Abstract
Traumatic brain injury (TBI) affects millions of people each year, causing impairment of physical, cognitive, and behavioral functions and death. Studies using Drosophila have contributed important breakthroughs in understanding neurological processes. Thus, with the goal of understanding the cellular and molecular basis of TBI pathologies in humans, we developed the High Impact Trauma (HIT) device to inflict closed head TBI in flies. Flies subjected to the HIT device display phenotypes consistent with human TBI such as temporary incapacitation and progressive neurodegeneration. The HIT device uses a spring-based mechanism to propel flies against the wall of a vial, causing mechanical damage to the fly brain. The device is inexpensive and easy to construct, its operation is simple and rapid, and it produces reproducible results. Consequently, the HIT device can be combined with existing experimental tools and techniques for flies to address fundamental questions about TBI that can lead to the development of diagnostics and treatments for TBI. In particular, the HIT device can be used to perform large-scale genetic screens to understand the genetic basis of TBI pathologies.
Keywords: Neuroscience, Issue 100, Drosophila melanogaster, High-Impact Trauma device, mortality, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is defined as injury to the brain from an external mechanical force. Most commonly, TBI results from closed head forces such as blunt forces and inertial acceleration and deceleration forces that cause the brain to strike the inside of the skull. In the United States, it is estimated that 50,000 individuals die each year from TBI and 2.5-6.5 million individuals are living with the consequences of TBI, including debilitating physical, cognitive, and behavioral problems1,2. The consequences of TBI are not only due to primary mechanical injuries to the brain but also to secondary cellular and molecular injuries to the brain as well as other tissues that occur over time3-5. The development of approaches to diagnose and treat TBI has proven to be difficult because TBI is a complex disease process. The variable nature of primary injuries, human physiology, and environmental factors results in heterogeneous secondary injuries and pathologies. Underlying variable factors include the severity of the primary injury, the time between repetitive primary injuries, and the age and genotype of the individual. Understanding how each variable factor contributes to the consequences of TBI is likely to aid in the development of approaches to diagnose and treat TBI6,7.
Here we describe a method for inflicting closed head TBI in Drosophila melanogaster (fruit flies) that can be used to delineate the contribution of variable factors to the consequences of TBI. The method is based on an initial observation that intensely hitting the side of a fly culture vial against the palm of a hand caused wild-type flies to become temporarily incapacitated, a likely consequence of TBI. Thus, we constructed the High-Impact Trauma (HIT) device to recapitulate the acceleration and deceleration forces from the hand-hitting action. A high-speed movie shows that a single strike from the HIT device causes flies to contact the vial wall several times with their head and body8. To some extent, all contacts are likely to cause the fly brain to ricochet and deform against the head capsule, similar to what happens to humans in falls and car crashes9. Accordingly, flies treated with the HIT device display phenotypes consistent with brain injury, including temporary incapacitation followed by ataxia, gradual recovery of mobility, gene expression changes in the head, and progressive neurodegeneration in the brain10. Thus, the HIT device makes it possible to study TBI using the enormous arsenal of experimental tools and techniques developed for flies.
Protocol
1. Construction of the HIT Device
Attach the spring to the board using two clamps and four screws (Figure 1A). Center the clamps relative to the width of the board and butt them up against one another with the outer clamp flush with the edge of the board. Prior to attaching the clamps, bend them using pliers to fit tightly over the spring. NOTE: See Table 1 for descriptions of the materials required for constructing the HIT device. The clamped end of the spring should be 1/8 inch (3.2 mm) from the edge of the board, and the free end should extend over the board by 3/4 inch (19 mm). Adjust the spring so that it lies parallel with the length of the board.
Wrap the free end of the spring once around with the adhesive strip of Velcro loops. The outer edge of the Velcro should be flush with the end of the spring. The Velcro is important because it is used to secure the vial to the spring by creating a tight compression fitting. The Velcro also permits easy connection and removal of vials, allowing many vials to be processed in a short period of time.
Place the ice bucket cover upside down, centered, tight against the wooden board. Orient the raised region of the ice bucket cover such that the long edge is parallel to the width of the board. Note that the raised region of the ice bucket is 1/2 inch (13 mm) higher than the wooden board, so that when a vial is attached to the spring the spring will not lie flat on the board.
Slide the whole device against a fixed object such as a wall, so that the ice bucket cover is wedged between the board and the object and does not move.
Tape the paper protractor to the bottom of a cardboard fly vial tray and stand it on edge against the length of the board so that the 90° mark is aligned with the spring when it is pulled back to a perfectly vertical position.
2. Operation of the HIT Device
Place between 1 and 60 CO2-anesthetized flies in an empty vial and stopper the vial using a tight-fitting cotton ball.
Confine the flies to the bottom 1 inch (2.5 cm) of the vial by pushing the cotton ball into the vial until it is 1 inch (2.5 cm) from the bottom. It is helpful to draw a line on the vial at the 1 inch (2.5 cm) mark. Note that confining flies to larger or smaller regions of the vial can affect the severity of phenotypes.
Wait 5 min for the flies to recover mobility from the CO2. Note that it is not known whether 5 min is sufficient to completely remove the effects of CO2.
Insert the end of the spring into the vial until the inner edge of the Velcro is flush with the top of the vial (Figure 1B). When the spring is lying flat, 1 inch (2.5 cm) of the vial should overlap the raised region of the ice bucket cover. Vials can be reused many times.
While sitting, hold the vial at the Velcro region using the thumb and forefinger of your left hand. Hold the board tight to the benchtop using your right hand. Alternatively, use C-clamps to hold the board tight to the benchtop.
Pull the spring perfectly straight back to the desired angle. Release the spring. Allow the spring to come to a complete stop.
Remove the vial from the spring and allow the flies to recover for ≥5 min. Subject the flies to another strike or transfer the flies to a vial with fly food. NOTE: A variety of assays can be used to evaluate the phenotypic effects of strikes from the HIT device. For example, effects on longevity can be determined by analyzing the percent of flies that survive at times after injury, effects on brain morphology can be determined by histological analysis of the head, and effects on gene expression can be determined by quantitative analysis of mRNA levels10.
Determine effects of the procedure that are not due to strikes by identically treating control flies that are not subjected to strikes. Wear hearing protection because the impact of the vial against the ice bucket cover produces a loud noise.
Representative Results
We are interested in understanding why flies die shortly after primary injury. To quantify death, we determined the Mortality Index at 24 hr (MI24), which is the percentage of flies that died within 24 hr of the primary injury. Flies subjected to strikes from the HIT device were incubated at 25 °C in a vial with fly food, and the number of dead flies was counted after 24 hr. We used this approach to identify factors that affect the MI24 and found that the MI24 is not affected by the number of flies in a vial (10 to 60 flies was tested), the time between repetitive strikes (1 to 60 min was tested), or the sex of the fly10. In contrast, we found that age at the time of the primary injury and genotype did affect the MI24. Older flies had a higher MI24 than younger flies, and flies of different genotypes had significantly different MI24s.
In Figure 2, we tested whether the severity of the primary injury affects the MI24. To alter the severity of the primary injury, we deflected the spring to different angles. For each angle, vials of either 0-7 or 20-27 day old w1118 flies (a standard laboratory strain) were subjected to four strikes with 5 min between strikes. Three vials of 60 flies were examined for each angle. Flies were transferred to vials with molasses food, incubated at 25 °C, and the number of dead flies was counted after 24 hr. The average MI24 and standard error of the mean was calculated for each angle. These data reveal that larger angles of deflection, i.e., more severe primary injuries, result in a higher MI24 than smaller angles of deflection, i.e., less severe injuries. This was observed for both 0-7 and 20-27 day old flies. Furthermore, in accord with Katzenberger et al. 10, 20-27 day old flies had a significantly higher MI24 than 0-7 day old flies at angles ≥50°. Thus, these data indicate that at multiple ages the MI24 correlates with the severity of the primary injury.
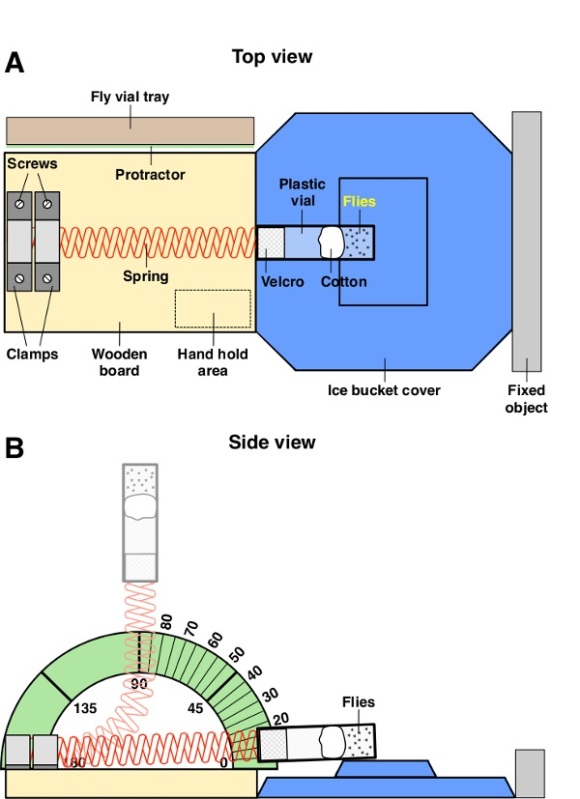 Figure 1: Diagram of the HIT device. (A) Illustration of the top view of the HIT device in the resting position. (B) Illustration of the side view of the HIT device. The spring is shown in the resting position (dark red) and deflected to 90° (light red).
Figure 1: Diagram of the HIT device. (A) Illustration of the top view of the HIT device in the resting position. (B) Illustration of the side view of the HIT device. The spring is shown in the resting position (dark red) and deflected to 90° (light red).
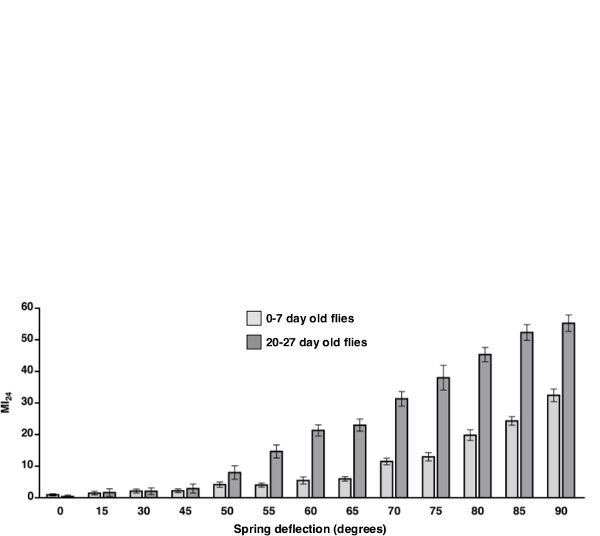 Figure 2: Primary injury severity correlates with the MI24. We determined the MI24 for 0-7 day old w1118 flies (light gray bars) and 20-27 day old w1118 flies (dark gray bars) that were subjected to four strikes from the HIT device with the spring deflected to the indicated angles in degrees. The 0 degree data are for flies not subjected to the HIT device. Shown are the average MI24 and the standard error of the mean. The MI24 of 0-7 and 20-27 day old flies was significantly different for 50° (P <0.05, one tailed t test) and angles > 50° (P <0.001, one tailed t test) but not for angles < 50°(P >0.1, one tailed t test).
Figure 2: Primary injury severity correlates with the MI24. We determined the MI24 for 0-7 day old w1118 flies (light gray bars) and 20-27 day old w1118 flies (dark gray bars) that were subjected to four strikes from the HIT device with the spring deflected to the indicated angles in degrees. The 0 degree data are for flies not subjected to the HIT device. Shown are the average MI24 and the standard error of the mean. The MI24 of 0-7 and 20-27 day old flies was significantly different for 50° (P <0.05, one tailed t test) and angles > 50° (P <0.001, one tailed t test) but not for angles < 50°(P >0.1, one tailed t test).
Discussion
The HIT device method is distinguished from other methods that inflict traumatic injury in flies by the fact that it causes closed head rather than penetrating TBI11. Furthermore, the HIT device method takes less time, effort, and skill to inflict TBI in many flies, so the method is more amenable than other methods to large-scale genetic screens. Lastly, the fact that primary injuries inflicted by the HIT device are not limited to the brain is both a limitation and an advantage. It is a limitation because additional studies are required to assess whether phenotypes are due to traumatic injury to the brain or to other body parts. On the other hand, polytrauma (traumatic injury to multiple body parts) often accompanies TBI and is thought to modulate TBI phenotypes, so the HIT device method can be used to investigate the contribution of polytrauma to TBI phenotypes. The fly TBI model also has advantages over rodent and non-human primate TBI models10. Large numbers of animals can be examined because flies breed rapidly (females lay ~100 eggs/day), have a short lifecycle (10 days from egg to mature adult at 25 °C), and are maintained in small vials and feed on inexpensive medium (~20¢/month/100 flies). In addition, TBI outcomes can easily be evaluated over the entire lifespan because adult flies have a relatively short lifespan (50-100 days at 25 °C). Lastly, an enormous arsenal of experimental tools and techniques developed from over 100 years of previous research on flies provides an unparalleled degree of experimental control for precise temporal and spatial regulation of gene expression; behavioral, developmental, and electrophysiological analyses; in vivo imaging etc.
This protocol describes the construction and operation of the HIT device, which is designed to inflict closed head TBI in flies. We have used the HIT device in conjunction with the MI24 assay to study organismal death following TBI7. However, the fly TBI model can be used in many other ways to understand death as well as other consequences of TBI. For example, physical consequences of TBI can be measured using assays for parameters such as climbing or flight, and behavioral consequences of TBI can be measured using assays for parameters such as sleep and learning and memory10,12-14. Structural effects on the brain can be determined using imaging techniques such as immunofluorescence microscopy and transmission electron microscopy. Molecular effects on the brain can be determined using genomic assays such as RNA-seq or proteomic assays such as mass spectrometry. Genetic effects on the brain can be determined using approaches such as conditional gene knockdown and overexpression. Environmental effects on the consequences of TBI can be determined by varying pre- or post-injury parameters such as incubation temperature and diet or parameters of the HIT device such as the angle of spring deflection or the time between repetitive strikes. Finally, the HIT device can be used for large-scale genetic screens to address fundamental questions such as why the consequences of TBI are worse in older individuals than younger individuals as well as for large-scale drug screens that can be used to identify treatments for the consequences of TBI.
When used in a consistent manner, we have found that the HIT device produces reproducible phenotypes among independent experiments and users. Parameters that can affect reproducibility are the set-up and operation of the HIT device. Phenotypes can be significantly affected by small changes in the position of the ice bucket cover relative to the board, the position of the cotton ball in the fly vial, and the position of vial relative to the Velcro strip. Furthermore, as shown in Figure 2, phenotypes can be significantly affected by small changes in the angle of spring deflection. Thus, new users of the HIT device should be monitored to ensure that the set-up and operation are identical to that of other users. We routinely calibrate new users by having them determine the MI24 of 0-7 day old w1118 flies. Lastly, the HIT device does not need to be identical to the one described in the protocol. We expect that most parts of the HIT device can be altered while maintaining the ability to inflict TBI in flies.
Disclosures
We have no conflicts of interest to disclose.
Acknowledgments
This work was supported by National Institutes of Health grant, R01 AG033620 (BG) and by Robert Draper Technology Innovation Funding (DAW).
References
- Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: A retrospective cohort study. Arch Phys Med Rehabil. 2009;90:1506–1513. doi: 10.1016/j.apmr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Coronado VG, et al. Surveillance for traumatic brain injury-related deaths – United States. MMWR Surveill Summ. 1997;60:1–32. [PubMed] [Google Scholar]
- Masel B, DeWitt DS. Traumatic brain injury: A disease process, not an event. J. Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Disease Models Mech. 2013;6:1307–1315. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon DK. Unique challenges in clinical trails in traumatic brain injury. Crit Care Med. 2009;37:S129–S135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsiger Z, Leudkte J, Mawer S, Willey M. HIT device high speed analysis. 2014. Available from: https://www.youtube.com/watch?v=hkmCXwPacBQ.
- Davceva N, Janevska V, Illevski B, Petrushevska G, Popeska Z. The occurrence of acute subdural haematoma and diffuse axonal injury as two typical acceleration injuries. J Forensic Leg Med. 2012;19:480–484. doi: 10.1016/j.jflm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Katzenberger RJ, Loewen CA, Wassarman DR, Petersen AJ, Ganetzky B, Wassarman DA. A Drosophila. model of closed head traumatic brain injury. Proc Natl Acad Sci USA. 2013;110:E4152–E4159. doi: 10.1073/pnas.1316895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Bonini NM. Axon degeneration and regeneration: insights from Drosophila .models of nerve injury. Annu Rev Cell Biol. 2012;28:575–597. doi: 10.1146/annurev-cellbio-101011-155836. [DOI] [PubMed] [Google Scholar]
- Babcock DT, Ganetzky B. An improved method for accurate and rapid measurement of flight performance in Drosophila. J Vis Exp. 2014. p. e51223. [DOI] [PMC free article] [PubMed]
- Tully T, Preat T, Boynton SC, Vecchio MD. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila.: moving beyond sleep time. Methods Enzymol. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]


