and healthy adaptive response, known as eustress, as opposed to a more aversive, hostile environment or maladaptive distress3,4. By promoting eustress and limiting harmful distress, mice show a variety of favorable phenotypes.
EE is a reliable experimental model with a wide range of applications, while being devoid of elements traditionally thought of as stressful1. The effects of EE on rodents have been studied for more than half a century. In the early 1960s studies showed that cortical cholinesterase levels decreased and subcortical cholinesterase levels increased in rats that were housed in environments with increased living space, increased number of rats, and a variety of wooden toys, mazes, and platforms5,6. In addition, EE is associated with many adaptive changes in the brain, behavior, and metabolism in mice of different ages and genetic backgrounds. Therefore, EE is a useful paradigm for a broad scope of experiments studying the effects of the environment on mice. EE effects can be observed in mice as quickly as one-week exposure, and can be seen in animals as old as eighteen months7. EE is often used in neuroscience and neurobehavioral experiments to study recovery of function, learning and memory2. Moreover, EE has considerable impact on the phenotype of a variety of toxin induced and transgenic animal models of human neurological diseases including Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, Epilepsy, Stroke, and more2.
However, over the years there has been inconsistency with the enrichment design making comparisons between studies from different laboratories difficult. For example, each of the studies on the EE effects relative to human neurological diseases referred to above had varying parameters to the enrichment. The study on Huntington’s disease housed 4-6 mice in large cages (44 x 28 x 12.5 cm3) containing cardboard boxes, tunnels, and sheets, as well as wooden and plastic objects that were changed every two days8,9. One of the studies done on Alzheimer’s housed 20 mice in a larger cage (1 m3) containing 2 running wheels, plastic tubes, cardboard boxes and nesting material that were changed or rearranged weekly10. Many setups of EE have similar themes such as objects to run on or hide in, yet the number of mice per EE housing, the living area, and the period of changing the environment vary much.
The inconsistent designs of EE have led to failed replication of EE effects. Our data on cancer and metabolism together suggest that the combination of stimulations provided in the following detailed EE housing protocol lead to significant anti-cancer and anti-obesity effects14,15. However, the difference of EE settings adopted by different labs might influence the outcomes of EE, as is seen in one failed attempt to reproduce EE effects on the growth of specific cancer tumors implanted in mice11. The different results could be due to a variety of reasons ranging from different growth rates of the tumor cell cultures to the type of detergent used for cleaning housing equipment11. In order to replicate the results seen in our experiments, a particular design of enrichment and animal husbandry is required. The following procedure details the EE housing that will allow other researchers to successfully replicate our efficacious enrichment model.
Protocol
The procedures described below have been approved by the Institutional Animal Care and Use Committee (IACUC) of the Ohio State University.
1. Preparation of Enriched Environment Prior to Housing Mice
- Cleaning and maintaining instruments.
- Run the large enrichment bin (120 cm x 90 cm x 76 cm) through a cage wash. A typical Animal Facility cage wash protocol includes 5 to 6 washing cycles, with the last cycle reaching 82 ºC. The whole washing should take approximately 40 min. Air-dry the bin.
- If the large enrichment bin, and any other housing instruments, cannot be sent through cage wash, then spray and wipe down the inside of the bin with sporicidal disinfectants following the manufacturer’s instructions. Wipe off the bin with a tap water soaked paper towel or 30% ethanol and dry with a paper towel to get rid of any scent from the previous disinfectant.
- Use plastic tubing, igloos with saucer type wheels, and other various plastic hutch-like toys in the EE. Wash these toys through a cage wash. If you do not have access to a cage wash follow step 1.1.1.1.
- Use metal running wheels in the EE. Wash wheels through a cage wash. If the paint on the wheels starts to fade so that the wheel becomes noisy when the animals run on it, apply a small amount petroleum jelly to where the wheel and axel connect.
- Use wooden logs in the EE. Clean the logs by removing any bedding or other debris by hand. Then sterilize the logs by autoclaving them on a dry cycle or textile cycle, which reaches a temperature of 121 ºC for 15 min.
- Use cages between dimensions of 29 cm x 18 cm x 12.5 cm and 30.5 cm x 19 cm x 14 cm to hold a feeding and drinking wire rack.Cut a hole at least 5 cm in diameter out of the front-bottom of the cage for mice to go in and out of from the EE. Clean these feeding cages through cage wash.
- Setting up the environment
- Pour ¼-inch corn cob laboratory animal bedding into the large enrichment bin. Cover the entire floor surface of the bin to a depth of 2-2.5 cm with bedding.
- Place two or three logs in one corner of the bin. Place logs in the corner to sequester an area of about 1,225 cm2. In the area sequestered by the logs, place three igloos with saucer wheels on top. Make sure the wheels are not touching the walls of the bin, the logs, or each other, so that there is enough room for the wheels to spin freely.
- Piece together the plastic tubing so that multiple arms branch out from the centeral connecting piece. Place this tubing in the middle of the bin.
- At two corners of the bin, place one feeding cage each filled with the same bedding as the bin, and the hole in the front of the feeding cage should be facing towards the center of the bin. On top of these feeding cages a clean food and water wire should be placed to hold food and water. Do not place any lid on the cage. Put food and water in the cage wires.
- Place three metal wheels in the bin, though not all next to each other. The three different types of wheels are one 11.5 cm wheel, one 20.5 cm wheel, and one 28 cm wheel. Then place 4-5 more plastic hutch-like toys or tunnels throughout the enrichment bin.
- Cover the bin with micro-isolator filter paper. Use binder clips to clip the filter paper to the bin.
- Novelty is important to EE. Use this setup as the framework of the EE and vary the placement of aforementioned cages, objects, and toys to create a novel environment for the animals.
2. Housing the Animals
Breed animals for experiments in regular laboratory housing and not EE. Optionally, place the animals in EE as early as 21 days of age upon weaning. Genotype and ear punch/tag animals prior to EE housing.
When placing animals into the enrichment bin from a standard cage, place the cage in the bin on its side so that the animals can move freely from the standard cage to the enrichment bin. Do not allow the nesting materials to be transferred into the enrichment bin.
House 10-20 animals of the same age, gender, and genetic strain in the EE.
3. Maintaining the Environment and Observing the Mice During Studies
- Daily
- Quietly observe the animals for signs of illness or distress. If necessary, gently handle the animals to further inspect health conditions. Record abnormal observations and consult a health technician or veterinarian staff if the animal displays conditions that require removal from the experiment.
- Provide topical treatment with triple anti-biotic ointment for small wounds or scabs. If an animal needs to be removed due to a chronic illness or injury, remove the animal from the experiment for euthanasia. If animals meet early removal criteria for the overseeing animal research care committee or veterinarian, do not return animals back to EE.
- Twice a Week
- Check the feed and water levels. Ensure that feed and water will last for the next 3-4 days. Straighten up running wheels and enrichment toys so that they are spread out and can be used by mice. Replace any that are too dirty.
- Weekly
- Replace water bottles with clean water bottles. Rearrange enrichment devices in a manner that follows the setup in Section 1.2. yet is different from the setup of the previous week. NOTE: If an experiment requires the animals to be removed from the enriched environment for an activity (recording body weight, blood draw, behavior test, etc.) it is best to rearrange the environment while the animals are away from the enrichment bin.
- Clean the feeding cages in the EE by hand or send it through cage wash as detailed in Section 1.1.5. Replace the wire racks that hold the food and water with clean wire racks as well.
- Follow the instruction in Section 2.2. when placing the animals back in enrichment. Spot check the rest of the large enrichment bin and clean, if necessary, by scooping out dirty litter and spreading down new clean bedding.
- Bi-weekly
- While performing the weekly husbandry, clean the toys by hand or send through cage wash as detailed in Section 1.1.2. If needed, place the mice in a standard micro-isolator cage for the time required to clean.
- Monthly
- Clean the majority of the litter out by moving enrichment toys and feeding cages and scooping out the litter. Replace with fresh litter. Ensure that there is one inch of bedding in all areas of the cage. If needed, place the mice in a standard micro-isolator cage for the time required to clean.
- Every Three Months (or At End of Experiment)
- Remove all bedding and toys and clean and disinfect the enrichment bin as described in Section 1.1.1. If the experiment is ongoing, replace with new bedding, feeding cages, assortment of toys and then return the mice to the bin.
Disinfect the entire enrichment bin more frequently than every three months if required by the research institution in which the EE is housed. If this is necessary and another large enrichment container is available, set up the additional enrichment container as detailed in Section 1.2. and transfer the animals to the new container, while the previous one is being cleaned.
4. Control Housing Conditions
House animals in the control group in micro-isolator cages of dimensions 30.5 cm x 19 cm x 14 cm. Include a wire rack in the cage for holding food and a water bottle, if the cage is not hooked up to a automatic watering system.
Provide the same corn cob bedding in the control cage as is in the EE. Provide a 4 cm x 4 cm nestlet for the mice to make a nest out of.
Replace and/or clean the cages, feeding wires, food and water once a week. Clean cages and wire racks through cage wash or as detailed in Section 1.1.1.1. Clean the cages more often if animals urinates more frequently or if the drinking water leaks onto the bedding.
Monitor the health of the animals in control housing daily. Assure the animals have plenty of food and water.
House 3~5 mice of the same gender in regular mouse cage. Do not provide any toy, tube, or hutch in the cage.
Institutional laboratory animal care facility health and laboratory technicians may provide husbandry for these control mice.
Keep animals on a 12 hr light/dark cycle as is for the mice in EE.
Representative Results
EE affects growth factor expression, increases neurogenesis and survival of cells within the central nervous system (CNS)12,13. More recent studies have shown that this technique of EE leads to a remarkable suppression of cancer growth in three mouse cancer models including B16 melanoma, MC38 colon cancer and APCmin/+ mouse that contains a germline mutation and is highly susceptible to spontaneous adenoma formation in the intestines14. The results in Figure 2 confirm the suppression of B16 melanoma tumor growth implanted in mice exposed to EE for 5 weeks(~80% decrease of tumor mass). Cancer growth suppression was even observed in mice when EE was initiated after the tumor had been established14. EE resulted in greater inhibition of melanoma growth in the high fat diet induced obesity mice (~70% decrease of tumor mass) than that in mice of normal weight (~50% decrease of tumor mass)14. EE induced signature changes in the serum biomarkers including robust drop of leptin, decrease of IGF-1, and increase of adiponectin levels14.
In addition, this technique of EE decreases adiposity, increases energy expenditure, leads to resistance against diet-induced obesity, and induces a white to brown fat phenotypic switch by activating the hypothalamic-sympathoneural-adipocyte axis15. The decrease in adiposity was seen as early as six days exposed to EE (Figure 3A, B) and again confirmed in animals exposed to EE for four weeks (Figure 3C, D). It is also important to note that, one, the anti-cancer and anti-obesity effects associated with EE cannot be accounted by physical activity alone, and, two, the EE effects can be reproduced by overexpression of brain-derived neurotrophic factor (BDNF) in the hypothalamus14,15.
 Figure 1: Enriched environment bin set up. The enriched environment has an area of 120 cm x 90 cm and includes two modified cages, running wheels, igloos with saucer wheels, plastic tubing, wooden logs, and other plastic enrichment toys.
Figure 1: Enriched environment bin set up. The enriched environment has an area of 120 cm x 90 cm and includes two modified cages, running wheels, igloos with saucer wheels, plastic tubing, wooden logs, and other plastic enrichment toys.
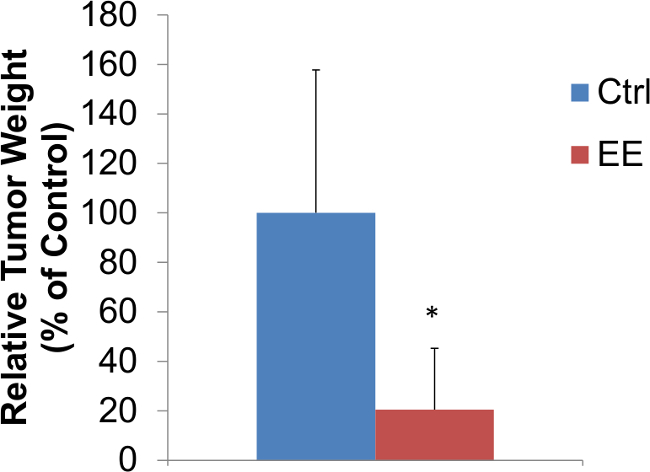 Figure 2: Enriched environment reduces tumor growth in C57BL/6 male mice. Three week old male mice were house in EE or control cages for 5 weeks prior to tumor inoculation. B16 melanoma tumor weights at 17 days after inoculation of 105 cells per animal subcutaneously above the right flank (n = 10 per group. Student’s t-test, * p <0.05).
Figure 2: Enriched environment reduces tumor growth in C57BL/6 male mice. Three week old male mice were house in EE or control cages for 5 weeks prior to tumor inoculation. B16 melanoma tumor weights at 17 days after inoculation of 105 cells per animal subcutaneously above the right flank (n = 10 per group. Student’s t-test, * p <0.05).
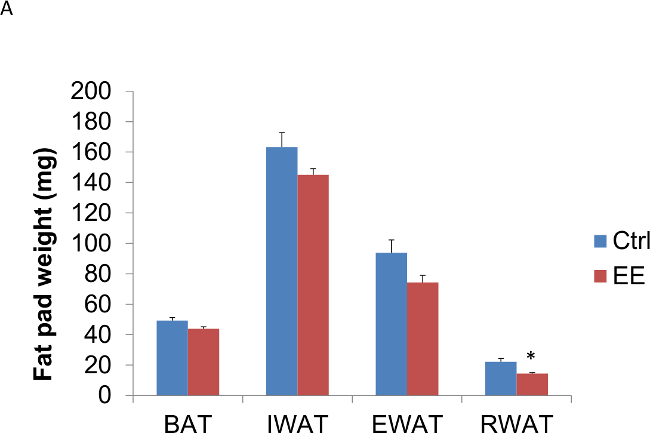
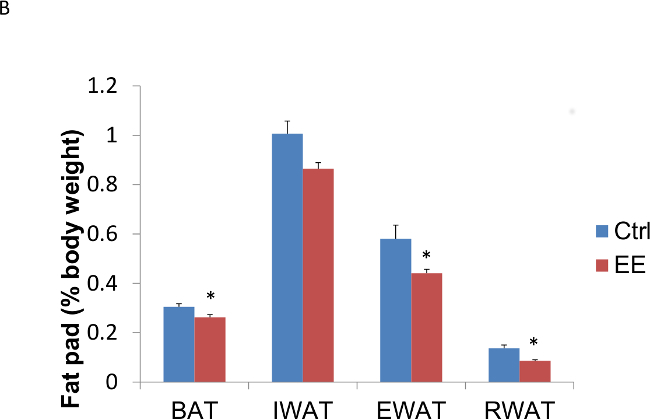
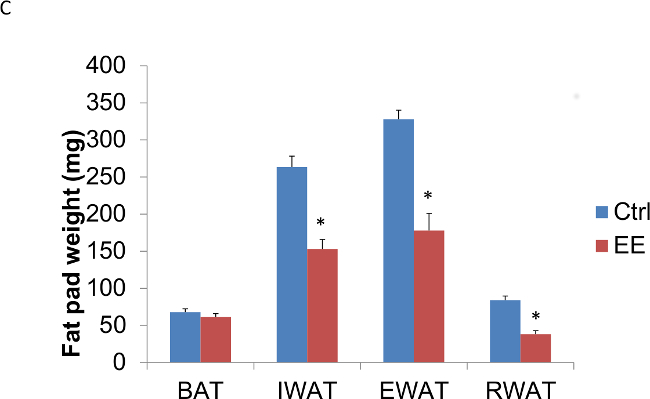
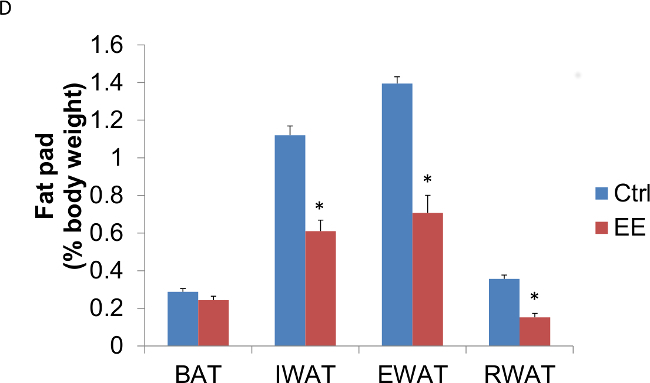 Figure 3: Enriched environment decreases adiposity in C57BL/6 male mice. Three week old mice were subjected to EE; one group for 6 days, another for four weeks. (A) Six days in EE decreased fat pad weights (n = 5 per group. Student’s t-test, *p <0.05). (B) Six days in EE decreased fat pad weights relative to body weight (n = 5 per group. Student’s t-test, *p <0.05). (C) Four weeks in EE decreased fat pad weights (n = 5 per group. Student’s t-test, *p <0.05). (D) Four weeks in EE decreased fat pad weights relative to body weight (n = 5 per group. Student’s t-test, *p <0.05). BAT: brown adipose tissue, IWAT: inguinal white adipose tissue, EWAT: epididymal white adipose tissue, RWAT: retroperitoneal white adipose tissue.
Figure 3: Enriched environment decreases adiposity in C57BL/6 male mice. Three week old mice were subjected to EE; one group for 6 days, another for four weeks. (A) Six days in EE decreased fat pad weights (n = 5 per group. Student’s t-test, *p <0.05). (B) Six days in EE decreased fat pad weights relative to body weight (n = 5 per group. Student’s t-test, *p <0.05). (C) Four weeks in EE decreased fat pad weights (n = 5 per group. Student’s t-test, *p <0.05). (D) Four weeks in EE decreased fat pad weights relative to body weight (n = 5 per group. Student’s t-test, *p <0.05). BAT: brown adipose tissue, IWAT: inguinal white adipose tissue, EWAT: epididymal white adipose tissue, RWAT: retroperitoneal white adipose tissue.
Discussion
The robust effects of EE on gene expression in the brain, decreased adiposity and cancer growth in mice depend heavily on the researcher’s ability to maintain the novel environment and handle the animals in a manner that promotes physical activity, social interaction, and cognitive challenges, yet does not distress the animals. There are two most important procedural steps to remember. First, remember to change the placement of the toys, objects, and cages every week. Even though the toys and objects might be the same from the previous week, the mice perceive the environment as novel. Change the toys and objects themselves with different ones every other week. Second, remember to keep the environment clean. If the soiled bedding does not get removed, the increased nitrogen levels may be a stressor to the animals.
In addition, there are many techniques to note in this EE protocol. The use of different or unique toys and different designs of plastic tube mazes is encouraged. The environment can be challenging but should not pose a threat to the animals’ health. Moreover, beware of toys that are dysfunctional, because the dysfunctional toys increase the risk of mouse limbs getting caught, or might be toxic to the animals if consumed by chewing on it. One constant to the EE is the use of three saucer wheels on top of igloos, sequestered to a corner or side of the bin by 2-3 logs. This setup of running saucers behind the logs has been present in all our enrichment studies. In terms of the use and placement of the other objects, the description of the layout above is a foundational blue print of the EE setup. Variation of the setup with this foundational framework of plastic tubing, running wheels, running saucers, cages and logs should provide a sufficient novel environment to reproduce the reported results.
If working with transgenic animals that have diabetes or other metabolic diseases, pay close attention to the food and water consumption to assure that the animals do not run out of food and water. If the animals have diabetes, they will urinate more, so the bedding of the EE housing may need to be spot checked and cleaned more frequently. The places in the EE housing that need bedding removed most often are the corners. Remember to adjust the wheels and other toys, so that they can be used by the animals. The animals are capable of running saucer wheels into the bedding and knocking running wheels over. If the animals are young, 3 to 5 weeks of age, they might dig under the cages in the EE and hide together under there, leaving the researcher clueless as to where the animals are. When removing soiled bedding, replacing toys, or cleaning cages, it is suggested to put the animals in a clean standard cage so as not to stress them out while adjusting their environment. It is easier to get all the animals in one cage if they have fewer places to hide in the environment. Therefore, removing toys prior to sequestering the animals in a cage makes the process easier. Furthermore, do not place more than 5-6 animals in a single standard cage while waiting for the cleaning or adjusting their EE environment. The male animals in EE can be aggressive particularly when EE is initiated in adulthood. Therefore, it is important to observe the environment for any wounded or dead animals.
In addition, the typical number of animals we house in EE for this large size of container is 10-20 mice. The number of animals other researchers use for EE vary, using around 10 or less mice, and the size of the cage or bin should be taken into consideration when determining the number of animals to house in EE. Having too many animals in a living environment can lead to aggression in male mice. The mice we have housed in EE have been of C57BL/6 backgrounds. Mice of other strains may be more or less aggressive, or more prone to health issues in this EE setup. This should be taken into consideration in determining the husbandry plan for the mice and the enriched environment.
Four weeks EE housing is sufficient to induce decrease of adiposity, drop of leptin level, and inhibition of B16 melanoma growth. Longer EE can lead to stronger effects on metabolism and cancer inhibition. If mice undergo surgical procedures during or before exposure to EE, they may be housed with sutures intact. Whether or not animals respond well to this particular EE setup with surgical staples or catheters intact has not been studied. Furthermore, we are currently investigating how this particular EE affects the lifespan or healthspan of aged animals. Preliminary data suggest that female C57BL/6 mice, 10 months of age, respond to EE similarly to young mice. One limitation of the EE housing is accommodating such a large bin in animal facility space, and preferentially in a gender specific room. Thus future studies on the effects of EE on a smaller scale, as well as finding toys that are easy to clean, endure high temperature cleaning and are safe to the animals health, will improve the applications of the EE in metabolic and cancer research.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
This work was supported by NIH grants CA163640, CA166590, and AG041250 to LC.
References
- Cao L, During MJ. What is the brain-cancer connection. Annu. Rev. Neurosci. 2012;35:331–345. doi: 10.1146/annurev-neuro-062111-150546. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Milsum JH. A model of the eustress system for health/illness. Behav. Sci. 1985;30:179–186. doi: 10.1002/bs.3830300402. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress Without Distress. Toronto: McClelland and Stewart; 1974. [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. J. Comp. Physiol. Psychol. 1960;53:509–519. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: A replication and extension. J. Comp. Physiol. Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Frick KM, et al. Effects of Environmental Enrichment on Spatial Memory and Neurochemistry in Middle-Aged Mice. Learn. Mem. 2003;10(3):187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington’s in mice. Nature. 2000;404:721–722. doi: 10.1038/35008142. [DOI] [PubMed] [Google Scholar]
- Spires TL, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J. Neurosci. 2004;24(9):2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci. 2005;25(21):5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westood JA, Darcy PK, Kershaw MH. Environmental Enrichment does not impact on tumor growth in mice. F1000Research. 2013;2:140. doi: 10.12688/f1000research.2-140.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning. Nat. Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits sponta- neous apoptosis, prevents seizures and is neuroprotective. Nat. Med. 1999;5(4):448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Cao L, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142(1):52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


